Abstract
The approach to the patient with relapsed or relapsed/refractory multiple myeloma requires a careful evaluation of the results of previous treatments, the toxicities associated with it, and an assessment of prognostic factors. The majority of patients will have received prior therapy with drug combinations, including a proteasome inhibitor and an immune-modulatory agent. It is the physician’s task to choose the right moment for the start of therapy and decide with the patient which goals need to be achieved. The choice of regimen is usually based on prior response, drugs already received, adverse effects, comorbidities of the patient, and expected efficacy and tolerability. Many double and triple drug combinations are available. In addition, promising new drugs such as pomalidomide, carfilzomib, and monoclonal antibodies are or will be available shortly, and other options can be explored in clinical trials. Finally, supportive care and palliative options need to be considered in later relapsed disease. Increasingly, it becomes important to consider the therapeutic options for the whole duration of the disease and integrate a systematic approach for the patient.
Learning Objectives
To consider a systematic approach for the diagnosis and treatment of patients with relapsed or refractory multiple myeloma
To take into account all relevant data on prior treatments, toxicities, tolerability, patient condition, and available options to make the optimal treatment choice
To learn about the clinical results of approved and investigational drugs in trials for patients with relapsed or refractory multiple myeloma
Introduction
Multiple myeloma (MM) is characterized by the presence of monoclonal plasma cells in bone marrow, extramedullary organs, or both. Symptomatic MM is defined by end organ damage such as lytic bone lesions, hypercalcemia, anemia, and renal impairment (CRAB). The International Myeloma Working Group (IMWG) has revised the criteria for MM. Progress in the treatment of newly diagnosed MM has been achieved through the introduction of high-dose therapy (HDT) with autologous stem cell transplantation (ASCT) and by the introduction of novel therapies. Significant improvement of progression-free survival (PFS) and overall survival (OS) is now a fact in younger and elderly patients. Despite the recent progress in OS, MM remains an incurable disease, and the majority of patients will relapse and will require additional treatment.
Definitions of relapsed and relapsed/refractory disease
The IMWG has published and revised the definitions of relapsed MM in 2015. Relapsed MM is defined as a recurrence of disease after prior response on the basis of objective laboratory and radiological criteria:
≥25% increase of the monoclonal protein (M-protein) in serum (absolute increase ≥ 0.5 g/dL) or urine (absolute increase ≥ 200 mg/d) or
≥25% difference between involved and uninvolved serum-free light chains (absolute increase > 10 mg/dL) or
>10% increase of the absolute percentage of bone marrow plasma cells or
development of new (extramedullary) plasmacytomas or hypercalcemia.
Relapsed/refractory MM (RRMM) is defined as disease that becomes nonresponsive or progressive on therapy or within 60 days of last treatment in patients who had achieved a minimal response or better on prior therapy.1 The most recent IMWG consensus defines the type of relapse according to clinical aggressiveness (Table 1).
International Myeloma Working Group diagnostic criteria for relapsed disease in multiple myeloma
| Nonaggressive relapse . | ||
|---|---|---|
| Biochemical relapse . | Symptomatic relapse . | Aggressive relapse . |
| Progression based on increase of M-protein | Slow inset of clinical symptoms and slowly increasing M-protein | Adverse cytogenetic abnormalities, eg, t(4;14), del(17p), ampl (1q21), hypodiploidy |
| No associated symptoms or MM-related organ dysfunction | Progressive disease with prominent symptoms and/or significant organ compromise | High β2M (>5.5 mg/L) or low albumin (<3.5 g/dL) |
| Presence of extramedullary disease | ||
| High LDH | ||
| Short duration of response or progression while on therapy | ||
| Aggressive clinical presentation including: | ||
| • Rapid onset of symptoms | ||
| • Extensive disease on laboratory, radiography, or pathology findings | ||
| • Disease-associated organ impairment | ||
| Circulating plasma cells | ||
| ISS stage II/III at relapse | ||
| Isotype transformation (light chain escape, hyposecretory disease) | ||
| Nonaggressive relapse . | ||
|---|---|---|
| Biochemical relapse . | Symptomatic relapse . | Aggressive relapse . |
| Progression based on increase of M-protein | Slow inset of clinical symptoms and slowly increasing M-protein | Adverse cytogenetic abnormalities, eg, t(4;14), del(17p), ampl (1q21), hypodiploidy |
| No associated symptoms or MM-related organ dysfunction | Progressive disease with prominent symptoms and/or significant organ compromise | High β2M (>5.5 mg/L) or low albumin (<3.5 g/dL) |
| Presence of extramedullary disease | ||
| High LDH | ||
| Short duration of response or progression while on therapy | ||
| Aggressive clinical presentation including: | ||
| • Rapid onset of symptoms | ||
| • Extensive disease on laboratory, radiography, or pathology findings | ||
| • Disease-associated organ impairment | ||
| Circulating plasma cells | ||
| ISS stage II/III at relapse | ||
| Isotype transformation (light chain escape, hyposecretory disease) | ||
LDH, lactate dehydrogenase.
Diagnostic procedures
At relapse the diagnostic evaluation should follow the full routine work-up of MM, including serum and urine electrophoresis and immunofixation, serum-free light chain analysis, and urine total protein. Other essential investigations include a complete blood count, renal function, serum calcium, and β-2-microglobulin. Bone marrow evaluation is strongly recommended, in particular in non- or oligosecretory MM, and should include morphology and fluorescence in situ hybridization (FISH) on CD138 selected plasma cells to detect cytogenetically unfavorable abnormalities. Finally, diagnostic imaging should include evaluation of skeletal lesions by conventional radiography or computer tomography or by magnetic resonance imaging. Positron emission tomography combined with computed tomography is recommended when extramedullary disease is suspected or for detection of new metabolic active lesions.
