Abstract
Primary central nervous system (CNS) lymphomas represent a subgroup of malignancies with specific characteristics, an aggressive course, and unsatisfactory outcome in contrast with other lymphomas comparable for tumor burden and histological type. Despite the high sensitivity to conventional chemotherapy and radiotherapy, remissions are frequently short lasting. Treatment efficacy is limited by several factors, including the biology and microenvironment of this malignancy and the “protective” effect of the blood-brain barrier, which limits the access of most drugs to the CNS. Patients who survive are at high risk of developing treatment-related toxicity, mainly disabling neurotoxicity, raising the question of how to balance therapy intensification with the control of side effects. Recent therapeutic progress and effective international cooperation have resulted in a significantly improved outcome over the past 2 decades, with a higher proportion of patients receiving treatment with curative intent. Actual front-line therapy consists of high-dose methotrexate-based polychemotherapy. Evidence supporting the addition of an alkylating agent and rituximab is growing, and a recent randomized trial demonstrated that the combination of methotrexate, cytarabine, thiotepa, and rituximab (MATRix regimen) is associated with a significantly better overall survival. Whole-brain irradiation and high-dose chemotherapy supported by autologous stem cell transplantation are 2 effective consolidation strategies in patients with a disease responsive to induction chemotherapy. Different strategies such as alkylating maintenance, conservative radiotherapy, and nonmyeloablative consolidation are being addressed in large randomized trials and a more accurate knowledge of the molecular and biological characteristics of this malignancy are leading to the development of target therapies in refractory/relapsing patients, with the overall aim to incorporate new active agents as part of first-line treatment. The pros and cons of these approaches together with the best candidates for each therapy are outlined in this article.
Learning Objectives
Learn the standard of care for different subgroups of patients with primary central nervous system lymphoma
Understand trends and developments resulting from recent and ongoing trials, in particular with regard to the role of novel drugs and new options
Introduction
International cooperation allowed a rapid development of efficient therapies in the field of primary central nervous system (CNS) lymphomas (PCNSLs), with a consequent outcome improvement.1 However, retrospective mono-institutional series showed relevant differences in survival figures between prospective trials and routine practice.2 Some methodologic limitations remain unsolved, and several factors are preventing further therapeutic progress. In particular, PCNSL patients often show impaired general conditions and poor performance status (PS) due to late diagnosis, which interferes with their inclusion in prospective trials and in the indication of a timely therapy. Current therapeutic knowledge is based on a few randomized trials; some single-arm, phase 2 trials; and many multicenter retrospective studies. This low level of evidence generates uncertainties when it comes to therapeutic decisions and lack of consensus on primary end points for future trials. Moreover, molecular and biological knowledge is insignificant compared with other diffuse large B-cell lymphomas, which limits the identification of new therapeutic targets.
Early diagnosis is the best treatment
Conventional and advanced neuroimaging techniques
Diagnostic specificity of conventional imaging techniques in PCNSL patients is low, and some advanced techniques have been used in an effort to narrow the differential diagnosis.3 PCNSL commonly shows restricted diffusion due to high cellularity, appearing hyperintense on diffusion-weighted imaging and hypointense on apparent diffusion coefficient maps,4 with lower apparent diffusion coefficient values than other primary and secondary CNS tumors.5 Apparent diffusion coefficient values may also play a prognostic role in PCNSL patients.6 Multiple diffusion tensor imaging metrics, including fractional anisotropy maps, seem to be a useful tool to distinguish PCNSLs from high-grade gliomas.7 When assessed by perfusion and permeability imaging, PCNSL lesions show absolute and relative cerebral blood flow values higher than those recorded in high-grade gliomas8 ; other differences between these tumors that could be used to support the suspicion of PCNSL have been reported with T2*-weighted, dynamic susceptibility–weighted, contrast-enhanced magnetic resonance imaging; T1-weighted, steady-state, dynamic contrast–enhanced magnetic resonance imaging; and susceptibility-weighted imaging sequences.9 Magnetic resonance spectroscopy provides in vivo measurement of different metabolic peaks, which can provide diagnostic information in various brain diseases; PCNSL usually displays extremely high lipid and macromolecule resonance but also high choline and lactate, low N-acetyl aspartate and creatine, and a high choline-to-creatine ratio.4 The diagnostic role of 18F-fluorodeoxyglucose positron emission tomography (PET) or 11C-methionine PET remains to be defined. 18F-fluorodeoxyglucose uptake by lymphoma can occasionally be masked by the high background uptake of gray matter tissues. 11C-methionine PET does not have this potential drawback because methionine uptake is low in normal brain tissues. Single-photon emission computed tomography with different radioisotopes such as 201Tl, N-isopropyl-123I-p-iodoamphetamine, and 99Tc(m)-sestamibi were investigated in HIV-positive patients with PCNSL.3
Early suspicion of PCNSL
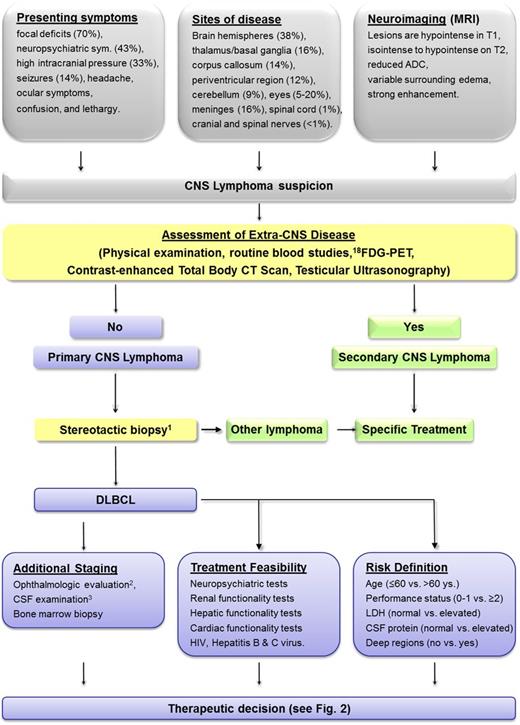
The early diagnosis of PCNSL is of great importance to start a timely and efficient treatment (Figure 1). Pathological confirmation of the diagnosis is mandatory and is usually performed on brain tissue; less commonly, diagnosis can be achieved by cytologic examination of cerebrospinal fluid (CSF) or vitrectomy samples. Stereotactic biopsy is the recommended procedure to provide suitable samples to expert pathologists. Importantly, most cases of PCNSL arise in the deep areas of the brain, basal ganglia, and periventricular regions, where geographical misses are common and the risk of bleeding is increased. Accordingly, stereotactic biopsy should be planned accurately, performed by expert neurosurgeons, and supported by robust clinical suspicion (Figure 1). Unfortunately, the diagnostic sensitivity of current strategies to formulate a clinical suspicion on the basis of neuroimaging, site of disease, and response to steroids is very poor. Several PCNSL patients receive steroids for months before biopsy to palliate symptoms and prevent complications. This strategy leads to confounding effects on neuroimaging, delayed and unsuitable biopsy, and higher risk of severe complications during therapy (eg, diabetes and other metabolic disorders, severe infections due to iatrogenic immunodepression). Half of the cases of early tumor progression are due to prolonged treatment interruptions caused by toxic complications, especially infections, often related to steroid pretreatment. Moreover, CNS tissue damage due to prolonged lymphoma infiltration is associated with disabling symptoms, loss of autonomy, and poor treatment tolerability, with consequent irreversible neurological impairment, even in the case of tumor remission, and negative effects on clinical trials, like slow accrual and increased risk of biased patient selection. Several investigators are putting their efforts into the identification of molecular and biological parameters useful to establish an early, reliable suspicion of PCNSL. Some chemokines (eg, CXCL13) and cytokines (eg, interleukin-10 [IL-10]) can be used, alone or in combination with neuroimaging, as useful diagnostic and prognostic biomarkers.10,11 Some microRNA (21, 19b, and 92a) can be detected in CSF with a high diagnostic sensitivity and specificity.12,13 High levels of IL-10 and/or a high IL-10-to-IL-6 ratio in ocular fluids are strongly suggestive of B-cell lymphomatous uveitis. However, despite being associated with a high diagnostic sensitivity, these tools are hardly used in routine practice, and they cannot replace histopathological confirmation.
