Abstract
Prevention and treatment of bleeding in hemophilia requires that plasma clotting factor activity of the replaced factor exceeds a defined target level. Most clinical decisions in hemophilia are based on implicit or explicit application of pharmacokinetic measures. The large interindividual variability in pharmacokinetics of factor concentrates suggests that relying on the average pharmacokinetic characteristics of factor concentrates would not allow optimizing the treatment of individual patients; for example, adjusting the frequency of infusions and targeting a specific clotting factor activity level on a case-by-case basis. However, individual pharmacokinetic profiles are seldom assessed as part of routine clinical care. Population pharmacokinetics provide options for precise and convenient characterization of pharmacokinetics characteristics of factor concentrates, simplified individual pharmacokinetic profiling, and individualized dosing. Population pharmacokinetics allow for the incorporation of determinants of interpatient variability and reduces the need for extensive postinfusion plasma sampling. Barriers to the implementation of population pharmacokinetics are the need for concentrate-specific pharmacokinetic models, Bayesian calculation power, and specific expertise for production, validation, and appraisal of forecasted estimates. Population pharmacokinetics provide an important theoretical and practical contribution to tailoring the treatment of hemophilia. The need remains for prospective exploration of the clinical impact of tailoring hemophilia treatment based on individual pharmacokinetics, and for the systematic validation of existing software solutions and concentrate-specific models.
Learning Objectives
Learn how knowledge of the individual profile of plasma clotting factor activity over time can help in setting treatment goals and regimens for patients with hemophilia
Learn how to efficiently obtain valuable individual profiles from sparse samples using modalities acceptable to patients and feasible in busy clinical settings
Established applications of PK in current management of hemophilia
The goal of treatment of patients with hemophilia A and B is to minimize the consequences of bleeding into their joints and vital organs and thus improve quality and length of life. Prophylactic administration of the deficient coagulation factor is the preferred approach to minimize the consequences of bleeding.1 Prophylaxis can be continuous, aiming at preventing spontaneous bleeds, or situational (ie, used before incurring an increase in bleeding risk), including surgery. The dose and frequency of factor-concentrate infusions required to minimize the number and severity of bleeding episodes vary largely among individuals, and may vary in the same individual over time.2 Measuring plasma clotting factor activity generated by the infused concentrate at selected times is the most basic and most meaningful of all pharmacokinetics (PKs) measures, and adjusting the dose accordingly is integral part of the treatment of hemophilia. The 3 most common measurements of plasma factor concentrate used to individualize treatment are the measurement of clotting factor activities in the setting of surgery (peaks and troughs); troughs for regular prophylaxis; and recovery and half-life to guide tapering of immune tolerance induction (ITI) treatment and assess its success.
Prevention of bleeding during surgery
To ensure safe surgery (ie, minimize perisurgical bleeding), national and international guidelines recommend maintaining plasma clotting factor activity above specific targets for specific durations of time.3 This goal can be achieved by repeated boluses or continuous infusion of factor concentrate. In either case, patients with hemophilia undergoing surgery require several measurements of plasma clotting factor concentrate activity and dose adjustments to reach and maintain the desired levels.4 These measurements can be done several times per day initially and less frequently once the PK are stable and the bleeding risk reduced. Perioperative dose adjustment based on sequential measurements of plasma clotting factor activities can be considered a simplified and empirical (ie, based on trial and error) PK-guided approach.
Prophylaxis
Besides clinical assessment of bleeding rates, the classical approach to verify the appropriateness of prophylaxis is to measure the plasma clotting factor activity just before the infusion of factor concentrate to ensure that it is still above the intended target (historically assumed to be 0.01 IU/mL, but sometimes less precisely referred to as 1%). Measurement of predose plasma clotting factor activity for patients on prophylaxis is a simplified and empirical PK-guided approach. Indeed, direct measurement of predose plasma clotting factor activity may be complicated because it requires synchronizing the infusion schedule and the visit time. Also, the optimal target level may be different for each patient,2 which can significantly increase the time required for individualization based on trial and error.
Assessment of success of ITI
ITI does not end when the inhibitor is no longer detectable (eg, negative Bethesda or Nijmegen titer). Complete success also requires a return to normal of the recovery and elimination half-life of the infused factor concentrate. Specific thresholds are suggested for defining complete or partial success, or failure.5 Calculation of the recovery or the half-life of the infused factor is another routine use of PK for tailoring individual treatment.
Variability as the rationale for using PK to individualize hemophilia therapy
All of the measurements discussed here and used in clinical practice are needed because of interindividual variability (IIV) in the disposal of factor concentrates and to ensure that clinical decisions are appropriate to the diverse characteristics of each patient. The extent of PK variability is shown in Table 1. PK variability of factor concentrates can be partitioned in several components.6 Some of the variability depends on the concentrate itself; some depends on the patient and can in turn be divided into variability among different patients (IIV) and within each patient over time (intraoccasion variability [IOV]).