Indications and timing of relapse treatment
The goal of relapse treatment is to relieve disease symptoms, to prevent new organ damage, and to achieve a second lasting disease remission. Second and later remissions tend to be shorter because disease may be more aggressive owing to the presence of different clones, which represent refractory disease.
Indications to start relapse treatment have been defined as the (re)appearance of one or more CRAB criteria or a rapid and consistent biochemical relapse as defined by the IMWG2 (Table 2).
Indications for treatment at relapse
| Type of relapse . | Indications . |
|---|---|
| Clinical relapse | Development of new soft-tissue plasmacytomas or bone lesions |
| • Definite increase (≥50%) in size of existing plasmacytomas or bone lesions | |
| • Hypercalcemia (≥11.5 mg/dL; 2.875 mmol/L) | |
| • Decrease in hemoglobin of ≥2 g/dL (1.25 mmol/L), or of <10 g/dL because of myeloma | |
| • Rise in serum creatinine by ≥2 mg/dL or more (≥177 mmol/L), due to myeloma | |
| • Hyperviscosity requiring therapeutic intervention | |
| Significant biochemical relapse in patients without clinical relapse | Doubling of the M-component in 2 consecutive measurements separated by 2 months with the reference value of 5 g/L, or |
| • In 2 consecutive measurements, any of the following increases: | |
| o the absolute levels of serum M-protein by ≥10 g/L, or | |
| o an increase of urine M-protein by ≥500 mg per 24 h, or | |
| o an increase of involved FLC level by ≥20 mg/dL (plus an abnormal FLC ratio) or 25% increase (whichever is greater) |
| Type of relapse . | Indications . |
|---|---|
| Clinical relapse | Development of new soft-tissue plasmacytomas or bone lesions |
| • Definite increase (≥50%) in size of existing plasmacytomas or bone lesions | |
| • Hypercalcemia (≥11.5 mg/dL; 2.875 mmol/L) | |
| • Decrease in hemoglobin of ≥2 g/dL (1.25 mmol/L), or of <10 g/dL because of myeloma | |
| • Rise in serum creatinine by ≥2 mg/dL or more (≥177 mmol/L), due to myeloma | |
| • Hyperviscosity requiring therapeutic intervention | |
| Significant biochemical relapse in patients without clinical relapse | Doubling of the M-component in 2 consecutive measurements separated by 2 months with the reference value of 5 g/L, or |
| • In 2 consecutive measurements, any of the following increases: | |
| o the absolute levels of serum M-protein by ≥10 g/L, or | |
| o an increase of urine M-protein by ≥500 mg per 24 h, or | |
| o an increase of involved FLC level by ≥20 mg/dL (plus an abnormal FLC ratio) or 25% increase (whichever is greater) |
In case of symptomatic relapse presenting with prominent new or worse CRAB symptoms, immediate treatment is required. The choice of treatment depends on many variables and is discussed below.
A biochemical relapse may require careful monthly monitoring of M-protein levels until significant progression occurs. Treatment of biochemical relapse is indicated as follows: a doubling of serum M-protein, increase of serum M-protein by ≥10 g/L, increase of urine M-protein by ≥500 mg per 24 hours or an increase of involved serum-free light chains (FLC) level by ≥200 mg/L (plus abnormal ratio) by 2 measurements, 2 months apart. In high-risk patients, such as those with aggressive disease at diagnosis or a short treatment-free interval with a suboptimal response to previous treatment or imminent risk for organ dysfunction such as previous light chain-induced renal impairment or new bone lesions or adverse cytogenetics [t(4;14), del17p, or both], treatment should be initiated early after biochemical relapse is diagnosed to avoid serious symptomatic disease.3
Risk factors to consider for choice of treatment of MM at relapse
Once relapse treatment is required, individual patient characteristics will influence the optimal treatment choice.4 The risk status has to be determined on the basis of established criteria. Twenty percent of patients have aggressive relapse according to IMWG criteria, on the basis of unfavorable cytogenetics [del17p, t(4;14), add 1q/del1p, t(14;16), high-risk gene expression profile, high β-2-M] or low-albumin, high-serum LDH (Table 2). Additional high-risk factors are plasma cell leukemia, short duration of previous remission, and rapid/aggressive progression/relapse.5,6 Of note, the International Staging System has not been validated as a prognostic variable in the relapse setting. These patients require immediate and intensive treatment with triplet drug regimens to achieve disease control and to improve survival. Patients with standard-risk or indolent disease are usually treated with less-intensive regimens or even monotherapy to which they were not previously exposed, respectively. Patient-related factors such as higher age, frailty, and comorbidities should be balanced against the treatment goals orer to avoid treatment-related morbidity (Table 3). In these patients, doublet rather than triplet combinations may be the preferred choice. It is important to consider the prior line (or lines) of therapy, prior responses, and adverse events (AEs) or cumulative toxicities that prohibit the use of certain drugs. The physical and emotional impact of hospitalization or frequent hospital visits for intravenous drug administration should be weighed. In addition, the occurrence of any grade treatment-related AEs should be balanced against the potential benefit. Treatment-related AEs are a frequent cause of premature treatment discontinuation, which will influence the outcome. It is important that the patient be able to complete the planned treatment to establish control of the disease. Quality of life is an additional goal, which should be discussed with the patient.