Flowchart of management of HIV-negative patients with brain masses suspected for lymphoma from presentation to therapeutic decision in ordinary clinical practice. 1Despite a strong suspicion of PCNSL, some patients suffering from large space-occupying lesions with acute symptoms of brain herniation could be eligible for surgical resection to reduce rapidly increased intracranial pressure; biopsy of extra-CNS organs is usually prefered in patients with positive staging as this procedure is associated with lower risk of severe complications. 2Ocular examination should include slit-lamp examination, indirect ophthalmoscopy, and ophthalmic ultrasonography. 3CSF evaluation should include cell counts, protein and glucose levels, cytology, and flow cytometry. IgHV gene rearrangement studies are optional. ADC, average diffusion coefficient; CT, computed tomography; Deep regions, basal ganglia, corpus callosum, periventricular areas, brain stem, and/or cerebellum; DLBCL, diffuse large B-cell lymphoma; 18FDG, 18F-fluorodeoxyglucose; IgHV, immunoglobulin heavy chain variable region; LDH, lactate dehydrogenase serum level; MRI, magnetic resonance imaging; sym., symptoms.
Flowchart of management of HIV-negative patients with brain masses suspected for lymphoma from presentation to therapeutic decision in ordinary clinical practice. 1Despite a strong suspicion of PCNSL, some patients suffering from large space-occupying lesions with acute symptoms of brain herniation could be eligible for surgical resection to reduce rapidly increased intracranial pressure; biopsy of extra-CNS organs is usually prefered in patients with positive staging as this procedure is associated with lower risk of severe complications. 2Ocular examination should include slit-lamp examination, indirect ophthalmoscopy, and ophthalmic ultrasonography. 3CSF evaluation should include cell counts, protein and glucose levels, cytology, and flow cytometry. IgHV gene rearrangement studies are optional. ADC, average diffusion coefficient; CT, computed tomography; Deep regions, basal ganglia, corpus callosum, periventricular areas, brain stem, and/or cerebellum; DLBCL, diffuse large B-cell lymphoma; 18FDG, 18F-fluorodeoxyglucose; IgHV, immunoglobulin heavy chain variable region; LDH, lactate dehydrogenase serum level; MRI, magnetic resonance imaging; sym., symptoms.
Induction therapy
Traditionally, surgery has not played a role in the treatment of PCNSL because of the multifocal and infiltrative nature of this tumor. The increased risk of postoperative morbidity in this population of patients has also contributed to discouraging tumor resection. Interestingly, an exploratory analysis of the German PCNSL Study Group 1 (G-PCNSL-SG1) randomized trial (see below) has shown significantly better survival in patients with subtotal or gross total resections compared with biopsied patients.14 However, this difference may be explained by a biased distribution of sites of disease between the 2 subgroups. In fact, biopsied patients more often had multifocal disease, large lesions, and/or deeply seated CNS lesions than resected patients; these unbalanced features may have contributed to the unfavorable outcome. Although the therapeutic role of tumor resection in PCNSL patients remains to be defined, in particular in series reflecting modern neurosurgical approaches, some patients suffering from large space-occupying lesions with acute symptoms of brain herniation could be eligible for surgical resection to reduce rapidly increased intracranial pressure, improve PS, and allow timely chemotherapy (Figure 1).
A modern approach to PCNSL includes 2 phases: induction and consolidation. Induction therapy consists of polychemotherapy containing methotrexate (MTX) delivered at doses >1 g/m2 (high-dose methotrexate [HD-MTX]). The optimal dose and administration schedule of MTX have not been determined. However, there is a growing consensus to deliver MTX at a dose ≥3 g/m2 by a 3-hour infusion. Currently, most treatment protocols combine HD-MTX with a variety of other chemotherapeutic agents, and MTX monotherapy has been progressively abandoned. The best evidence to support this approach comes from the International Extranodal Lymphoma Study Group–20 (IELSG20) randomized trial that demonstrated that the addition of high-dose cytarabine is associated with a significantly improved complete remission rate (CRR) and progression-free survival (PFS) compared with MTX monotherapy.15
Chemotherapy induction in “young” patients
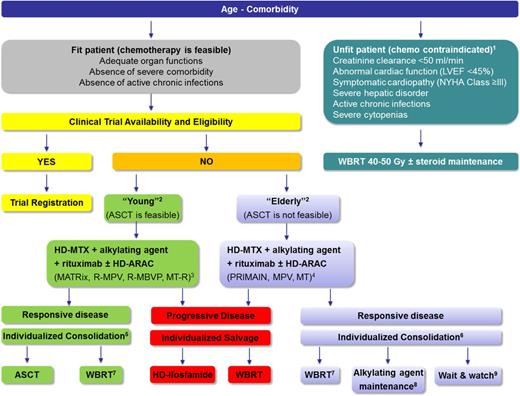
Although age is the main prognostic factor in PCNSL patients, it should not be considered as an exclusive parameter when a therapeutic decision must be taken (Figure 2). Comorbidity and related organ dysfunctions are important factors to define eligibility for HD-MTX–based chemotherapy, and for myeloablative chemotherapy in particular. Age is an important parameter for comparing published studies and for addressing iatrogenic neurotoxicity. Nevertheless, a reliable cutoff to distinguish young and elderly patients is yet to be defined. Different age subgroups were considered together in the very first prospective trials, but the definition of age upper limit became an increasingly pressing issue to address more intensified induction therapies and myeloablative, high-dose chemotherapy supported by autologous stem-cell transplantation (HDC/ASCT). On the basis of the International Prognostic Index, some investigators considered 60 years of age as the cutoff to distinguish elderly patients not eligible for HDC/ASCT in prospective trials,16 resulting in potential undertreatment of some fit patients >60 years. On the other hand, stating the upper limit to 70 years of age in trials addressing HDC/ASCT showed that half of patients >65 years do not complete the planned treatment.17 Nevertheless, several centers use HDC/ASCT in selected patients >70 years with excellent results in routine practice.18 Therefore, a large variability in comorbidity and neurological conditions exists in patients aged between 65 and 75 years, resulting in a diffused use of personalized treatments.