Pharmacokinetic characteristics of various factor concentrates
| Single-arm studies . | |||||
|---|---|---|---|---|---|
| Concentrate . | Reference . | Patient age (years) . | t1/2 (mean ± SD, hours) . | Cl (mean ± SD, dL h−1/kg−1) . | Vss (mean ± SD, mL/kg−1) . |
| Single-arm studies | |||||
| Plasma-derived FVIII | Wolf, 2004 | 11-16 | 11.8 ± 4.2 | 0.15 ± 0.05 | NA |
| Saez, 1999 | 18-44 | 12.5 ± 5.4 | 0.51 ± 0.03 | NA | |
| Powell, 2000 | 14-46 | 16.0 ± 3.1 | NA | NA | |
| Advate | Tarantino, 2004 | 10-65 | 11.2 ± 2.5 | 0.30 ± 0.01 | 46.0 ± 10.0 |
| Blanchette, 2004 | <6 | 9.7 ± 1.9 | 0.44 ± 0.01 | 51.4 ± 12.3 | |
| Valentino, 2012 | 7-59 | 13.9 ± 5.3 | 0.39 ± 0.01 | NA | |
| Malhangu, 2014 | ≥12 | 12.4 ± 2.0 | 0.30 ± 0.02 | NA | |
| Kogenate | Abshire, 2000 | 12-60 | 16.8 ± 4.7 | NA | NA |
| Rotschild, 2002 | 12-59 | 14.4 ± 2.7 | NA | NA | |
| ReFacto | Lusher, 2003 | NA | 14.5 ± 5.3 | NA | NA |
| Morfini, 2003* | ≥12 | 17.4 ± 13.0 | 0.38 ± 0.01 | 78.5 ± 25.6 | |
| Santoro, 2009* | 21-69 | 12.9 ± 4.7 | 0.50 ± 0.01 | 46.6 ± 12.1 | |
| Recht, 2009 | 12-60 | 11.2 ± 5.0 | 0.45 ± 0.02 | NA | |
| NovoEight | Jimenez-Yuste, 2015 | ≥12 | 11.2 ± 4.2 | 0.35 ± 0.01 | 46.7 ± 9.6 |
| N8-GP | Tiede, 2013 | 2-60 | 19.0 ± 5.5 | 0.18 ± 0.09 | 45.3 ± 17.8 |
| Eloctate | Malhangu, 2014 | ≥12 | 19.7 ± 2.3 | 0.20 ± 0.01 | NA |
| Crossover studies | |||||
| Monoclonate-P | Deitcher, 1999 | 27-41 | 15.5 ± 2.8 | 0.28 ± 0.06 | 77.1 ± 13.9 |
| Bioclate | 14.9 ± 2.9 | 0.40 ± 0.04 | 95.0 ± 21.9 | ||
| ReFacto | Kessler, 2005 | 18-44 | 14.8 ± 5.6 | NA | NA |
| Hemofil | 13.7 ± 3.7 | ||||
| ReFacto | Di Paola, 2007 | 19-72 | 13.0 ± 3.1 | 0.39 ± 0.14 | 58.6 ± 13.7 |
| Advate | 13.6 ± 3.8 | 0.40 ± 0.14 | 61.7 ± 18.6 | ||
| BAY 79-4980 | Powell, 2008 | 12-60 | 11.4 ± 2.5 | 0.31 ± 0.08 | 50.0 ± 9.5 |
| Recombinate | 11.6 ± 2.5 | 0.31 ± 0.07 | 49.4 ± 9.6 | ||
| N8 | Martinowitz, 2011 | 13-54 | 10.8 ± 4.9 | 0.41 ± 0.11 | 59.8 ± 11.7 |
| Advate | 11.1 ± 3.5 | 0.42 ± 0.12 | 61.3 ± 7.9 | ||
| Benefix | Negrier, 2011 | NA | 19.3 | 0.69 | 194.98 |
| N9-GP | 96.2 | 0.07 | 99.5 | ||
| Plasma derived | 17.8 | 0.55 | 140.58 | ||
| BeneFIX | Santagostino, 2012 | 15-58 | 17.2 ± 2.3 | 0.05 ± 0.01 | 132.5 ± 34.1 |
| F9-fusion protein | 91.6 ± 20.7 | 0.05 ± 0.001 | 99.1 ± 15.5 | ||
| Plasma derived | 14.6 ± 1.7 | 0.007 ± 0.002 | 91.6 ± 15.0 | ||
| BeneFIX | Powell, 2013 | 12-71 | 33.8 ± 2.4 | 0.06 ± 0.004 | 261.1 ± 19.5 |
| Alprolix | 82.1 ± 5.5 | 0.03 ± 0.002 | 314.8 ± 18.8 | ||
| Alphanine | Lissitchov, 2013 | 15-45 | 32.7 ± 7.4 | 0.04 ± 0.001 | 134.0 ± 42.0 |
| BeneFIX | 36.0 ± 12.8 | 0.05 ± 0.001 | 175.0 ± 52.0 | ||
| Single-arm studies . | |||||
|---|---|---|---|---|---|
| Concentrate . | Reference . | Patient age (years) . | t1/2 (mean ± SD, hours) . | Cl (mean ± SD, dL h−1/kg−1) . | Vss (mean ± SD, mL/kg−1) . |
| Single-arm studies | |||||
| Plasma-derived FVIII | Wolf, 2004 | 11-16 | 11.8 ± 4.2 | 0.15 ± 0.05 | NA |
| Saez, 1999 | 18-44 | 12.5 ± 5.4 | 0.51 ± 0.03 | NA | |
| Powell, 2000 | 14-46 | 16.0 ± 3.1 | NA | NA | |
| Advate | Tarantino, 2004 | 10-65 | 11.2 ± 2.5 | 0.30 ± 0.01 | 46.0 ± 10.0 |
| Blanchette, 2004 | <6 | 9.7 ± 1.9 | 0.44 ± 0.01 | 51.4 ± 12.3 | |
| Valentino, 2012 | 7-59 | 13.9 ± 5.3 | 0.39 ± 0.01 | NA | |
| Malhangu, 2014 | ≥12 | 12.4 ± 2.0 | 0.30 ± 0.02 | NA | |
| Kogenate | Abshire, 2000 | 12-60 | 16.8 ± 4.7 | NA | NA |
| Rotschild, 2002 | 12-59 | 14.4 ± 2.7 | NA | NA | |
| ReFacto | Lusher, 2003 | NA | 14.5 ± 5.3 | NA | NA |
| Morfini, 2003* | ≥12 | 17.4 ± 13.0 | 0.38 ± 0.01 | 78.5 ± 25.6 | |
| Santoro, 2009* | 21-69 | 12.9 ± 4.7 | 0.50 ± 0.01 | 46.6 ± 12.1 | |
| Recht, 2009 | 12-60 | 11.2 ± 5.0 | 0.45 ± 0.02 | NA | |
| NovoEight | Jimenez-Yuste, 2015 | ≥12 | 11.2 ± 4.2 | 0.35 ± 0.01 | 46.7 ± 9.6 |
| N8-GP | Tiede, 2013 | 2-60 | 19.0 ± 5.5 | 0.18 ± 0.09 | 45.3 ± 17.8 |