Relative risk of novel doublet and triplet combinations in relapsed MM
| Combination . | HR for PFS . | PFS (mo) . | ≥VGPR, % . | ≥CR, % . | Remarks . |
|---|---|---|---|---|---|
| Elotuzumab-Rd vs Rd | 0.71 | 19 | 35 | 5 | Effective after 2-3 lines of therapy |
| Carfilzomib-Rd vs Rd | 0.69 | 26.3 | 38 | 32 | Effective after 1 line |
| Daratumumab-Rd vs Rd | 0.37 | NR at 18 mo | 76 | 43 | Most effective in high-risk cytogenetics |
| Ixazomib-Rd vs Rd | 0.82 | 20.6 | 48 | 32 | Effective after 2−3 lines |
| Daratumumab-Vd vs Vd | 0.39 | NR at 12 mo | 28 | 10 | More effective than carfilzomib-dexamethasone |
| Panobinostat-Vd vs Vd | 0.63 | 12 | 28 | 11 | Available for ≥3 lines |
| Carfilzomib-d vs bortezomib-d | 0.53 | 18.3 | 54 | 13 | More affordable, less toxic |
| Combination . | HR for PFS . | PFS (mo) . | ≥VGPR, % . | ≥CR, % . | Remarks . |
|---|---|---|---|---|---|
| Elotuzumab-Rd vs Rd | 0.71 | 19 | 35 | 5 | Effective after 2-3 lines of therapy |
| Carfilzomib-Rd vs Rd | 0.69 | 26.3 | 38 | 32 | Effective after 1 line |
| Daratumumab-Rd vs Rd | 0.37 | NR at 18 mo | 76 | 43 | Most effective in high-risk cytogenetics |
| Ixazomib-Rd vs Rd | 0.82 | 20.6 | 48 | 32 | Effective after 2−3 lines |
| Daratumumab-Vd vs Vd | 0.39 | NR at 12 mo | 28 | 10 | More effective than carfilzomib-dexamethasone |
| Panobinostat-Vd vs Vd | 0.63 | 12 | 28 | 11 | Available for ≥3 lines |
| Carfilzomib-d vs bortezomib-d | 0.53 | 18.3 | 54 | 13 | More affordable, less toxic |
d, dexamethasone; NR, not reached.
Goal of treatment: achievement of response
During the first relapse, clinically relevant responses can be achieved in 40% to 50% of patients. A clinical response is an important goal of treatment, because it is needed for disease and symptom control and also is a first step toward a meaningful disease-free interval. An important question is whether the depth of response affects long-term outcome in relapsed MM (for a review, see Lonial and Anderson7 ). In the APEX trial, achievement of complete response (CR) with bortezomib was associated with a longer time to next treatment than it was with very good partial response (VGPR) or partial response (PR). In the MM009 and MM010 trials using lenalidomide plus dexamethasone, time to progression (TTP) and OS were significantly longer in patients who achieved VGPR or better than it was in those with PR (TTP: 27 vs 12 months; OS: not reached vs 44 months). At subsequent relapses and in RRMM, virtually no impact of CR/VGPR on OS or TTP is observed, as was demonstrated in the MM003 trial with pomalidomide plus low-dose dexamethasone.8 Recently, several new drugs were evaluated in relapsed MM, that is, carfilzomib combined with dexamethasone (Endevour) or Rd (Aspire); panobinostat combined with bortezomib and dexamethasone (Vd) (Panorama); elotuzumab combined with Rd (Eloquent-2); ixazomib combined with Rd (Tourmaline); daratumumab alone or combined with Rd (Pollux) or Vd (Castor). In these trials, with the exception of Eloquent-2, a significant number of patients achieved a complete response, which indicates that CR can be achieved in relapsed MM. These data emphasize that in first relapse, CR may be a relevant and realistic treatment goal, which can be actively pursued in fit patients. In second relapse and beyond the goal of treatment is to prevent organ impairment and to achieve disease control.
Prior treatment and outcome
Most patients with newly diagnosed MM who are transplant eligible receive a triple drug induction regimen that contains a proteasome inhibitor (PI) plus dexamethasone and a third agent followed by HDT supported by ASCT. Nontransplant-eligible patients receive either continuous lenalidomide plus dexamethasone (Rd) or melphalan plus prednisone and bortezomib. Until recently, second-line options were Rd for patients who received a PI as a first-line option or a PI-based regimen for those who were treated before with Rd. The IMWG has conducted a survey of the risk of progression in patients relapsing after prior therapy with immune-modulatory agents (IMiDs) and bortezomib, showing that that the median OS and event-free survival were 9 months and 5 months, respectively.9 In 2 studies, single-agent bortezomib or bortezomib plus pegylated doxorubicin and dexamethasone were compared. A lower response rate was observed in thalidomide-exposed patients than in thalidomide-naïve patients with bortezomib monotherapy as salvage treatment but not when bortezomib was combined with pegylated doxorubicin and dexamethasone.10 In an analysis of the MM009 and MM010 trials comparing lenalidomide plus dexamethason with dexamethasone alone in relapsed MM, thalidomide-naïve patients had a significantly better overall response (ORR) than did patients who had received thalidomide. In other trials a similar difference was observed between thalidomide-naïve and thalidomide-exposed patients who received subsequent treatment with bortezomib, lenalidomide, dexamethasone (VRD); bortezomib, cyclophosphamide, dexamethasone; or bortezomib, thalidomide, dexamethasone (VTD). In the VISTA trial, patients who had been treated upfront with bortezomib, melphalan, and prednisone (VMP) had a similar or better outcome after relapse treatment with bortezomib-based or IMiD-based regimens than did MP-treated patients.11 Bortezomib retreatment may be effective, during which 40% of patients achieved a response with a TTP of 8 months and a better outcome in patients who achieved a complete response (CR). In conclusion, retreatment with previously administered drugs is feasible and useful if a substantial and lasting response was obtained in the absence of cumulative or irreversible toxicities.
Age and frailty
Patients of higher age, with comorbidities, or both should be considered candidates for treatment when relapse occurs. The primary goal of treatment should be relief of symptoms and prevention of new CRAB lesions. In general these patients do not tolerate standard dose and schedule of (combination) treatment. The IMWG has issued an algorithm for the treatment approach in elderly or frail patients.12 Mitigated schedules are preferred, for example, with weekly dosing of bortezomib or reduced dose. Toxic combinations and cumulative toxicity should be avoided at all cost. Frequently occurring AEs that must be prevented are grade 3-4 peripheral neuropathy caused by PIs, vinca alkaloids and IMiDs, cardiotoxicity and hypertension (carfilzomib, anthracyclins), fatigue and wasting (PIs), depression and myopathy (dexamethasone), and deep myelosuppression.