Flowchart of therapeutic management of HIV-negative patients with PCNSL. 1Other clinical and biochemical parameters may be considered. An overall evaluation of an experienced multidisciplinary team is recommended. 2An undebatable cutoff to define “elderly” population does not exist. Age should not be used as an exclusive parameter to define therapeutic approach. Age, comorbidity, and ASCT feasibility should be considered together to define treatment. 3Several chemotherapy regimens for young patients are available; some examples are reported in parenthesis. The MATRix regimen is supported by the highest level of evidence as assessed in an international randomized trial. 4Several chemotherapy regimens for elderly patients are available; some examples are reported in parenthesis. The PRIMAIN regimen has been assessed in the largest single-arm phase 2 trial; MPV and MT have been addressed in a randomized trial performed in the pre-rituximab era. The effect of the addition of this antibody both on toxicity and efficacy remains to be assessed. 5Randomized trials suggest both WBRT and ASCT are effective as consolidation therapies in young patients, with a higher risk of neurotoxicity after WBRT. The discussion with selected patients about the pros and cons of the use of consolidation WBRT or ASCT is recommended. 6Randomized trials focused on consolidation therapies in elderly patients are not available. All consolidation options are supported by single-arm phase 2 trials. 7Radiation field and dose should be chosen on the basis of response to primary chemotherapy. WBRT dose reduction to 23.4 Gy is recommended in patients who achieved a complete remission after induction of chemoimmunotherapy. 8Encouraging data with maintenance with temozolomide or procarbazine are available. Lenalidomide maintenance seems to be an interesting experimental option. 9Watchful waiting is suggested only in patients in complete remission after well-documented induction chemoimmunotherapy. ASCT, myeloablative chemotherapy supported by ASCT; HD-ARAC, high-dose cytarabine; HD-MTX, high-dose methotrexate; LVEF, left ventricular ejection fraction; MPV, HD-MTX/procarbazine/vincristine; MT, HD-MTX/temozolomide; MT-R, rituximab/HD-MTX/temozolomide; NYHA, New York Hospital Association; PRIMAIN, rituximab/HD-MTX/procarbazine; R-MBVP, rituximab/HD-MTX/carmustine/etoposide/HD-ARAC; R-MPV, rituximab/HD-MTX/procarbazine/vincristine.
Flowchart of therapeutic management of HIV-negative patients with PCNSL. 1Other clinical and biochemical parameters may be considered. An overall evaluation of an experienced multidisciplinary team is recommended. 2An undebatable cutoff to define “elderly” population does not exist. Age should not be used as an exclusive parameter to define therapeutic approach. Age, comorbidity, and ASCT feasibility should be considered together to define treatment. 3Several chemotherapy regimens for young patients are available; some examples are reported in parenthesis. The MATRix regimen is supported by the highest level of evidence as assessed in an international randomized trial. 4Several chemotherapy regimens for elderly patients are available; some examples are reported in parenthesis. The PRIMAIN regimen has been assessed in the largest single-arm phase 2 trial; MPV and MT have been addressed in a randomized trial performed in the pre-rituximab era. The effect of the addition of this antibody both on toxicity and efficacy remains to be assessed. 5Randomized trials suggest both WBRT and ASCT are effective as consolidation therapies in young patients, with a higher risk of neurotoxicity after WBRT. The discussion with selected patients about the pros and cons of the use of consolidation WBRT or ASCT is recommended. 6Randomized trials focused on consolidation therapies in elderly patients are not available. All consolidation options are supported by single-arm phase 2 trials. 7Radiation field and dose should be chosen on the basis of response to primary chemotherapy. WBRT dose reduction to 23.4 Gy is recommended in patients who achieved a complete remission after induction of chemoimmunotherapy. 8Encouraging data with maintenance with temozolomide or procarbazine are available. Lenalidomide maintenance seems to be an interesting experimental option. 9Watchful waiting is suggested only in patients in complete remission after well-documented induction chemoimmunotherapy. ASCT, myeloablative chemotherapy supported by ASCT; HD-ARAC, high-dose cytarabine; HD-MTX, high-dose methotrexate; LVEF, left ventricular ejection fraction; MPV, HD-MTX/procarbazine/vincristine; MT, HD-MTX/temozolomide; MT-R, rituximab/HD-MTX/temozolomide; NYHA, New York Hospital Association; PRIMAIN, rituximab/HD-MTX/procarbazine; R-MBVP, rituximab/HD-MTX/carmustine/etoposide/HD-ARAC; R-MPV, rituximab/HD-MTX/procarbazine/vincristine.
There is increasing evidence suggesting an important role of the addition of an alkylating agent and rituximab combined with MTX, with or without high-dose cytarabine, in PCNSL patients aged ≤70 years17,19-21 (Figure 2). A combination of rituximab, MTX, procarbazine, and vincristine followed by low-dose whole-brain radiotherapy (WBRT) was addressed in 52 patients, with an overall response rate (ORR) of 79% and a 2-year PFS of 57% (intention to treat).21 The same combination followed by consolidative ASCT was investigated in 33 patients aged <65 years, reporting an ORR of 94% and a 2-year PFS of 79%.19 A combination of MTX, temozolomide, and rituximab followed by consolidative nonmyeloablative chemotherapy with high doses of cytarabine and etoposide and without radiotherapy was tested in 44 patients, with an ORR of 77% and a 2-year PFS of 59%.20 Unfortunately, no conclusions can be drawn on the effect of each drug (eg, alkylating agent and rituximab) in these single-arm phase 2 trials, and similar results have been reported in some studies addressing HD-MTX monotherapy. Conversely, the randomized phase 2 trial known as IELSG32 demonstrated that the addition of thiotepa and rituximab to an HD-MTX–cytarabine combination (MATRix regimen) significantly improved the outcome in comparison with combinations of high doses of MTX and cytarabine with or without rituximab, with an ORR of 87% and a 2-year PFS and an overall survival (OS) of 62% and 67%, respectively.17 This trial also suggests that the addition of rituximab to HD-MTX–based chemotherapy is associated with a significant improvement in ORR, with unchanged tolerability17 ; however, the role of rituximab in upfront treatment of PCNSL will be eventually established by an ongoing randomized phase 2 trial of the Dutch-Belgian Cooperative Trial Group for Hematology Oncology and the Australasian Leukaemia and Lymphoma Group (HOVON/ALLG) (Dutch trial register www.trialregister.nl; NTR2427). Presently, a number of combinations of HD-MTX, alkylating agents, and rituximab, with or without cytarabine, are being successfully used as standard of care in different countries (Figure 2). Importantly, the IELSG32 trial was conducted in 53 centers in 5 European countries, covering an extensive geographical area, which favors results generalizability and leads me to recommend the MATRix regimen as a new standard combination in young patients with newly diagnosed PCNSL.