| Eloctate | Malhangu, 2014 | ≥12 | 19.7 ± 2.3 | 0.20 ± 0.01 | NA |
| Crossover studies | |||||
| Monoclonate-P | Deitcher, 1999 | 27-41 | 15.5 ± 2.8 | 0.28 ± 0.06 | 77.1 ± 13.9 |
| Bioclate | 14.9 ± 2.9 | 0.40 ± 0.04 | 95.0 ± 21.9 | ||
| ReFacto | Kessler, 2005 | 18-44 | 14.8 ± 5.6 | NA | NA |
| Hemofil | 13.7 ± 3.7 | ||||
| ReFacto | Di Paola, 2007 | 19-72 | 13.0 ± 3.1 | 0.39 ± 0.14 | 58.6 ± 13.7 |
| Advate | 13.6 ± 3.8 | 0.40 ± 0.14 | 61.7 ± 18.6 | ||
| BAY 79-4980 | Powell, 2008 | 12-60 | 11.4 ± 2.5 | 0.31 ± 0.08 | 50.0 ± 9.5 |
| Recombinate | 11.6 ± 2.5 | 0.31 ± 0.07 | 49.4 ± 9.6 | ||
| N8 | Martinowitz, 2011 | 13-54 | 10.8 ± 4.9 | 0.41 ± 0.11 | 59.8 ± 11.7 |
| Advate | 11.1 ± 3.5 | 0.42 ± 0.12 | 61.3 ± 7.9 | ||
| Benefix | Negrier, 2011 | NA | 19.3 | 0.69 | 194.98 |
| N9-GP | 96.2 | 0.07 | 99.5 | ||
| Plasma derived | 17.8 | 0.55 | 140.58 | ||
| BeneFIX | Santagostino, 2012 | 15-58 | 17.2 ± 2.3 | 0.05 ± 0.01 | 132.5 ± 34.1 |
| F9-fusion protein | 91.6 ± 20.7 | 0.05 ± 0.001 | 99.1 ± 15.5 | ||
| Plasma derived | 14.6 ± 1.7 | 0.007 ± 0.002 | 91.6 ± 15.0 | ||
| BeneFIX | Powell, 2013 | 12-71 | 33.8 ± 2.4 | 0.06 ± 0.004 | 261.1 ± 19.5 |
| Alprolix | 82.1 ± 5.5 | 0.03 ± 0.002 | 314.8 ± 18.8 | ||
| Alphanine | Lissitchov, 2013 | 15-45 | 32.7 ± 7.4 | 0.04 ± 0.001 | 134.0 ± 42.0 |
| BeneFIX | 36.0 ± 12.8 | 0.05 ± 0.001 | 175.0 ± 52.0 | ||
The table shows PK parameters reported in selected studies. Variability across concentrates can be appraised by comparing values across different rows, whereas the variability among patients is represented by the standard deviations on each of the row. An approximate estimate of the lowest and highest value found in 95% of the population can be calculated as mean minus twice the SD or mean plus twice the SD, respectively. For the half-life of Advate in the Tarantino study, for example, half-life would go from 6.2 to 16.2 hours.
Cl, clearance; NA, not available; t1/2, terminal half-life; SD, standard deviation; Vss, plasma volume at the steady state.
1 stage clotting test, generic standard.
Variability in PK among different concentrates
PK were used for many years to prove the bioequivalence of new concentrates to established ones for the purpose of obtaining marketing approval. The concept of bioequivalence is the PK equivalent to noninferiority, and the goal was to show that PK were preserved in new, safer products. With the introduction of the first bioengineered concentrate (B-Domain Deleted Factor VIII, intended to have higher production efficiency and lesser immunogenicity), PK became a distinctive characteristic of a concentrate. In the case of B-Domain Deleted Factor VIII, PK were called on to explain differential efficacy, diverse effect on laboratory tests, and need for higher doses.7 With the identification of several mechanisms to prolong the half-life of factor VIII and factor IX concentrates, the PK of concentrates became a critical competitive characteristic. The core set of characteristics of new products grew with the addition of the enhanced PK profile to claims of higher safety and hopes of lower immunogenicity. Science progressed in 2 separate, but interrelated, directions. On one side, the capacity for engineering new molecules progressed enormously. On the other side, the approach to PK assessment of factor concentrates changed dramatically. Moving from noncompartmental (the historical reference standard) to multicompartmental assumptions led to an increase in the complexity of PK analyses and reports and added a new source of variability in the PK characteristics of different concentrates. Comparing concentrates based on their PK characteristics would require comparable methods of assessment, which is not always the case in the published literature.