Cytogenetics
Until recently, the impact of unfavorable cytogenetics at the diagnosis of relapse on the outcome of treatment had not been consistently evaluated. Therefore, the results of cytogenetic subgroups should be taken with caution. In a prospective trial comparing VRD with lenalidomide plus dexamethasone (RD) in relapsed MM, the presence of +1(q21) was associated with shorter OS in the RD group, whereas the impact of t(4;14) and del13q was less clear, and del17p had a poor outcome in both groups.13 In an analysis by the Intergroup Francophone de Myelome (IFM) t(4;14) had a negative impact on OS in relapsed MM patients treated with lenalidomide plus dexamethasone. In the MM003 trial in relapsed and refractory MM, there was a benefit of response and PFS (3 vs 1 months) in patients with t(4;14) or del17p who received pomalidomide plus LD dexamethasone in comparison with dexamethasone alone.
During the past years, several randomized clinical trials with new drugs in doublet or triplet combinations were performed in patients with first or later relapse of MM. In the following section these trials are discussed in more detail, because they set the stage for effective second and later line treatments in patients with relapsed MM.14-18 First, the Aspire trial evaluating carfilzomib plus Rd showed no difference in PFS and TTP across subgroups with high-risk versus standard-risk cytogenetics.18 The same observation was made in other recent trials, which evaluated elotuzumab combined with Rd (Eloquent-2), ixazomib combined with Rd (Tourmaline), and daratumumab alone or combined with Rd (Pollux) or with Vd (Castor). In these trials, cytogenetics by FISH were included in the diagnostic panel, and poor- versus standard-risk FISH groups were prospectively analyzed as prognostic variables. In Table 4 the impact of high-risk cytogenetics on the outcome with these treatments is shown. It should be noted that the cytogenetic data were obtained with different assays and cutoff values, which makes comparisons difficult.
PFS in patients with high-risk cytogenetics in recent trials for relapsed multiple myeloma
| . | Median PFS . | ||
|---|---|---|---|
| Regimen . | All high risk . | Del(17p) . | t(4;14) . |
| KRd vs Rd19 | 23.1 vs 13.9 mo (HR = 0.70) | 24.5 vs 11.1 mo (HR = NA) | 23.1 vs 16.7 mo (HR = NA) |
| Kd vs Vd35 | 8.8 vs 6.0 mo (HR = 0.646) | 7.6 vs 4.9 mo (HR = NA) | 10.1 vs 6.8 mo (HR = NA) |
| Elotuzumab-Rd vs Rd17 | NA | 21.2 vs 14.9 mo (HR = 0.70) | 15.8 vs 5.5 mo (HR = 0.52) |
| IRD vs Rd37 | 21.4 vs 9.7 mo (HR = 0.543) | 21.4 vs 9.7 mo (HR = 0.596) | 18.5 vs 12 mo (HR = 0.645) |
| DRd vs Rd16 | NR vs 10.2 (HR = 0.44) | NA | NA |
| . | Median PFS . | ||
|---|---|---|---|
| Regimen . | All high risk . | Del(17p) . | t(4;14) . |
| KRd vs Rd19 | 23.1 vs 13.9 mo (HR = 0.70) | 24.5 vs 11.1 mo (HR = NA) | 23.1 vs 16.7 mo (HR = NA) |
| Kd vs Vd35 | 8.8 vs 6.0 mo (HR = 0.646) | 7.6 vs 4.9 mo (HR = NA) | 10.1 vs 6.8 mo (HR = NA) |
| Elotuzumab-Rd vs Rd17 | NA | 21.2 vs 14.9 mo (HR = 0.70) | 15.8 vs 5.5 mo (HR = 0.52) |
| IRD vs Rd37 | 21.4 vs 9.7 mo (HR = 0.543) | 21.4 vs 9.7 mo (HR = 0.596) | 18.5 vs 12 mo (HR = 0.645) |
| DRd vs Rd16 | NR vs 10.2 (HR = 0.44) | NA | NA |
DRd, daratamumab, lenalidomide, dexamethasone; IRD, ixazomib, lenalidomide, dexamethasone; Kd, carfilzomib, dexamethasone; KRd, carfilzomib, lenalidomide, dexamethasone; NA, not applicable.
In general, the new combinations of carfilzomib, ixazomib, elotuzumab, and daratumumab, each combined with a backbone of either Rd or Vd, partly or completely abrogated the negative impact of t(4;14) or del(17p) to an extent that these abnormalities were not an independent variable for the primary endpoint PFS in these trials. Hence, at first relapse, FISH analysis contributes to a correct evaluation of the patient risk status.
Treatment options for relapsed MM
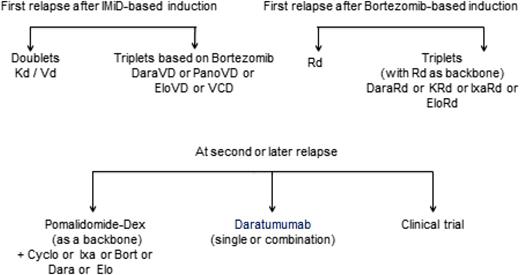
For 10 to 15 years, thalidomide, bortezomib, and lenalidomide as single agents or in combination with dexamethasone have been used in patients with relapsed MM. These agents have set the stage for the development of next-generation IMiDs, PIs, monoclonal antibodies, and histone deacetylase inhibitors in relapsed and refractory disease.8,18 In addition, several novel agents were recently approved in the United States and Europe for treatment of relapsed MM: (a) pomalidomide in combination with dexamethasone; (b) bortezomib in combination with pegylated doxorubicin; (c) carfilzomib alone or combination with dexamethasone or Rd; (d) panobinostat combined with Vd; (e) elotuzumab combined with Rd; (f) ixazomib combined with Rd; and (g) daratumumab monotherapy. In Europe, bendamustin with dexamethasone has also been approved. However, many other drugs may also be used that were not all approved for this indication, such as thalidomide, alkylator, and anthracyclin combinations. Recently, IMWG published recommendations for global myeloma care at relapse.19 Figure 1 gives a general strategy for treatment selection, which is discussed below. The reader is also referred to updated European Society for Medical Oncology guidelines (Figure 2), a recent review, and the IMWG consensus.4,20,21
European Society for Medical Oncology guidelines 2017 for treatment of relapsed/refractory MM. Source: P. Moreau et al. Multiple myeloma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up, Annals of Oncology 2017;28(suppl 4):iv52–iv61; doi:10.1093/annonc/mdx096. Reproduced with permission of Oxford University Press on behalf of the European Society for Medical Oncology. Please visit: www.esmo.org.