Chemotherapy induction in “elderly” patients
Despite recent therapeutic progress in this field, the prognosis of elderly PCNSL patients remains poor, with a median OS of <2 years in most prospective studies.22 Generally speaking, HD-MTX–based induction therapy is well tolerated by older patients, providing that adequate supportive measures and careful check of renal function are met. However, 7% to 10% of elderly patients treated with an MTX dose ≤3.5 g/m2 require treatment discontinuation and 26% to 44% need dose reduction due to decreased renal function.23-25 A meta-analysis in 783 immunocompetent patients aged ≥60 years with newly diagnosed PCNSL suggests that there is no difference in survival between patients treated with HD-MTX plus oral alkylating agents and more intensive intravenous combinations.22 Accordingly, recent prospective trials are focused on a combination of HD-MTX and an oral alkylating agent, with or without rituximab (Figure 2). A recent randomized phase 2 trial of the Association des Neuro-Oncologue d'Expression Française (ANOCEF) and the Groupe Ouest-Est d'Etude des Leucémies et Autres Maladies du Sang (GOELAMS) intergroup showed no significantly better outcome with the combination of MTX, procarbazine, vincristine, and cytarabine, known as “MPV-A,” in comparison with an MTX-temozolomide combination, with ORRs of 82% and 71%, respectively; a 1-year PFS of 36% in both arms; and 2-year OS rates of 39% and 58%, respectively.26 Both regimens were associated with similar moderate toxicity and improvement in quality of life (QoL), suggesting that these poorly prognostic patients are eligible for treatment. However, this trial presents some criticisms; in particular, the age lower limit was 60 years, which means that some patients may have been undertreated, and adequate historical controls were unavailable, leading investigators to use previous retrospective studies with “similar” characteristics as comparators. Moreover, the trial was performed in the pre-rituximab era, and results could change with the addition of this antibody. The PRIMAIN trial includes the largest series of elderly PCNSL patients (n = 107) treated in the rituximab era.27 Enrolled patients were ≥65 years and received 3 courses of a combination of HD-MTX, 2 oral alkylators (procarbazine and lomustine), and rituximab followed by 4 weekly procarbazine maintenance courses; lomustine was omitted during the study due to recurrent infectious complications. The CRR was 36% and 2-year PFS was 37%; both parameters remained unchanged after the exclusion of lomustine, but tolerability was remarkably improved. These studies suggest that induction with HD-MTX, an alkylating agent, and rituximab is advisable for patients aged >65 years. In elderly patients with poor neurological conditions and in very old (>80 years) patients, comorbidities and frequent admissions to the hospital due to toxicity need to be individually weighed against a limited survival benefit.
Management of intraocular and CSF disease
CSF and vitreous humor are 2 chemotherapy sanctuaries in which tumor cells grow undisturbed. The largest reported series of unselected cases suggests that CSF and intraocular disease are recorded in 16% and 13% of cases, respectively, at presentation.28 CSF dissemination is underestimated if assessed only by conventional cytology examination, whereas flow cytometry is able to improve diagnostic sensitivity.29 Ocular involvement is usually bilateral and, conversely to CSF dissemination, is often diagnosed as the exclusive site of disease (the so-called primary vitreoretinal lymphoma).30 There are a few pharmacokinetic studies focused on the bioavailability of intravenously delivered drugs in these areas in PCNSL patients, often suggesting that drug concentrations and half-life are unpredictable. Some authorities suggested including intrathecal and/or intravitreal chemotherapy as part of first-line treatment of PCNSL patients, with controversial results and substantial side effects. However, these strategies have not been prospectively investigated and their efficacy in PCNSL remains unclear. Some retrospective studies did not demonstrate benefit from the addition of intrathecal chemotherapy in patients treated with MTX dosed at 3 g/m2.28,31 The comparison of 2 consecutive single-arm trials performed by the same multicenter group seems to suggest some benefit of intraventricular chemotherapy.32,33 In the first trial, MTX-cytarabine–based chemotherapy plus intraventricular chemotherapy resulted in a median event-free survival of 21 months, with half of young patients alive at a median follow-up of 100 months; however, 19% of patients experienced Ommaya reservoir infections.33 To reduce the incidence of reservoir infections, a similar group of patients was treated with the same intravenous chemotherapy but without intraventricular treatment, which resulted in an unexpectedly high rate of early relapses, which was attributed to the omission of intraventricular chemotherapy.32 Importantly, recently reported or ongoing PCNSL trials do not use intrathecal and/or intraventricular chemotherapy.8-10,13,14 We use intrathecal chemotherapy, often including rituximab, and preferably by intraventricular route through an Ommaya reservoir, in patients with CSF disease with insufficient response to intravenous HD-MTX–based chemotherapy or in patients who are not able to receive a MTX dose ≥3 g/m2. We recommend intravitreous chemotherapy for a presenting or recurrent disease confined to the eyes in patients with contraindications to receive HD-MTX–based chemotherapy.
Consolidation therapy
WBRT
Radiotherapy plays a central role as consolidative approach in PCNSL patients who achieve tumor response after induction therapy, whereas it is not curative when used alone. Due to the microscopically diffused and multifocal nature of PCNSL, the advised irradiated volume should include the whole brain, the first 2 cervical segments of the spinal cord, and the eyes. Doses ≤50 Gy to the whole brain, with or without a tumor bed boost, were used, but a progressive dose reduction, maintaining standard fractionation (1.8–2 Gy/fraction), was introduced in recent trials.
Only 1 randomized trial comparing WBRT with observation after chemotherapy has been published34 (Table 1). In this study, called G-PCNSL-SG1, patients who achieved a complete remission after HD-MTX, with or without ifosfamide, were randomly allocated between consolidating WBRT, 45 Gy in 30 fractions, and observation. The use of WBRT was associated with a significantly better PFS, whereas there were no differences in OS.34 Importantly, at a median follow-up of 81 months, a survival plateau was not shown in any of the assessed subgroups, suggesting unsatisfactory efficacy of the therapies used.34 As many authors pointed out,35,36 the G-PCNSL-SG1 trial presented several interpretative caveats, major protocol violations in one-third of randomized patients, and the predetermined primary end point for noninferiority was not met. These shortcomings led some authorities to continue to recommend consolidation WBRT as the standard of care36 (Figure 2). Preliminary results of 2 recent randomized trials comparing WBRT and HDC/ASCT as consolidation options in patients with PCNSL responsive to HD-MTX–containing induction16,37 are in line with this recommendation. In the IELSG32 trial,21 patients aged ≤70 years treated with HD-MTX–based induction followed by WBRT had a 2-year PFS and OS of 72% and 85%, respectively. In the PRECIS trial,16 patients aged ≤60 years treated with HD-MTX–based induction followed by WBRT had a 2-year PFS and OS of 63% and 86%, respectively. Importantly, the predetermined efficacy threshold was achieved in both studies, demonstrating that WBRT is an effective consolidation option.