Variability in PK among different individual patients
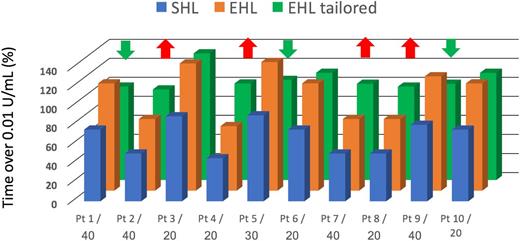
The PK variability among patients can be seen by looking at the standard deviations for the PK parameters associated with each concentrate in Table 1. The range of observed values is generally wide enough to produce a large overlap across most of the concentrates, at least in comparisons within the same “class” (eg, standard or extended half-life). The variability among concentrates can be appraised by comparing averages values of PK parameters across different rows. The variability appears to be greater among different individuals than among different concentrates (at least within the same class).8,9 This observation implies that the combination of dose and dosing interval required by different patients to maintain a specific target varies considerably within each concentrate. A concentrate with more favorable PK will produce “on average” more favorable results in a population of patients and will increase the proportion of patients reaching the desired target, but it will not ensure the best possible result for most patients with optimal use of the available resources (Figure 1). Basing decisions on population averages has little to do with the concept of treatment individualization, which is adjusting the treatment according to an individual patient’s PK profile. It is impossible to predict the individual requirements of each patient based only on the average PK of any of the currently marketed concentrates. Rather, prediction of individual patient requirements necessitates measuring postinfusion plasma clotting factor activities in each patient to establish individual PK profiles.10
Comparing use of average PK parameters to individualized PK profiles. The figure represents the % time spent over 0.01 IU/mL for 10 hypothetical patients. In blue, the time spent by each patient was calculated when they were treated with a standard half-life factor VIII; below each patient label, the U/kg administered to obtain the displayed results. On average, the population of patients was treated with 30 UI/kg and spent 68% of their time above 0.01 IU/mL, with a minimum of 45 and a maximum of 90. The orange columns show the % time spent over 0.01 IU/mL obtained by switching all patients to the same dosage of an extended half-life concentrate; the average time spent over 0.01 IU/mL increases to 100, ranging from 68% to >100%. The green columns show the % time spent over 0.01 IU/mL obtained by switching all patients to the extended half-life concentrate adjusting their dose to their PK (the 5 patients identified by the green arrows had their dose decreases, those identified by the red arrows had their dose increased); the average time spent over 0.01 IU/mL increases to 100, ranging from 95% to >100%. Note that we have hypothesized not to decrease the dose of patient number 3 because it is treated with 20 IU/kg. The average dose in the population would remain 30 IU/kg.
Comparing use of average PK parameters to individualized PK profiles. The figure represents the % time spent over 0.01 IU/mL for 10 hypothetical patients. In blue, the time spent by each patient was calculated when they were treated with a standard half-life factor VIII; below each patient label, the U/kg administered to obtain the displayed results. On average, the population of patients was treated with 30 UI/kg and spent 68% of their time above 0.01 IU/mL, with a minimum of 45 and a maximum of 90. The orange columns show the % time spent over 0.01 IU/mL obtained by switching all patients to the same dosage of an extended half-life concentrate; the average time spent over 0.01 IU/mL increases to 100, ranging from 68% to >100%. The green columns show the % time spent over 0.01 IU/mL obtained by switching all patients to the extended half-life concentrate adjusting their dose to their PK (the 5 patients identified by the green arrows had their dose decreases, those identified by the red arrows had their dose increased); the average time spent over 0.01 IU/mL increases to 100, ranging from 95% to >100%. Note that we have hypothesized not to decrease the dose of patient number 3 because it is treated with 20 IU/kg. The average dose in the population would remain 30 IU/kg.
Variability in PK in the same individual over time and across different concentrates
For some drugs, dose–response varies not only between individuals, but over time in the same individual. A typical example in the field of hemostasis is warfarin, which requires periodic assessment of the international normalized ratio to ensure the dose is tailored to the needs of the patient. Fortunately, the IIV in the PK profile of factor concentrates is limited. Clinical studies reassessing individual PK after 6 to 12 months show that once the PK profile is established, it remains stable over time11-15 ; other studies comparing concentrates in a crossover design9,16,17 show that there is more similarity in within-patient PK profiles for different concentrates than for different patients on the same concentrate. The stability of the PK profile over time extends to the individual PK parameters (area under the curve, clearance, half-life) and shape of the profile (eg, 1- vs 2-compartment disposition).18,19
In summary, the value of using PK to individualize treatment in hemophilia is based on factor concentrates having, relative to their therapeutic window, a large IIV and small IOV. Assessing the individual PK is of value in optimizing the treatment regimen. Using PK, even in the form of simple trough measurements, will be quicker and more efficient than proceeding by trial and error (eg, increasing or decreasing the dose based on the occurrence of bleeding events). Using a formal individual PK profiling approach will be quicker and more efficient than simply using trough measurements.
Goals and advantages of tailoring hemophilia treatment using PK
At both individual and population levels, the most important result of tailoring treatment should be maximizing the net benefit of treatment.20 If the goal was to maximize efficacy, without concerns about the harms of supratherapeutic plasma clotting factor activities, the easiest solution would be to treat all patients with a very high dose to ensure complete protection from bleeding. Even discounting the increased risk of complications (eg, thrombotic events, development of inhibitors), maximizing efficacy would not be affordable or sustainable. Including cost considerations changes the goal to maximizing the cost–benefit ratio of treatment. That is, the goal is to identify the treatment regimen that maximizes the net benefit while keeping the cost in the affordable range. In the Western world, cost goes beyond direct costs to include indirect costs and missed opportunities. Ultimately, cost is replaced by the construct of value.21,22 Value incorporates an economic component as well as entire sets of individual and social preferences and values that may encompass a much broader spectrum of real-life situations.
Optimizing resource use and increasing equitable access to treatment
Replacement therapy for hemophilia is expensive, and there are insufficient resources to cover treatment needs globally. Adoption of PK-tailored prophylaxis can help to optimize use of resources in many ways. First, it holds the promise of making treatment more effective. Regardless of the lower limit of plasma factor concentrate needed to prevent bleeding in each patient, maximizing the time patients spend above that level will decrease risk and, more importantly, the short- and long-term consequences of bleeding. Any reduction in the efficacy of treatment translates to cost inefficiency. Sometimes, some inefficiency is unavoidable, and a modest loss is accepted as a reasonable tradeoff.23 More often, maximum efficiency is associated with optimal use or resources. However, increases in the intensity of treatment beyond that needed for maximum efficiency would be a waste. PK can help by suggesting the dose needed to ensure that the critical threshold is exceeded at any point in time. In the absence of resource constraints, it can indicate how much to increase the dose to obtain the desired target; in resource-constrained settings, it can indicate the highest threshold that can be obtained by adjusting the infusion frequency of any given fixed amount of factor concentrate. In an equity-centered setting, should an extended half-life (EHL) concentrate be affordable for only 50% of the population, individual profiling could be used to identify hemophilia patients with short half-life, which would be “normalized” by the 50% increase. Whether the guiding value should be to ensure that all patients have the infusion frequency they desire or to achieve a target level appropriate for the desired intensity of physical activity, PK could be used to achieve these goals.