European Society for Medical Oncology guidelines 2017 for treatment of relapsed/refractory MM. Source: P. Moreau et al. Multiple myeloma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up, Annals of Oncology 2017;28(suppl 4):iv52–iv61; doi:10.1093/annonc/mdx096. Reproduced with permission of Oxford University Press on behalf of the European Society for Medical Oncology. Please visit: www.esmo.org.
Retreatment
Retreatment with an agent used previously is considered feasible, if the treatment produced a clinically meaningful response of adequate duration and with acceptable toxicity. In general, the minimal depth of the initial response should be partial response, whereas the minimal duration of response should have been at least 6 months. Trials and retrospective analyses have shown that retreatment with bortezomib is feasible and effective and does not incur cumulative toxicity. Lenalidomide retreatment is also feasible and may induce responses in up to 44% of relapsed patients and is better than retreatment with thalidomide. Tolerability is another important consideration. Residual toxicity from prior treatment such as neuropathy, myelosuppression, and thrombosis may prohibit retreatment. For some patients a change to a less-intensive schedule or dose may make the treatment better tolerated. For bortezomib, changing to a weekly and subcutaneous schedule will reduce toxicity. If an effective alternative treatment is available at relapse, switching drug class is preferable, and previously used agents may then be considered again at a later relapse. Patients may even become sensitive to (escalated dosages of) drugs to which they were previously refractory, on the basis of the appearance of different tumor clones during subsequent stages of the disease.
IMiDs
Thalidomide-based treatments.
A meta-analysis of thalidomide monotherapy limited to trials of ≥50 patients reported an ORR of 28% (CR 2% and PR 26%).22 A phase 3 comparison of low-dose (100 mg) and high-dose (400 mg) thalidomide showed similar activity, with a better safety profile associated with low-dose thalidomide. Retreatment with thalidomide was associated with a 30% ORR. Combining thalidomide with dexamethasone improves its efficacy in relapsed MM, which is further improved when thalidomide is combined with VTD; dexamethasone and cyclophosphamide (CTD); bortezomib plus melphalan and prednisone or dexamethasone; lenalidomide, melphalan, and prednisone; or VTD with pegylated liposomal doxorubicin. These regimens have an ORR of 63% to 90% with CR being reported in 2% to 35% of patients. In conclusion, thalidomide combined with dexamethasone is an option for relapsed patients who are thalidomide-naïve, when an oral treatment schedule is needed and who are not eligible for bortezomib or lenalidomide-based treatment. Thalidomide is now less frequently used because of relatively poor tolerability, the risk of venous thrombotic events, fatigue, and peripheral neuropathy as well as the availability of more effective alternative treatments. In spite of this, thalidomide plus dexamethasone may be an effective and affordable alternative in specific environments.
Lenalidomide-based treatments.
Lenalidomide is active as a single agent, and it is well tolerated in patients with relapsed MM with 25% to 27% ORR and 23 to 27 months OS. Its main toxicities are myelosuppression, diarrhea, and risk of venous thrombosis. Adding dexamethasone to lenalidomide further improves response rates by 30%. In the MM-009 and MM-010 trials, patients were treated with 25 mg of lenalidomide plus dexamethasone until disease progression or unacceptable toxicity. An analysis of pooled data from MM-009 and MM-010 after a follow-up of 48 months confirmed the improved outcomes with lenalidomide plus dexamethasone, which significantly improved ORR (60.6% vs 21.9%), duration of response (15.8 vs 7 months), and median TTP (13.4 vs 4.6 months).23 For maximum PFS benefit, patients should be treated for at least 12 months with full-dose lenalidomide plus dexamethasone, followed by lower-dose continued therapy. Addition of cyclophosphamide to lenalidomide and dexamethasone for continuous treatment has clinical value in patients with suboptimal reponse.24 Lenalidomide enhances the antimyeloma effect of bortezomib, and these agents have been evaluated as combination therapy with dexamethasone (RVd) in patients with relapsed MM. The combination of lenalidomide, bortezomib, and dexamethasone (RVd) is an active and well-tolerated regimen in patients with relapsed MM and can overcome drug resistance in patients previously treated with lenalidomide, bortezomib, thalidomide, or ASCT. With a follow up of >2 years, minimal response or better was achieved by 78% of patients, including PR or better in 64% and CR or near CR in 25% of patients; median PFS was 9.5 months, and median OS was 26 months. The European Myeloma Network has defined a consensus statement for the use of lenalidomide.25 In conclusion, lenalidomide combined with dexamethasone is currently the most valuable option for relapsed MM and may be combined with bortezomib, cyclophosphamide, or other agents. The recommendation is to give full dose with corticosteroids during reinduction and to continue with a lower dose until progression.
Pomalidomide.