Completed and ongoing randomized clinical trials investigating the role of consolidative WBRT in patients with newly diagnosed PCNSL
| Trial, scientific group (clinicaltrials.gov ID) . | Recruitment . | Patients, n . | Eligibility criteria . | Control arm . | Intervention arm . | Primary end point . | Comments . |
|---|---|---|---|---|---|---|---|
| G-PCNSL-SG-1 | Completed | 551 | Newly diagnosed PCNSL; age ≥18 y; ECOG PS ≤3 | HD-MTX ± ifosfamide → WBRT 45 Gy | HD-MTX ± ifosfamide | OS | Several methodologic flaws, noninferiority margin not met; no definite conclusions can be drawn |
| IELSG32, International Extranodal Lymphoma Study Group (NCT01011920) | Completed | 219* and 118† | Newly diagnosed PCNSL; age 18–65 y (ECOG ≤ 3); age 65–70 y if ECOG ≤2 | Induction → WBRT 36 Gy | Induction treatment → HDC/ASCT | CRR* and PFS† | First randomization proved efficacy of MATRix regimen; both consolidation therapies (WBRT and HDC/ASCT) are effective |
| RTOG 1114, Radiation Therapy Oncology Group (NCT01399372) | Completed | 89 | Newly diagnosed PCNSL; age ≥18 y; ECOG PS ≤3 | R-MPV → HD-ARAC | R-MPV → WBRT 23.4 Gy → HD-ARAC | PFS | Estimated completion regarding primary end point is December 2022 |
| PRECIS, ANOCEF/GOELAMS (NCT00863460) | Completed | 140 | Newly diagnosed PCNSL; age 18–60 y | R-MBVP → R-HD-ARAC→ WBRT 40 Gy | R-MBVP → R-HD-ARAC → HDC/ASCT | 2-y PFS | Results reported as meeting abstract |
| Trial, scientific group (clinicaltrials.gov ID) . | Recruitment . | Patients, n . | Eligibility criteria . | Control arm . | Intervention arm . | Primary end point . | Comments . |
|---|---|---|---|---|---|---|---|
| G-PCNSL-SG-1 | Completed | 551 | Newly diagnosed PCNSL; age ≥18 y; ECOG PS ≤3 | HD-MTX ± ifosfamide → WBRT 45 Gy | HD-MTX ± ifosfamide | OS | Several methodologic flaws, noninferiority margin not met; no definite conclusions can be drawn |
| IELSG32, International Extranodal Lymphoma Study Group (NCT01011920) | Completed | 219* and 118† | Newly diagnosed PCNSL; age 18–65 y (ECOG ≤ 3); age 65–70 y if ECOG ≤2 | Induction → WBRT 36 Gy | Induction treatment → HDC/ASCT | CRR* and PFS† | First randomization proved efficacy of MATRix regimen; both consolidation therapies (WBRT and HDC/ASCT) are effective |
| RTOG 1114, Radiation Therapy Oncology Group (NCT01399372) | Completed | 89 | Newly diagnosed PCNSL; age ≥18 y; ECOG PS ≤3 | R-MPV → HD-ARAC | R-MPV → WBRT 23.4 Gy → HD-ARAC | PFS | Estimated completion regarding primary end point is December 2022 |
| PRECIS, ANOCEF/GOELAMS (NCT00863460) | Completed | 140 | Newly diagnosed PCNSL; age 18–60 y | R-MBVP → R-HD-ARAC→ WBRT 40 Gy | R-MBVP → R-HD-ARAC → HDC/ASCT | 2-y PFS | Results reported as meeting abstract |
Data were updated from reference 59. ECOG, Eastern Cooperative Oncology Group; HD-ARAC, high-dose cytarabine; HD-MTX, high-dose methotrexate; R-MBVP, rituximab/HD-MTX/carmustine/etoposide/HD-ARAC; R-MPV, rituximab/HD-MTX/procarbazine/vincristine.
First randomization.
Second randomization.
The major concern preventing a broader use of consolidation WBRT in PCNSL patients regards the increased risk of severe neurotoxicity. This disabling complication obscures treatment benefit in long-term survivors and was imputed to WBRT in a few retrospective studies.38 Overall, reports on long-term sequelae of WBRT are compromised by low patient numbers, old-fashioned radiotherapy modalities, differing radiation fields and doses, and varying combinations of WBRT and chemotherapy. In early studies addressing HD-MTX–based chemotherapy and “full-dose” WBRT, neurotoxicity was defined only through clinical observation, which resulted in a 5-year cumulative incidence of 25% to 35%, with a related mortality of 30%, and higher rates in patients aged >60 years. Later on, the impact of WBRT on cognitive functions was assessed by the use of a panel of neuropsychological tests established by the International PCNSL Collaborative Group in a few mono-institutional studies.19,21 Only recently, a prospective assessment of cognitive functions was performed in large randomized trials, but follow-up seems to be still short to draw definitive conclusions.16,37 In line with previous studies,38,39 preliminary results of the IELSG32 trial show that, after a rapid improvement in cognitive functions, patients treated with consolidative WBRT experienced a progressive decline in some attention/execution functions.37 In this trial, the severity of cognitive decline after WBRT seems to be lower than previously reported,38,39 which may be explained by a shorter follow-up, which does not allow verification of more delayed effects on cognitive impairment and, more importantly, of the use of lower radiation doses (eg, 36 Gy in the IELSG32 trial and ≥45 Gy in previous studies38,39 ). WBRT dose is an important neurotoxicity-determining factor. In fact, a recently reported exploratory analysis of subjective QoL questionnaires (EORTC-QLQ-C30 and BN20) and objective Mini-Mental Status Examination (MMSE) testing on 57% of PCNSL patients enrolled in the above-mentioned G-PCNSL-SG1 trial revealed that QoL and cognition were affected by postchemotherapy WBRT at 45 Gy, with significant increased rates of fatigue, appetite loss, and hair loss and lower MMSE values.40 Conversely, a single-arm phase 2 trial suggested that WBRT dose reduction to 23 Gy is associated with stable cognitive functions for up to 2 years.21 However, these results should be interpreted with caution because few elderly patients, who represent the subgroup associated with the highest risk, were considered. The impact of reduced-dose WBRT both on survival and cognitive functions is being addressed in the Radiation Therapy Oncology Group (RTOG-1114) randomized trial.
The available literature suggests that iatrogenic cognitive decline is underestimated in PCNSL patients and that WBRT should be used with caution, especially in elderly patients, and calls for the implementation of formal neuropsychometric testing in clinical trials. Importantly, the impact of cognitive decline on patients’ everyday life remains an unexplored issue. Routine practice shows that the same deficit may have a varied weight in patients’ lives according to the age group, instruction level, professional qualification, and other variables.
Myeloablative conditioning and ASCT
There is growing evidence supporting the use of HDC/ASCT as part of first-line therapy in PCNSL patients (Table 2). Early studies using a BEAM (carmustine [BCNU], etoposide, cytarabine, and melphalan) combination, the most commonly used conditioning regimen in relapsed diffuse large B-cell lymphoma, have been associated with disappointing survival figures, which has been imputed to the low CNS bioavailability of used drugs at the doses delivered. Conversely, results reported with thiotepa-based conditioning regimens are encouraging.41 Although direct comparison between used conditioning regimens is difficult, the BCNU-thiotepa combination seems to be equally effective but less toxic that the thiotepa-busulfan-cyclophosphamide combination.41 In the largest single-arm phase 2 trial addressing HDC/ASCT in PCNSL patients aged ≤65 years,42 the CRR after ASCT was 77%, with a 3-year PFS and OS of 67% and 81%, respectively. At a median follow-up of 57 months, unexpected toxicity has not been reported, and treatment-related mortality was 5%.