Education, adherence, and compliance
Widespread adoption of individual PK profiling would make both physicians and patients more knowledgeable about the strengths and limitations of PK. The calculations required to predict plasma clotting factor activities at various postinfusion times for products with complex disposal patterns (eg, multicompartmental PK) are beyond the capacity of the average patient with hemophilia.10 Determining the effects of dose changes requires complex mathematical calculations involving individual PK estimates. Comparisons of “best educated guesses” and formally calculated postinfusion clotting factor activities resulting from changes in doses or intervals would be helpful. Smartphone apps showing the decay of plasma clotting factor activity after each infusion would allow patients to check whether it was safe to undertake some physical activity or determine which booster dose would be needed to safely participate in a sport at any given time. Alerting functions triggered by reaching risky plasma estimated clotting factor activity levels could minimize the chance of skipping infusions, particularly for regimens with a low frequency of infusion. Adherence to treatment would potentially increase. Behavioral theory supports the construct that deeper knowledge of the physiopathology of disease and the mechanism of action of treatment has a positive effect on compliance, adherence, and persistence.24
Understanding the nuances of the variability in bleeding phenotypes
Knowledge of the estimated plasma clotting factor activity over time would allow patients and physicians to finely correlate bleeding events with predicted plasma clotting factor activities. This could help to identify individualized thresholds for occurrence of bleeding, which could guide the selection of personalized targets for individualized treatment.2 This is a more sophisticated, but similar, approach to determining if spontaneous bleeding events in patients on prophylaxis are correlated with the time elapsed after the previous infusion (eg, they always happen on the preinfusion day) or the omission of a planned dose (nonadherence). When the individual PK profile and treatment and bleeding logs of an individual patient are available, the existence of a correlation between bleeding events and the predicted plasma clotting factor activity can be assessed.25
In summary, knowledge and availability at the point of care of individual plasma clotting factor activities over time can enable physicians and patients to make better informed decisions about the optimal treatment regimen for individualized goals. Reviewing the effect obtained by treatment with knowledge of estimated plasma clotting factor activities at time of events may guide in selecting effective and safe treatment targets. Information technology could assist in scaling the individual plasma clotting factor activity over time profile accordingly to changing a treatment regimen and make it available and actionable at decision points to physicians in the clinic and educated patients in the course of everyday life.
Practicalities and approaches to estimating and using individual PK profiles
If individual PK profiling holds the promise of being useful for optimizing individualized treatment regimens in various contexts, why is its adoption so sketchy in the hemophilia community? There are several possible explanations.
The complexity of performing a structural individual PK study
First and foremost, performing individual PK profiling is cumbersome for patients and clinicians.8,10 Under a multicompartmental assumption, the optimal number of samples needed to estimate the plasma clotting factor activity over time profile is large and scattered over many days. Until recently, there have been few systematic attempts to perform individual PK profiles in clinical practice, even though they are recommended by national and international guidelines.3,26 When attempts were made, assessment of the PK properties of a factor concentrate was usually based on the classical structural approach. In a nutshell, this approach requires several postinfusion samples to define the slope underlying different compartment components of the drug disposition as well as sampling until complete disappearance of the concentrate from the patient’s plasma or at least 5 half-lives have elapsed. In practice, this means sampling 9 to 11 times over 48 to 72 hours.27 Once the plasma clotting factor activity in those samples is measured in the laboratory, a nonlinear regression approach is used to fit a plasma clotting factor activity-over-time curve, assuming 1 or more compartments. A graphical representation of the plasma clotting factor activity-over-time profile curve and the capacity to predict plasma clotting factor activity at given times are the main features of the approach. The capacity to derive PK parameters such as clearance, area under the curve, and terminal half-life is an additional asset. The calculated PK parameters may allow, with help of specialized software, simulation of the effect of administering a different dose or calculation of the appropriate time for reinfusion when targeting a specific predose level. Unfortunately, the number of required samples and technical complexity of the estimation make a traditional PK based approach beyond the reach of most clinicians and unacceptable to most patients.
Barriers to performing structural PK studies in clinical practice
The single most important barrier to the adoption of PK-tailored treatment using the classical PK approach is the need for many samples over several days, which is a significant burden on clinicians and patients (and their families). For this reason, simplified sampling schemas have been suggested, particularly in pediatric settings.28 Another important barrier imposed by a structural PK study is the need for a washout period. Reinfusing a patient before the residual factor concentrate has been completely cleared from the plasma—which, in theory, would require an infusion interval ≥5 times the elimination half-life—results in a small but significant accumulation of concentrate until the steady state is reached.29 Performing the PK study on the first infusion of a new concentrate, particularly if a washout was done, may be less informative for the purpose of personalizing treatment.10 Finally, not every clinical center can manage the complexity of performing a structural analysis under multicompartmental assumptions. Even if dedicated software and expertise are available, performing the calculations needed to explore the effect of changes in treatment regimen is a not trivial task. As well, the approach is sensitive to measurement errors and dependent on operator expertise, both of which can be improved by practice and experience.