Pomalidomide alone or combined with dexamethasone was approved by the US Food and Drug Administration and the European Medicines Agency for patients who have received at least 2 prior therapies, including lenalidomide and bortezomib, and have disease progression.26 The standard dose of pomalidomide is 4 mg on days 1 to 21 in a 28-day schedule. Its toxicities include myelosuppression and peripheral neuropathy. The pivotal trial demonstrating the superiority of pomalidomide with dexamethasone was MM003, which showed a better PFS (4 vs 1.9 months) and OS (12 vs 8 months) in spite of crossover from the dexamethasone arm for patients who did not respond.8 Recently, an expert panel consensus statement was published on the optimal use of pomalidomide in RRMM.27 Pomalidomide has activity against MM carrying the del17p abnormality. Pomalidomide is the only approved agent with demonstrated clinical activity in end-stage disease in patients who are refractory to bortezomib and lenalidomide. It should be continued until progression, preferably combined with dexamethasone. In case of insufficient response, pomalidomide can be combined with cyclophosphamide, or bortezomib and dexamethasone, or a combination of both.
Proteasome inhibitors
Bortezomib.
Bortezomib monotherapy is effective in patients with relapsed MM, as was demonstrated in pivotal studies.28 It can safely and effectively be administered to patients with renal impairment. Its main toxicities include peripheral neuropathy, which may preclude further treatment, gastrointestinal symptoms, and transient thrombocytopenia. Bortezomib, in comparison with dexamethasone, improved outcomes in patients with RRMM in the APEX trial.29 Patients treated with bortezomib have higher ORRs (38% vs 18%; CR 6% vs <1%; P < .001 for each), longer TTP (6.2 months vs 3.5 months; P < .001), and better 1-year OS (80% vs 66%; P = .003) than did those treated with dexamethasone. The drug is now routinely administered subcutaneously, which improves tolerance while keeping efficacy.30 It is now routinely used in a weekly schedule in elderly patients.31 Retreatment with bortezomib has clinical value if the patients were responsive before and if the response lasted more than 6 months.32 Bortezomib is effective in combination with other agents such as anthracyclins. Bortezomib in combination with dexamethasone and pegylated liposomal doxorubicin showed improved TTP (9.3 vs 6.5 months) and OS (76% vs 65%) and good tolerability. Bortezomib can be combined with weekly oral cyclophosphamide (300-500 mg/m2) and dexamethasone or prednisone.33 Bortezomib is also effective as part of triple or quadruple drug salvage regimens (ORR 56% to 88%; CR 6% to 46%; VGPR or better, 34% to 55%), and favorable response rates have been reported of CTD (VCTD); bortezomib plus melphalan and prednisone; and bortezomib with doxorubicin, dexamethasone, and lenalidomide.
Bortezomib may enhance the effects of the IMiDs thalidomide and lenalidomide. Bortezomib plus thalidomide/dexamethasone (VTD) was more effective than was thalidomide/dexamethasone for TTP (19.5 vs 13.8 months), CR/near CR (45% vs 25%), and duration of response (17.2 vs 13.4 months) in patients who relapsed after ASCT. Currently, one of the most effective and widely used regimens is bortezomib plus lenalidomide and dexamethasone. In a phase 1 trial in RRMM, ORR was 61% and OS of 37 months.34 Grades 3 and 4 toxicities were myelosuppression, whereas only grades 1 and 2 polyneuropathy was observed. In conclusion, bortezomib combined with dexamethasone is an effective treatment of RRMM. Its efficacy is increased when combined with thalidomide, lenalidomide, cyclophosphamide, or an anthracyclin. Patients should be carefully monitored for peripheral neuropathy, in case the dose and schedule should be reduced.
Two next-generation PIs became available in 2016, that is, intravenous carfilzomib and oral ixazomib. Carfilzomib irreversibly binds to the proteasome subunit and has been dosed at levels ranging from 27 mg/m2 to 70 mg/m2 weekly. Its safety profile is dominated by cardiovascular toxicity, including hypertension and congestive heart failure. Carfilzomib has been compared as single agent with dexamethasone (FOCUS trial) without a clear PFS benefit. This also suggests that carfilzomib must be combined with other agents. Carfilzomib plus dexamethasone was superior to bortezomib plus dexamethasone for PFS (16 months vs 9 months) in the Endeavor trial in patients who may have been treated with bortezomib before but were not refractory.35 Good results were achieved with carfilzomib combined with lenalidomide and dexamethasone (Rd) in the ASPIRE trial, leading to a better PFS than with Rd (PFS 26.3 vs 17.6 months; hazard ratio [HR] 0.69; P = .0001). ORR was 87% versus 67% and ≥ VGPR at 38% versus 31%.18 The combination was well tolerated, and there was a clinical benefit across different risk groups, including higher age, adverse FISH, and high International Staging System (ISS) stage. Carfilzomib has been combined with pomalidomide and dexamethasone and proven highly effective in RRMM.36 Carfilzomib is currently indicated for patients who received ≥2 prior treatments, including bortezomib and an IMiD. It has been approved for relapsed MM in combination with Rd.
Ixazomib is an oral boron-based PI that has been combined with dexamethasone alone or with Rd or with melphalan/prednisone. It is generally well tolerated, and its safety profile is comparable with bortezomib, though with only limited polyneuropathy. In the Tourmaline trial, ixazomib combined with Rd had a superior PFS to Rd (20 vs 15.9 months, HR 0.82; P = .05).37 ORR was 78% versus 72% and ≥ VGPR at 48% versus 39%. The superior outcome was observed across poor-risk cytogenetic groups and patients of different ages. The role of ixazomib for continuous treatment in RRMM is currently under clinical investigation. Other PIs under clinical development include marizomib and oprozomib.
Corticosteroids and conventional agents.