Reported studies focused on ASCT in PCNSL
| Ref . | N* . | Median age (range), y . | Therapy line . | Therapy (induction→intensification) . | CRR to induction,† % . | Transplanted patients,† % . | Conditioning regimen . | WBRT . | Median follow-up, mo . | OS, y (%) . | Neuro toxicity,† % . | TRM,† % . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 60 | 22 | 53 (27–64) | Salvage | ARAC+VP16 | 36 | 91 | Bu/TT/Cy | No | 41 | 3 (64) | 32 | 4 |
| 61 | 43 | 52 (23–65) | Salvage | ARAC+VP16 | 35 | 63 | Bu/TT/Cy | No | 36 | 2 (45) | 5 | 12 |
| 62 | 45 | 57 (19–72) | Salvage | ICE‡ | 51 | 40 | Bu/TT | No | 53 | 5 (40) | NR | 5 |
| 63 | 28 | 53 (25–71) | First | HDMTX→ARAC | 29 | 50 | BEAM | No | 28 | 2 (55) | 0 | 4 |
| 64 | 25 | 52 (21–60) | First | MBVP→IFO+ARAC | 44 | 68 | BEAM | Yes | 34 | 4 (64) | 8 | 4 |
| 65 | 6 | 53 (30–66) | First | MBVP→IFO+ARAC | 2 of 6 patients | 6 of 6 patients§ | BEAM | Yes | 41 | 2 (40) | 2 of 6 patients | 0 of 6 patients |
| 66 | 11 | 52 (33–65) | First | HDMTX→ARAC | 8 of 11 patients | 11 of 11 patients§ | BUCYE | Yesll | 25 | 2 (89) | 3 of 11 patients | 0 of 11 patients |
| 67 | 13 | 56 (35–65) | First | MPV→ARAC | 31 | 46 | LEED | Yesll | 44 | 3 (76) | 0 | 0 |
| 68 | 23 | 55 (18–69) | First | HDMTX→ —# | 13 | 69 | Bu/TT | Yesll | 15 | 2 (48) | 39 | 13 |
| 19 | 33 | 57 (23–67) | First | R-MPV→ —# | 66 | 81 | Bu/TT/Cy | No | 45 | 3 (81) | 0 | 12 |
| 69,70 | 21 | 56 (34–69) | First | MPV→ARAC | 24 | 100§ | Bu/TT/Cy | No | 60 | 5 (44) | 0 | 24 |
| 71 | 30 | 54 (27–64) | First | HDMTX→ARAC+TT | 33 | 77 | BCNU/TT | Yes | 63 | 5 (69) | 17 | 3 |
| 72,73 | 13 | 54 (38–67) | First | HDMTX→ARAC+TT | 31 | 85 | BCNU/TT | Yesll | 72 | 5 (77) | 0 | 0 |
| 16 | 38¶ | 55 (25–60) | First | R-MBVP→R-ARAC | 38 | 62 | Bu/TT/Cy | No | 33 | 4 (65) | NR | 5 |
| 37 | 59¶ | 58 (26–70) | First | HDMTX+ARAC±R±TT | 54 | NR | BCNU/TT | No | 40 | 2 (71) | 0 | 3 |
| Ref . | N* . | Median age (range), y . | Therapy line . | Therapy (induction→intensification) . | CRR to induction,† % . | Transplanted patients,† % . | Conditioning regimen . | WBRT . | Median follow-up, mo . | OS, y (%) . | Neuro toxicity,† % . | TRM,† % . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 60 | 22 | 53 (27–64) | Salvage | ARAC+VP16 | 36 | 91 | Bu/TT/Cy | No | 41 | 3 (64) | 32 | 4 |
| 61 | 43 | 52 (23–65) | Salvage | ARAC+VP16 | 35 | 63 | Bu/TT/Cy | No | 36 | 2 (45) | 5 | 12 |
| 62 | 45 | 57 (19–72) | Salvage | ICE‡ | 51 | 40 | Bu/TT | No | 53 | 5 (40) | NR | 5 |
| 63 | 28 | 53 (25–71) | First | HDMTX→ARAC | 29 | 50 | BEAM | No | 28 | 2 (55) | 0 | 4 |
| 64 | 25 | 52 (21–60) | First | MBVP→IFO+ARAC | 44 | 68 | BEAM | Yes | 34 | 4 (64) | 8 | 4 |
| 65 | 6 | 53 (30–66) | First | MBVP→IFO+ARAC | 2 of 6 patients | 6 of 6 patients§ | BEAM | Yes | 41 | 2 (40) | 2 of 6 patients | 0 of 6 patients |
| 66 | 11 | 52 (33–65) | First | HDMTX→ARAC | 8 of 11 patients | 11 of 11 patients§ | BUCYE | Yesll | 25 | 2 (89) | 3 of 11 patients | 0 of 11 patients |
| 67 | 13 | 56 (35–65) | First | MPV→ARAC | 31 | 46 | LEED | Yesll | 44 | 3 (76) | 0 | 0 |
| 68 | 23 | 55 (18–69) | First | HDMTX→ —# | 13 | 69 | Bu/TT | Yesll | 15 | 2 (48) | 39 | 13 |
| 19 | 33 | 57 (23–67) | First | R-MPV→ —# | 66 | 81 | Bu/TT/Cy | No | 45 | 3 (81) | 0 | 12 |
| 69,70 | 21 | 56 (34–69) | First | MPV→ARAC | 24 | 100§ | Bu/TT/Cy | No | 60 | 5 (44) | 0 | 24 |
| 71 | 30 | 54 (27–64) | First | HDMTX→ARAC+TT | 33 | 77 | BCNU/TT | Yes | 63 | 5 (69) | 17 | 3 |
| 72,73 | 13 | 54 (38–67) | First | HDMTX→ARAC+TT | 31 | 85 | BCNU/TT | Yesll | 72 | 5 (77) | 0 | 0 |
| 16 | 38¶ | 55 (25–60) | First | R-MBVP→R-ARAC | 38 | 62 | Bu/TT/Cy | No | 33 | 4 (65) | NR | 5 |
| 37 | 59¶ | 58 (26–70) | First | HDMTX+ARAC±R±TT | 54 | NR | BCNU/TT | No | 40 | 2 (71) | 0 | 3 |
Data were updated from reference 41. ARAC, cytarabine; BCNU, carmustine; BEAM, carmustine, etoposide, cytarabine, and melphalan; Bu, busulfan; BUCYE, busulfan, cyclophosphamide and etoposide; Cy, cyclophosphamide; HDMTX, high-dose methotrexate; ICE (regimen), ifosfamide, carboplatin, etoposide; IFO, ifosfamide; LEED, cyclophosphamide, etoposide and melphalan; MBVP (regimen), methotrexate, carmustine, etoposide, and methylprednisolone; MPV (regimen), methotrexate, vincristine, and procarbazine; NR, not reported; R-ARAC, rituximab/cytarabine; Ref, reference; R-MPV (regimen), MPV plus rituximab; TRM, treatment-related mortality; TT, thiotepa; VP16, etoposide.