The practical and theoretical advantages of the population PK approach
More recently, a novel method to estimate the individual PK profile of factor concentrates has been adopted in the field of hemophilia. This new method has been facilitated by the adoption of the population PK approach30 to the study of the PK properties of factor concentrates.31,32 As previously mentioned, the largest variability in PK parameters in hemophilia is among individuals (high IIV), whereas the PK profile is reasonably stable over time within the same individual (low IOV). The population PK approach addresses IIV, or part of it, by including relevant covariates (eg, age, body mass, blood group, von Willebrand levels) in a multivariable regression model.11,31-33 If more than 1 sample per patient is available, a hierarchical (mixed-model) approach is adopted, in which correlations are tested on curve segments provided by the same individuals (Figure 2) rather than on single measurements. All of the information in the derivation cohort is used in the modeling phase to generate structured knowledge about the general shape of the PK profile for the concentrate and the effect of patient-level covariates. With the classical structural approach, one can learn about the PK of the concentrate and its variability in the population. With the population PK approach, one can learn about the concentrate and about the role of patient characteristics in determining the IIV in PK. This knowledge can then be used to estimate individual PK parameters from a reduced set of measurements (both plasma clotting factor activity and covariates) by performing a Bayesian regression. The probability of specific patterns of PK parameters (describing specific plasma clotting factor activity-over-time profiles) and the probability of their association with patient-level covariates (from the population) are used to draw an individual PK profile using sparse measures of plasma factor activity and knowledge of the covariates (from the individual). Consider the analogy of buying a suit. At one end of the spectrum, one can choose to buy a dress “off the rack,” that is, multiple copies of the same dress design are manufactured in different sizes based on the average weight and body composition of the population in the country (nonindividualized approach). At the other end of the spectrum, one could commission a master tailor to create a dress to fit him perfectly by taking all of the relevant measurements and using these to create the dress (structural approach). Between these 2 ends of the spectrum is a middle option: One could take a few simple measurements (eg, waist and hip circumference, height) and identify some “covariates” (eg, “fit:” slim, regular, large; age; intended use of the suit) and use that information to find the best suit among the available selection (population-based approach). This last suit might not fit as well as the tailor-made one, yet it would fit much better than the generic off-the-rack suit; it would also probably be less expensive and take less time to make than the tailor-made suit. Similarly, by adopting a population PK approach, 2 to 3 blood samples drawn at selected time points would give the same precision as a full set of 9 to 11 measurements.34,35 No washout is needed,35 and samples can be drawn after different infusions and analyzed together. The Bayesian regression produces credibility intervals around the most likely profile, quantifying the uncertainty in their precision. Finally, some of the software capable of Bayesian regression would also allow one to simulate the effect of administering a different dose or to calculate the appropriate time for reinfusion when targeting a specific predose level.36 Of note, participation to the Web-Accessible Population Pharmacokinetic Service-Hemophilia (WAPPS)36 collaborative effort to adopt population PK tailoring has been recently suggested by the United Kingdom Haemophilia Centre Doctors’ Organisation guidelines on usage of EHL concentrates in routine clinical practice.26
Derivation of a population PK model by hierarchical multivariable regression. Dots represent postinfusion plasma measurements of clotting factor activity; each different color identifies a different patient; when more than 1 time point is available for a patient, the association among them is considered in the regression model. The red dashed line represents the average regression line; the blue dotted regression lines represent the upper and lower boundaries of the regression.
Derivation of a population PK model by hierarchical multivariable regression. Dots represent postinfusion plasma measurements of clotting factor activity; each different color identifies a different patient; when more than 1 time point is available for a patient, the association among them is considered in the regression model. The red dashed line represents the average regression line; the blue dotted regression lines represent the upper and lower boundaries of the regression.
How can an individual PK profile be used to individualize treatment?
Answering this question is the final step in the decision to adopt PK-based individualization. What is involved in interpreting the results of the individual PK estimation? How much technical knowledge of PK is needed? How complicated is translating an individual PK profile into a clinical decision?
The complete definition of the PK of a factor concentrate comprises several technical parameters, each defining 1 or more specific aspect of the plasma clotting factor activity-over-time profile. The focus of this discussion is not to determine which PK parameters are best suited to compare different drugs, predict their clinical utility at a population level, or describe their most appropriate use and the optimal regimen on theoretical grounds; rather, the goal is to individualize treatment. For this scope, individual PK parameters are needed only to allow drawing the individual plasma clotting factor activity-over-time profile, which has all of the information needed to shape a prophylactic regimen for an individual patient and is easy to communicate and understand. More and more often, PK assessments are reported in terms of plasma clotting factor activity at specific times, times required to reach the target plasma clotting factor activity, or proportion of time spent above a given threshold of plasma clotting factor activity.12-15 After performing an individual PK assessment using population-based PK software,36 the software can simulate the effects of administering different doses, and/or the adoption of different infusion frequencies, and/or the dose and frequency required to achieve levels previously selected targets. An interactive interface can draw in real-time plasma clotting factor activity-over-time profiles corresponding to specific treatment choices (Figures 3 and 4). Graphical profiles provide all of the required information in a format easier to understand and communicate than any PK parameters. Challenges posed by individualizing treatment according to bleeding phenotype, bleeding history, level of physical activity, adherence to treatment, and willingness to infuse with higher or lower frequency20 can be addressed by examining the individual plasma clotting factor activity-over-time profile. The concept will be explained further with the help of 3 clinical examples. Each case is unique, which is why tailoring treatment is important. Some cases, however, follow common patterns and as such have a higher educational value.