Dexamethasone is added to most therapies at a weekly dose of 40 mg or 20 mg in frail and elderly patients. Its adverse effects, that is, osteoporosis and infections, prohibit prolonged use of the drug. However, in patients who have exhausted other options, weekly dexamethasone or continuous low-dose (20 mg) prednisolone may be considered. Cyclophosphamide is an alkylating agent that usually is well tolerated and can be given orally or intravenously. It is often combined with bortezomib in the bortezomib, cyclophosphamide, dexamethasone or the cyclophosphamide and dexamethasone schedules or with lenalidomide and pomalidomide, but it can also be taken alone in a 300 mg/m2 weekly regimen. Also, standard-dose intermittent oral melphalan may be a valuable option for economic reasons or when patients have no other treatment options. High-dose conventional chemotherapy such as dexamethasone, cyclophosphamide, etoposide, cisplatin (DCEP) and dexamethasone, thalidomide, cisplatin, doxorubicin, cyclophosphamide, etoposide (DT-PACE) can be given in RRMM with ORR of 63%, although toxicity is common, and responses are usually of short duration.
Bendamustine.
This bifunctional alkylating agent was approved for the treatment of chronic lymphocytic leukemia, indolent B-cell non-Hodgkin’s lymphoma, and newly diagnosed MM in patients who cannot tolerate thalidomide or bortezomib because of neuropathy. However, it is more frequently used when combined with corticosteroids in RRMM. Early studies have reported an ORR rate of 55% and PFS of 8 months and good tolerability with a regimen of bendamustine (60-100 mg/m2) alone or in combination with thalidomide, in combination with Rd, or combined with bortezomib and dexamethasone in patients with relapsed MM.
Recently approved novel agents
Histone deacetylase inhibitors.
Panobinostat and vorinostat are epigenetic drugs that can be combined with other agents.38,39 Vorinostat combined with bortezomib and dexamethasone showed a PFS advantage of only 1 month in comparison with bortezomib and dexamethasone (Vd). Panobinostat is a pandeacetylase inhibitor that demonstrated combined synergy with Vd (Panorama 2).40 In a phase 3 trial (Panorama 1), PFS was longer (11.9% vs 8.1 months), and CR was better (27.6% vs 15.7%), whereas OS and ORR were not different from Vd with placebo. The main treatment related toxicities were thrombocytopenia, gastrointestinal symptoms, and fatigue.39 Panobinostat has been approved for the treatment of RRMM in patients who failed bortezomib and lenalidomide.
Monoclonal antibodies.
Recently, 2 monoclonal antibodies, elotuzumab and daratumumab, were introduced, which have a unique mechanism of action in the treatment of relapsed MM.
Elotuzumab is a monoclonal antibody that targets SLAMF-7, which is present on the surface of plasma cells. It has little single-agent activity in RRMM, but elotuzumab combined with lenalidomide and low-dose dexamethasone showed more than 80% ORR without significant toxicity. The lower dose of 10 mg/kg is associated with a longer PFS than is the dose of 20 mg/kg (33 vs 19 months). In the phase 3 trial ELOQUENT 2, elotuzumab (10 mg/kg) with Rd was compared with the Rd backbone in relapsed MM. PFS at 3 years was 27% versus 19%, ORR 78.5% versus 65.5%, and ≥ VGPR at 34.0% versus 28.6%. PFS by predefined subgroups was superior, with elotuzumab independent from age, high-risk FISH, prior bortezomib, and prior response type.16 Overall survival was 60% versus 53%, respectively. Adverse events were mild with grade 3 or 4 anemia (15%), neutropenia (34%), and fatigue (8%) occurring most frequently. Elotuzumab has been combined with pomalidomide/dexamethasone in RRMM and with checkpoint inhibitors in ongoing trials. Elotuzumab in combination with Rd has been approved for use in first and later relapse MM.
Daratumumab is an antibody that targets CD38 and kills plasma cells through antibody-dependent cellular cytotoxicity– and complement-dependent cytotoxicity–mediated mechanisms.41 In a phase 1 study in RRMM patients of whom 75% were refractory to bortezomib and lenalidomide, an ORR of 42% was observed.42 Adverse events were modest and mainly respiratory infusion reactions during the first administration. These results were confirmed in a phase 2 trial.43 On the basis of preclinical studies, lenalidomide has been identified as a synergistic partner for daratumumab.44 Clinical trials have been performed using daratumumab with Rd (Pollux trial) or with Vd (Castor trial) as comparator arms.15,17 In the Pollux trial, continuous treatment was given, and in Castor there was a fixed duration of treatment. At a median follow-up of 17 months in Pollux, daratumumab with Rd given until progression was superior for ORR (93% vs 76%), greater than CR (46% vs 20%), 18 months PFS (79% vs 49%), whereas OS was not different. In patients who were refractory to the last line of therapy (28%), ORR was 87% versus 64% and PFS 65% versus 34%.17 The better outcome for PFS was observed across cytogenetic subgroups, age groups, and ISS stages. Additional analysis for minimal residual disease confirmed the proportion of patients with deep responses at the 10−4 to 10−6 level.45 In Castor a fixed number of 8 cycles given Daratumumb plus Vd had superior 12-month PFS (60% vs 22%), ORR (84% vs 63%), and CR (26% vs 10%).15 In patients with ≥2 lines of therapy, PFS was 44% versus 22%. The superior outcome with daratumumb plus Vd was observed regardless of cytogenetic risk. Also in Castor a significant proportion of patients achieved minimal residual disease negativity. These trials have set the stage for use of daratumumab combinations in relapsed and RRMM. Daratumumab has been approved as monotherapy in patients with RRMM, who have failed PI and IMiD therapy. Isatuximab (SAR650984) and MOR202 are other anti-CD38 antibodies, which are currently being investigated in clinical trials. These and other novel agents are discussed in the third presentation of this session.
Cross-comparison of novel triplet regimens in relapsed MM.
Several triplets with novel agents have shown superior activity across cytogenetic and other subgroups. Cross-comparison of these regimens is hampered by the different inclusion criteria and risk groups, which makes treatment choice a challenge. The relative hazard ratios of these triplets give an indication about their potential benefit in general (Table 3) and in adverse cytogenetic groups (Table 4). First of all, the choice should be based on prior exposure to PIs and IMiDs, taking into account the response, duration of response, and (cumulative) toxicities. If multiple options are available, the choice should be based on efficacy and expected duration of response. In a network meta-analysis of all published phase 3 trials in relapsed and RRMM, the probability of having the best combination for PFS across patient groups was 99% for daratumumab plus Rd, 1% for daratumumab plus Vd, and 0% for the other combinations.46 In conclusion, monoclonal antibodies have significant clinical activity in particular when combined with other agents while showing limited toxicity. It is expected that antibodies will acquire an important place in the future treatment of relapsed MM.