*Number of assessable patients.
†Values are percentages unless otherwise indicated.
Some patients with relapsed disease were retreated with HD-MTX.
Performed ASCT was a selection criterion.
llOnly for patients not achieving a complete remission.
Randomized trials: data regard patients allocated to the ASCT arm.
These patients did not receive intensification.
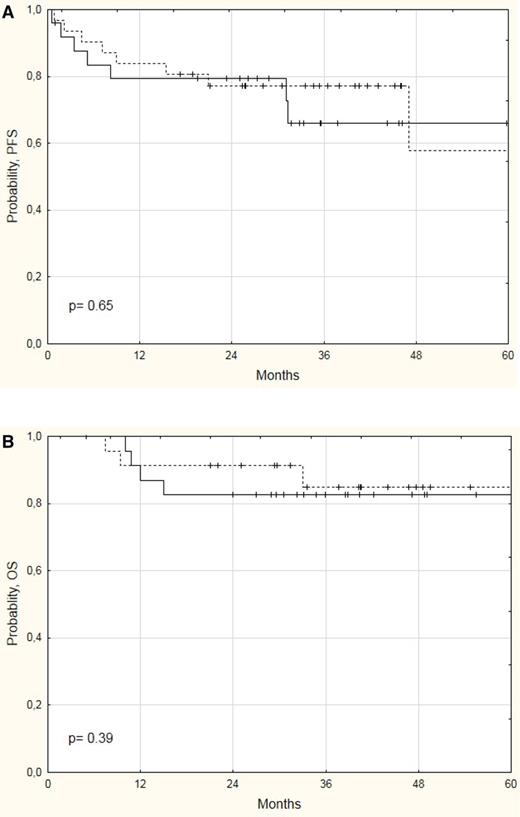
Comparison of HDC/ASCT with WBRT as consolidation therapy after front-line HD-MTX–based chemotherapy is being investigated in the above-mentioned IELSG32 and PRECIS trials. Preliminary results of these randomized trials agree that HDC/ASCT is an effective approach. In the IELSG32 trial, patients aged ≤70 years with PCNSL responsive to MTX-cytarabine–based combinations and managed with consolidative BCNU-thiotepa conditioning ASCT achieved a 93% CRR, with a 2-year PFS and OS of 63% and 71%, respectively.37 Importantly, patients responsive to MATRix induction achieved a 4-year OS of >80% in both WBRT and ASCT arms (Figure 3), which represents an excellent life expectancy for these high-risk patients. These encouraging results were achieved with a 3% transplant-related mortality and significant improvement in cognitive functions and QoL.37 Preliminary results of the PRECIS trial show that patients aged ≤60 years had a 2-year PFS and an OS of 86% in the ASCT arm.16 Data on treatment impact on cognitive functions and QoL are pending.
Survival curves of PCNSL with disease responsive to MATRix chemoimmunotherapy. PFS (A) and OS (B) curves of PCNSL patients with disease responsive to a MATRix regimen and consolidated with WBRT (dotted lines) or HDC/ASCT (continuous lines) in the IELSG32 trial.37
Survival curves of PCNSL with disease responsive to MATRix chemoimmunotherapy. PFS (A) and OS (B) curves of PCNSL patients with disease responsive to a MATRix regimen and consolidated with WBRT (dotted lines) or HDC/ASCT (continuous lines) in the IELSG32 trial.37
Advanced age and poor general condition of most PCNSL patients are the major obstacles for a widespread use of HDC/ASCT. A recent multicenter retrospective study that focused on 52 PCNSL patients aged ≥65 years treated with consolidative HDC/ASCT suggests that some selected elderly patients can be managed safely with this strategy.18 Although only one-third of patients received ASCT in complete remission, the 2-year PFS and OS rates were 62% and 71%, respectively, with a 4% transplant-related mortality. These encouraging results should be taken into account with caution due to the risk of selection biases in this type of retrospective study, which is suggested by the fact that more than half of patients were younger than 70 years, with a median Karnofsky score of 80%.
Nonmyeloablative chemotherapy
The Cancer and Leukemia Group B (CALGB) 50202 multicenter phase 2 trial reported promising results using high doses of cytarabine and etoposide as nonmyeloablative consolidation, without WBRT, after induction therapy with MTX, temozolomide, and rituximab.20 The CRR after induction was 66%, with a 2-year PFS of 57% and a 2-year OS of 70%. Toxicity was mild, with a treatment-related mortality of 2% and with no cases of severe neurotoxicity. Although supporting evidence is still limited, nonmyeloablative chemotherapy seems worthy of further research because, as an alternative to HDC/ASCT, it could allow the use of a de-escalated consolidation therapy applicable to a wide population of patients. The efficacy and tolerability of this approach are under investigation in 2 ongoing randomized trials using HDC/ASCT as comparator (NCT01011920 and NCT00863460).
Maintenance therapy
Recent trials addressed the role of maintenance therapy in PCNSL patients, which is of particular importance in elderly patients who are often unsuitable candidates for other consolidation strategies, such as WBRT or HDC/ASCT. Three prospective trials used oral alkylating agents (eg, temozolomide, procarbazine), whereas some role for oral immunomodulators (eg, lenalidomide) was reported in a retrospective study. Temozolomide was assessed as maintenance drug in 2 studies, showing a maximum tolerated dose of 100 mg/m2 per day with hepatic and renal dose-limiting toxicities. Patients treated with MTX-temozolomide-rituximab followed by hyperfractionated WBRT and temozolomide maintenance had a 2-year OS rate of 80%.43 The Nordic Group has investigated the role of temozolomide at 150 mg/m2 per day, days 1–5 every 28 days, for 1 year or until progression in patients aged >65 years enrolled in a phase 2 trial and treated with a front-line age-adjusted MTX-cytarabine regimen.44 This strategy was associated with a 2-year OS of 60%, which was similar to survival figures achieved without maintenance in patients aged <65 years. Procarbazine maintenance was assessed in patients aged >65 years responsive to a rituximab-procarbazine-MTX combination, resulting in a 2-year OS of 47%.27 Unfortunately, the lack of a suitable control group in these trials does not allow researchers to establish the contribution of alkylator maintenance in the light of these encouraging results. A retrospective series reported only as a meeting abstract suggested a contribution of maintenance with lenalidomide in 12 patients with relapsed PCNSL.45 Treatment was feasible, even among patients >70 years old, and the duration of response with lenalidomide was longer than time to progression after the first-line therapy in most patients, even after local salvage therapy (eg, stereotactic radiotherapy, tumor resection). Comprehensively, these studies suggest that single-drug maintenance could be a proper strategy to prolong survival, mostly in elderly patients who are often unsuitable candidates for WBRT or HDC/ASCT. A randomized trial called FIORELLA, comparing 2 different maintenance strategies, such as procarbazine and lenalidomide, after an HD-MTX-procarbazine-rituximab combination in patients aged >70 years but eligible for chemotherapy will start shortly.