Individualized plasma clotting factor activity over time profile: case 1. The red line with hollow points shows the measured plasma clotting factor activities used to estimate the PK profile for the patient (green dashed line). The solid line shows the predicted PK profile for the simulated regimen. The further away the predicted (solid) line is from the measured (hollow point) line and from the estimated (dashed) predicted individual PK profile, the lower our confidence in the precision of the calculation. (A) The threshold after 2000 IU on Monday-Wednesday-Friday. (B) After 2000 units every third day. (C) Every 3.5 days (Sunday morning and Thursday night). The calculations include the weekly average consumption, which is 6000 U with the current regimen, 5000 with solution B, 4000 with solution C. The plots were produced with the calculator function of the WAPPS-Hemo software, a freely available, registration based online tool providing individualized PK profiles using a Bayesian population approach (www.wapps-hemo.org). The interactive graph relative to this example is available at https://demo.wapps-hemo.org/PatientResult.aspx?PIR=Tb1LL6RgexRq8cZ2K5-pEA.
Individualized plasma clotting factor activity over time profile: case 1. The red line with hollow points shows the measured plasma clotting factor activities used to estimate the PK profile for the patient (green dashed line). The solid line shows the predicted PK profile for the simulated regimen. The further away the predicted (solid) line is from the measured (hollow point) line and from the estimated (dashed) predicted individual PK profile, the lower our confidence in the precision of the calculation. (A) The threshold after 2000 IU on Monday-Wednesday-Friday. (B) After 2000 units every third day. (C) Every 3.5 days (Sunday morning and Thursday night). The calculations include the weekly average consumption, which is 6000 U with the current regimen, 5000 with solution B, 4000 with solution C. The plots were produced with the calculator function of the WAPPS-Hemo software, a freely available, registration based online tool providing individualized PK profiles using a Bayesian population approach (www.wapps-hemo.org). The interactive graph relative to this example is available at https://demo.wapps-hemo.org/PatientResult.aspx?PIR=Tb1LL6RgexRq8cZ2K5-pEA.
Individualized plasma clotting factor activity over time profile: case 2. The red line with hollow points shows the measured plasma clotting factor activities used to estimate the PK profile for the patient (green dashed line). The solid line shows the predicted PK profile for the simulated regimen. The further away the predicted (solid) line is from the measured (hollow point) line and from the estimated (dashed) predicted individual PK profile, the lower our confidence in the precision of the calculation. (A) Individual PK profile on the current dose of 1000 IU on Monday and Thursday. (B) The effect of increasing the dose to 2000 IU. (C) The dose that would be required on the Friday infusion to obtain a trough >0.025. (D) The booster dose that would be required on Sunday to obtain a trough >0.025. The plots were produced with the calculator function of the WAPPS-Hemo software, a freely available, registration-based online tool providing individualized PK profiles using a Bayesian population approach (www.wapps-hemo.org). The interactive graph relative to this example is available at https://demo.wapps-hemo.org/PatientResult.aspx?PIR=pVyUp4UHO0ib9GzrozEx2g, and the interactive graph relative to case 3 is available at https://demo.wapps-hemo.org/PatientResult.aspx?PIR=QYLy46pQOGEEn9PewiBvHA.
Individualized plasma clotting factor activity over time profile: case 2. The red line with hollow points shows the measured plasma clotting factor activities used to estimate the PK profile for the patient (green dashed line). The solid line shows the predicted PK profile for the simulated regimen. The further away the predicted (solid) line is from the measured (hollow point) line and from the estimated (dashed) predicted individual PK profile, the lower our confidence in the precision of the calculation. (A) Individual PK profile on the current dose of 1000 IU on Monday and Thursday. (B) The effect of increasing the dose to 2000 IU. (C) The dose that would be required on the Friday infusion to obtain a trough >0.025. (D) The booster dose that would be required on Sunday to obtain a trough >0.025. The plots were produced with the calculator function of the WAPPS-Hemo software, a freely available, registration-based online tool providing individualized PK profiles using a Bayesian population approach (www.wapps-hemo.org). The interactive graph relative to this example is available at https://demo.wapps-hemo.org/PatientResult.aspx?PIR=pVyUp4UHO0ib9GzrozEx2g, and the interactive graph relative to case 3 is available at https://demo.wapps-hemo.org/PatientResult.aspx?PIR=QYLy46pQOGEEn9PewiBvHA.
Case 1
A.B. is a young adult with severe hemophilia A. He has been on primary prophylaxis and has been successful in preserving most of his joint function. He was compliant with a Monday-Wednesday-Friday treatment pattern until he completed high school. Adherence decreased during his graduate studies, and he now struggles to keep up with the infusion schedule after getting a job in a law firm. He is considering switching to an EHL concentrate to gain more flexibility. His primary goal is to be able to skip 1 day without significantly increasing the risk of a breakthrough bleed. An important secondary goal is to reach a higher trough when he is able to keep infusing on the proper days. In the past, he has performed a PK assessment on his current concentrate, and he presents a clearance of 0.29 mL kg−1/min−1 and a terminal half-life of about 10:30 hh:mm. He wants to know what to expect with the switch. We discuss the concept that more variability exists for a given concentrate among different patients than within the same patient among different concentrates. A crossover study of 15 patients with hemophilia showed that the average increase in terminal half-life for patients switching from Advate to Eloctate was about 50% (for A.B., assuming he was an “average” individual, this would be going from 10:30 hh:mm to 15:755 hh:mm). We hypothesize that, switching from Advate to the same dose of Eloctate, he should gain over 24 hours before getting to the same postinfusion trough plasma clotting factor activity. This might allow him to infuse every third day while maintaining a safe trough or to keep his current infusion pattern and increase his trough. We agree on switching, but also on the opportunity to test if the prediction based on the “average” assumption is correct. After a few infusions on the new concentrate, A.B. comes to clinic one afternoon to draw a 7-hour sample, and 1 more time before work to draw a 49-hour sample. The PK estimate is shown in Figure 3. The terminal half-life is estimated at 17:30 hh:mm, which is a 65% increase over Advate. The data confirm that he can infuse on Monday-Wednesday-Friday with a trough >0.07 IU/mL on the short interval and >0.03 IU/mL on the long interval (Figure 3A); every third day with a trough of 0.03 (Figure 3B); or on a Sunday morning to Thursday evening scheme with a trough >0.02 (Figure 3C).