HDT with ASCT or allogeneic SCT.
In transplant-eligible patients, HDT followed by ASCT may be considered. In patients who did not yet receive HDT before, it is the treatment of choice if stem cells can be obtained. In general, a second HDT plus ASCT may be considered in patients who had responded to a previous HDT with 18 to 24 months of PFS. Several trials have addressed the possibility to omit HDT in first line and to apply HDT/ASCT at first relapse. The IFM group demonstrated that HDT/ASCT is superior to VRD for PFS.47 The European Myeloma Network and the GIMEMA group reported similar outcomes. Hence HDT/ASCT should not be delayed until relapse but can be repeated. Such a strategy requires careful planning, and stem cells for a second transplant should be collected as early as possible.
Allogeneic transplantation is an experimental option for use in clinical trials in patients with high-risk disease and unfavorable FISH. The European Group for Blood and Bone Marrow Transplantation published a long follow-up of newly diagnosed and relapsed MM patients treated with autologous/reduced intensity allogeneic SCT versus autologous SCT, showing a superior OS with the combination (47% vs 31% at 96 months).48 Most investigators currently consider allogeneic SCT an option only for younger, fit patients with high-risk disease in first relapse.
Supportive care.
Patients with RRMM are at risk owing to the presence of disease, exposure to chemotherapy, myelosuppression, exposure to corticosteroids, and organ impairment. Frequent infections and bone disease are common and should be adequately prevented and treated. Intravenous zoledronate or pamidronate should be restarted at relapse, with calcium and vitamin D supplements. Low-dose local radiotherapy (20-40 Gy) may be administered to local bone lesions in case of pain or imminent fractures. Infections should be managed proactively. Prophylactic vaccination is recommended for influenza A and B, pneumococci, and Haemophilus influenza. Anemia may be treated with erythropoietin (40 000 U weekly) or darbopoietin (500 μg every 3 weeks) or with transfusion. Patients with increased risk of venous thrombotic events and those who are treated with thalidomide or lenalidomide should receive prophylaxis with aspirin (1 risk factor) or low-molecular-weight heparin (≥2 risk factors). Treatment of polyneuropathy and pain should be administered carefully. For detailed guidelines the reader is referred to the IMWG consensus.19
Specific treatment recommendations for first and later relapse
First relapse
The following practical suggestions may be considered:
The goal of treatment at first relapse is to achieve a maximum response and a durable disease-free interval.
In patients with relapse after previous good and lasting response of at least 18 months, treatment-free interval of 6 to 9 months, or both, retreatment with the original schedule can be considered.
In the majority of patients a switch to an alternative regimen is more practical because possible resistant clones are more likely to respond.
For transplant-eligible patients, HDT plus ASCT should be considered, especially if they have not received this treatment in first line.
In patients with suboptimal response to relapse retreatment, escalation to at least 1 agent that was not previously used should be considered.
Patients with high-risk relapse should be treated with a 3- or 4-drug regimen, preferably including a PI, an IMiD and dexamethasone, or both, plus one of the recently approved novel agents.
Carfilzomib and pomalidomide can be used in patients who were primary refractory to bortezomib and lenalidomide, respectively.
Continuous treatment should be considered when possible.
Allogeneic stem cell transplantation should be reserved for young and fit patients with high-risk disease. Given the significant risk of transplant-related mortality, graft-versus-host disease and lack of superior efficacy evidence, allogeneic transplant should only be performed under strict clinical conditions such as a clinical trial.
All eligible patients should be offered participation in a clinical trial.
Second and later relapse
The goal of relapse treatment is to achieve disease control and symptom release.
Patients should be treated with a regimen containing at least 1 or preferably more agents to which they were not previously exposed.
Daratumumab monotherapy is a valuable and approved option for these patients. Daratumumab may be combined with Rd or Vd for better efficacy in countries where these combinations have been approved. Alternative treatments include elotuzumab plus Rd, panobinostat plus Vd, carfilzomib plus Rd, and ixazomib plus Rd.
Retreatment with agents, which were used in first line and to which patient has responded, can be considered.
Patients should receive ongoing therapy until next relapse/progression, when a switch to an alternative regimen is recommended.
Eligible patients should be considered for trial participation with new drugs.
Conclusions and future directions
Relapse treatment has improved markedly. Continuous or repeated therapy with new drug combinations is well tolerated and leads to durable clinical responses, PFS, and OS. The majority of adverse events associated with these novel agents are hematologic and can be managed. Continuous therapy from first relapse to disease progression has the potential to maintain suppression of residual disease, prolong the time to subsequent relapse, and extend OS. Long-term treatment with novel agents may, however, lead to the emergence of drug-resistant MM clones, especially in patients with adverse FISH cytogenetics. Therefore, well-designed studies with long-term follow-up are needed to extend the clinical benefits and safety of new treatment approaches. This treatment strategy may generate prolonged control of the disease. If this can be achieved in RRMM patients, it will represent a paradigm shift, allowing MM to be managed as a chronic illness.
Correspondence
Pieter Sonneveld, Department of Hematology, Erasmus Medical Center Cancer Institute, Room Na824, PO Box 2040, 3000 CA, Rotterdam, The Netherlands; e-mail: p.sonneveld@erasmusmc.nl.
References
Competing Interests
Conflict-of-interest disclosure: The author has consulted for and received honoraria from Celgene Corporation, Amgen, Janssen, Karyopharm, and Skyline.
Author notes
Off-label drug use: None disclosed.