Novel agents
The high frequency of somatic mutations in genes involved in important pathways such as B-cell receptor (BCR), Toll-like receptor, and NF-κB play central roles in PCNSL.46-48 These recurrent mutations result in pathway deregulation, which seems to drive mechanisms in PCNSL tumorigenesis. Some of these cellular molecules and pathways as well as microenvironment factors can be exploited therapeutically. The more advanced examples regard drugs targeting the BCR pathway, immunomodulatory agents, and antibodies targeting immune checkpoint molecules inhibiting the antitumor immune response.
Losses and deletions of chromosome 6p21 (HLA locus) are the most frequent genomic aberrations in PCNSL. Some candidate genes are linked to chromosome 6q, including PRDM1, PTPRK, and A20 (TNFAIP3), a key negative regulator of NF-κB signaling. Increased MALT1 copy number and activating mutations of CARD11 and MyD88 (76% of cases) suggest that aberrant activation of the NF-κB pathway is common in PCNSL.47 Accordingly, agents that attenuate proximal signals promoting NF-κB may hold promise in the treatment of PCNSL. Ibrutinib, a potent Bruton tyrosine kinase (BTK) inhibitor, which shows some activity in activated-like DLBCL, is being addressed in at least 3 PCNSL trials. Preliminary results of these trials and a retrospective study show a good tolerability at 560- and 840-mg/day doses, with meaningful concentrations in the CSF and an ORR of 55% to 68%.49-51 Although activation of invasive aspergillosis generated major concerns and the fact that responses were usually short lived (median PFS: 4–5 months), a trial addressing the MTX-ibrutinib combination is ongoing.
MUM1 may contribute to the pathogenesis of B-cell lymphomas via transcriptional upregulation of MYC and other genes. Immunomodulators, such as lenalidomide and pomalidomide, mediate therapeutic efficacy via downmodulation of MUM1/IRF-4 in a cereblon-dependent manner,52 suggesting that these drugs may have significant activity in PCNSL. A trial of pomalidomide in CNS lymphoma is ongoing (NCT01722305), and preliminary results in 25 accrued patients show an ORR of 43%, with grade 3/4 hematologic and nonhematologic toxicity in one-third of patients and a maximum tolerated dose of 5 mg/day for 21 of 28 days.53 After a few case reports of patients with relapsed PCNSL successfully treated with lenalidomide,54 a phase 1 trial with a rituximab-lenalidomide combination in recurrent/refractory CNS and intraocular lymphomas was designed (NCT01542918). Preliminary results show that lenalidomide achieved meaningful concentrations in the CSF with an objective response of lesions present in the brain, eyes, and CSF.45 Preliminary results of a phase 2 trial in 50 patients with relapsed PCNSL treated with 8 courses of lenalidomide 20–25 mg/day plus rituximab followed by 12 courses of lenalidomide 10 mg/day showed an ORR of 67% (36% after induction) and a 1-year PFS of 20%.55 Although only one-third of patients completed induction and 42% required a dose reduction, the median duration of response to lenalidomide was 8 months compared with 4 months after the previous treatment line, suggesting that this drug deserves to be further investigated in PCNSL.
PCNSL is often accompanied by a robust inflammatory response containing activated macrophages and T cells,56 which suggests a hypothetical role of immunotherapies potentiating T-cell–mediated immune surveillance. PCNSL exhibits frequent 9p24.1 copy number alterations, infrequent translocations of 9p24.1, and increased expression of PD-1 ligands.57 The activity of PD-1 blockade in other lymphomas with 9p24.1 alterations prompted some investigators to treat patients with relapsed PCNSL with the anti-PD1 antibody nivolumab. To date, supporting evidence is constituted by responses lasting ≥13, 14, 17, and ≥17 months in 4 treated patients.58 More consistent data will be provided by an ongoing phase 2 trial addressing nivolumab in patients with relapsed PCNSL (NCT02857426).
Molecular knowledge aimed at establishing other target therapies in PCNSL is growing. Results of ongoing trials addressing novel agents, if confirmed in a large series, might pave the way to classes of agents never used before in this setting, with different mechanisms of action from classic chemotherapeutic drugs that can be delivered by oral route. Trials addressing new first-line therapies including these novel agents should be the next step.
How I treat PCNSL
A modern treatment of PCNSL includes induction and consolidation phases.
Patients younger than 65 without relevant comorbidity should be treated with a combination of MTX-cytarabine-thiotepa-rituximab (MATRix regimen) as induction chemotherapy.
Patients aged >70 years without relevant comorbidity should be treated with a combination of methotrexate, an alkylating agent (eg, procarbazine, temozolomide), and rituximab (eg, rituximab/HD-MTX/procarbazine/vincristine [R-MPV], rituximab/HD-MTX/procarbazine [PRIMAIN], rituximab/HD-MTX/temozolomide [MTR] regimens) as induction chemotherapy.
Both induction and consolidation phases are personalized in patients aged >65 and <75 years; comorbidity, PS, and prognostic factors are important decisional tools.
Elderly patients in poor neurological conditions and very old (>80 years) patients with contraindications to chemotherapy should be treated with primary radiotherapy alone.
In patients with “borderline” conditions, the use of reduced doses of well-established chemotherapy regimens should be preferred to unverified “homemade” combinations.
Patients who are suitable candidates for full-dose modern induction therapy should not be managed with intrathecal and/or intraventricular chemotherapy.
Treatment should include intrathecal chemotherapy, preferably by intraventricular route, in patients with meningeal disease who are not able to receive an MTX dose ≥3 g/m2 or who achieved insufficient response to intravenous chemotherapy.
Intravitreous chemotherapy should be considered for presenting or recurrent disease confined to the eyes in patients with contraindications to receive intravenous chemotherapy.
Consolidative WBRT and HDC/ASCT are 2 effective consolidation options; therapeutic choice should be based on age, comorbidity, and tolerability to induction chemotherapy. Pros and cons should be discussed with the patient and his/her relatives.
HDC/ASCT is an excellent option for patients <65 years or selected patients <75 years, with responsive disease, without relevant comorbidity and good tolerability to induction chemotherapy.
Consolidative WBRT should be preferred for patients <60 years, with responsive disease, with relevant comorbidity, and/or poor induction tolerability. WBRT is an unavoidable option for poor autologous peripheral blood stem cell mobilizers.
If consolidative radiotherapy is indicated, the whole brain, the first 2 segments of the spinal cord, and the eyes should be irradiated, with a dose of 23 to 30 Gy and standard fractionation.
Elderly patients are often unsuitable candidates for both WBRT and HDC/ASCT; maintenance with oral alkylating agents or immunomodulators deserves to be investigated.
Lenalidomide and ibrutinib are 2 experimental drugs that deserve further investigation.
Correspondence
Andrés José María Ferreri, Unit of Lymphoid Malignancies, Department of Onco-Haematology, IRCCS San Raffaele Scientific Institute, Via Olgettina 60, 20132 Milan, Italy; e-mail: ferreri.andres@hsr.it.
References
Competing Interests
Conflict-of-interest disclosure: A.J.M.F. declares no competing financial interests.
Author notes
Off-label drug use: thiotepa, temsirolimus, lenalidomide, nivolumab, and ibrutinib.