Case 2
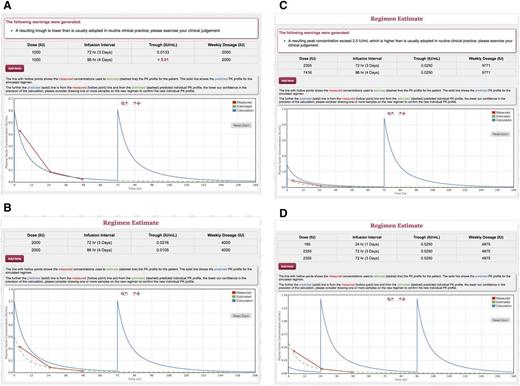
E.F. is a 11-year-old boy with severe hemophilia A. He is currently on a Monday and Thursday treatment regimen with 1000 IU of Kovaltry (22 IU/kg for 45 kg, with a measured trough of 0.03 IU/mL at 48 hours). He presents a severe bleeding phenotype, has developed a target joint at the left ankle, and not uncommonly bleeds into the right ankle. He adds 1 or 2 extra doses when he bleeds or adds an extra infusion if he (or his mother) thinks he has overused the left ankle. He reports no changes in his sedentary lifestyle, but bleeds have become more frequent. His mother is worried about his future and wonders if it is time to increase the dose. She is reluctant to consider a switch to a long-acting product at this time. We review the treatment log of bleeds and infusions for the past 3 months. E.F. reports a worrisome count of 3 bleeds, 2 of which resulted in ankle joints requiring treatment on Sundays, and 1 less “certain” elbow bleed noticed upon wakening on a Thursday morning. Using the individual PK profile on file from 6 months earlier, the plasma clotting factor activity at the time of bleed is always ≤0.02 IU/mL, which he reaches around Sunday at noon. We start by estimating the trough at 96 hours with the current dose of 1000 IU, which results <0.01 IU/mL (Figure 4A); the effect of increasing the dose to 2000 IU, which produces a trough of 0.02 IU/mL at 72 hours, but 0.01 IU/mL on Sunday evening (Figure 4B); the increase of the Friday dose that would be required to keep the trough >0.025 on the longer infusion interval (Figure 4C); the booster dose to be given on Sunday morning to get to the same result (Figure 4C). From Figure 4A, we can also estimate the trough we would obtain by switching the patient to 1000 IU every other day, which would be about 0.033 IU/mL. For each of the regimen, we also obtain the estimate of the weekly usage of factor concentrate.
Case 3
C.D. is a 22-year-old patient living with severe hemophilia B. He is on biweekly infusions with BeneFIX, Sundays and Thursdays, and he is currently treating with 50 IU/kg (∼5000 IU/wk for 53 kg). He is not a diligent diary keeper, and he has had a few breakthrough bleeds, mostly related to participating in sports. He plays tennis regularly, mostly on Mondays and Thursdays. He has set up clinic appointment to discuss whether and how to intensify his treatment while preparing for a nonprofessional tennis tournament. We invite him to have his clotting factor IX activity measured once on Monday and once on Tuesday to perform a PK study; the measured plasma clotting factor activity levels are 0.07 IU/mL at 26 hours and 0.04 at 57 hours after infusion. After obtaining the individual profile, we meet to discuss the results. He plans to play tennis 4 times per week. We agree that we will need to check clinically for any bleeding, but we assume it would be safe for him to play when his factor IX is >0.10 IU/mL2. Based on the simulated graph, he would be safe playing on postinfusion days 1 and 2 (Sunday, Monday, Thursday, and Friday), but would be at excessive risk on postinfusion days 3 and 4 (Tuesday, Wednesday, and Saturday). We decide to simulate regimens allowing a trough of 0.10 IU/mL, which results in 75 IU/kg (4000 IU) every third day (14 400 IU/wk), or 30 IU/kg every second day (1500 IU, 9500 IU/wk), and the booster dose needed on any third or fourth days if continuing on the current regiment to keep the trough >0.10 IU/mL during that day, which results in 15 IU/mL (750 IU). He decides to adopt the every-other-day regimen, waiting for EHL factor IX to become available in his setting. Links to interactive graphs for the 3 cases are provided in the legends to the figures.
Concluding remarks
Individual PK profiling on limited sparse data collected during routine treatment, without the need for washout, and merging data obtained after different infusions is now an available option for most concentrates on the market. Individual interactive plasma clotting factor activity-over-time profiles can be easily generated and should be adopted as the standard way of using PK to individualize treatment, particularly for EHL concentrates.26 Individualized treatment does not benefit the patient only. The adoption of Web-available solutions such as WAPPS for individual PK profiling supports the collection of large sets of data, which can be used to explore yet-unanswered questions on factor concentrates PK. Research questions could address the effects of laboratory sources of variability (measurement error, different sensitivity of different reagents, use of internal or external standards)37,38 ; identification of PK properties common to all concentrates or concentrate subclasses; and the performance of generic models incorporating the variability introduced by each concentrate as a regression covariate. Examples of unmet clinical needs are the identification of optimal sampling times, guidance for using PK to individualize treatment in patients with a previous history of inhibitors or current subclinical inhibitors, and the possibility of predicting individualized profiles on new concentrates before switching. Finally, the net clinical and economic benefit of applying PK-tailored prophylaxis in terms of reduced bleeding rate, increased compliance, and reduced waste of factor concentrate need to be demonstrated in prospective clinical observations.
Correspondence
Alfonso Iorio, McMaster University, 1280 Main St West, Hamilton, ON L8S 4K1, Canada; e-mail: iorioa@mcmaster.ca.
References
Competing Interests
Conflict-of-interest disclosure: The author declares no competing financial interests.
Author notes
Off-label drug use: None disclosed.