Abstract
In the last decade, the treatment of higher-risk myelodysplastic syndromes (MDS) has revolved around the azanucleosides, azacitidine and decitabine, which at lower doses are postulated to work predominantly via their effects on inhibition of DNA methyltransferases and consequent DNA hypomethylation. For patients who relapse after, or do not respond to, hypomethylating agent therapy, the outcome is dismal, and new agents and approaches that have the potential to alter the natural history of these diseases are desperately needed. Allogeneic stem cell transplant is the only known potentially curative approach in MDS, but its applicability has been limited by the advanced age of patients and attendant comorbidities. There is now an increasing array of new agents under clinical investigation in MDS that aim to exploit our expanding understanding of molecular pathways that are important in the pathogenesis of MDS. This review focuses on a critical appraisal of novel agents being evaluated in higher-risk MDS that go beyond the conventional hypomethylating agent therapies approved by the US Food and Drug Administration.
Learning Objectives
To understand the current treatment landscape in higher-risk MDS and the limitations of conventional hypomethylating agent therapy
To gain an insight into the novel agents and approaches under clinical investigation in higher-risk MDS
Introduction
The most notable development in the treatment of higher-risk myelodysplastic syndromes (MDS) in the last several years was the approval by the US Food and Drug Administration (FDA) of the hypomethylating agents (HMAs) 5-azacytidine (azacitidine) and 5-aza-2′deoxycytidine (decitabine) in 2004 and 2006, respectively. The use of these agents at lower doses, where their effects on DNA methyltransferase (DNMT) inhibition are postulated to predominate, results in objective responses including complete (CR) and partial (PR) responses in approximately 15% to 20% of patients. An additional 20% to 30% achieve hematologic improvement (HI) in blood counts.1-3 Similar response rates have been demonstrated in clinical trials focused solely on higher-risk MDS (intermediate-2 or high-risk by International Prognostic scoring system [IPSS]). For example, in the landmark study by Fenaux et al, in which azacitidine was compared with conventional care regimens, the CR plus PR rate was 29%. The overall response rate (ORR) defined as CR, PR, and HI was 49%.4 Responses are gradual in onset, with a median onset to response of 2 to 4 months and median time to best response of 5 to 6 months. Responses can occur as late as 12 months, although the majority of responses (>90%) would be expected to occur by 6 months5,6 )
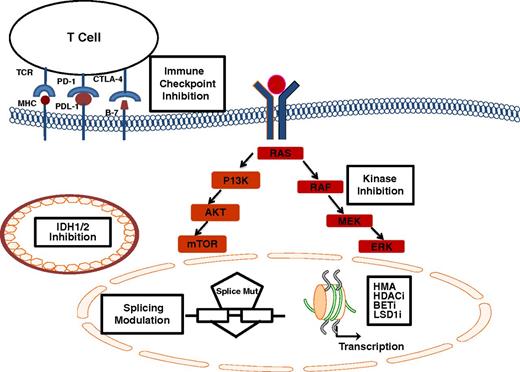
Limitations of treatment with HMAs are therefore obvious; namely, many patients have primary resistant disease, time to onset of response and achievement of best response can take several months, and myelosuppression before onset of response is nearly universal. Furthermore, despite the fact that a survival benefit has been demonstrated with azacitidine in higher-risk MDS,4 these agents are not curative. The median duration of response is in the 10- to 14-month range.3,4,6 Outcome after failure of HMAs is particularly dismal, with a median survival of less than 6 months.7,8 Therefore, the development of new agents and strategies beyond the traditional HMAs approved by the FDA represents a significant area of unmet need at this time. This review will focus on novel agents and combinations under investigation, including novel formulations of HMAs, novel epigenetic modulators, immunotherapeutic approaches, and therapies targeting specific molecular pathways in higher-risk myelodysplastic syndromes (Figure 1).
Novel agents and pathways under investigation in MDS. Epigenetic modulators including HMA, HDACis, BET inhibitors, and LSD1i affect chromatin structure and transcription; immune checkpoint inhibition with a variety of monoclonal antibodies targeting the PD-1/PDL1 interaction, or CTLA-4 and its corresponding ligand facilitate antigen (MHC) recognition by T-cell receptors; IDH1/2 inhibition affects the mutant enzyme within mitochondria, splicing modulation acts preferentially on cells harboring mutations in splicing factors (splice mut); kinase inhibitors downregulate key signaling pathways including the RAS/MAPK and the PI3-K/AKT/m-TOR pathway.
Novel agents and pathways under investigation in MDS. Epigenetic modulators including HMA, HDACis, BET inhibitors, and LSD1i affect chromatin structure and transcription; immune checkpoint inhibition with a variety of monoclonal antibodies targeting the PD-1/PDL1 interaction, or CTLA-4 and its corresponding ligand facilitate antigen (MHC) recognition by T-cell receptors; IDH1/2 inhibition affects the mutant enzyme within mitochondria, splicing modulation acts preferentially on cells harboring mutations in splicing factors (splice mut); kinase inhibitors downregulate key signaling pathways including the RAS/MAPK and the PI3-K/AKT/m-TOR pathway.
Next-generation hypomethylating agents
Because HMAs are S-phase-specific, a more prolonged exposure to the drug may allow greater incorporation into DNA. If used at relatively low doses, this would be hypothesized to lead to more sustained hypomethylation. A longer schedule of parenteral administration of decitabine and of azacitidine have been associated with significant activity in both acute myeloid leukemia (AML) and MDS, including poor prognosis subsets, lending some credence to that hypothesis.6,9-11 These considerations, along with the very short half-lives (less than 30 minutes) of conventional HMAs, coupled with the need for chronic administration to achieve or maintain a response, has spurred the development of the next generation of hypomethylating agents12 (Table 1). These include oral formulations of existing HMAs and/or novel compounds, rationally designed, with a view to increasing or prolonging cellular exposure to HMA therapy and ultimately improving therapeutic outcome.
Next-generation HMAs and inhibitors of other posttranscriptional/posttranslational marks
| Agents . | Mechanism of action . | Phase of development . | Comments . | Reference . |
|---|---|---|---|---|
| ASTX727 | DNMT inhibition | Phase 1/2 | Intermediate 2/high-risk MDS-HMA failure in phase 1, HMA-naive in phase 2 | NCT02103478 |
| Guadecitabine (SGI-110) | DNMT inhibition | Phase 3 | Intermediate 2/high-risk MDS-HMA failure | NCT02907359 |
| Pracinostat azacitidine | HDAC inhibition DNMT inhibition | Phase 2 | MDS-HMA naive; high or very high-risk; stage 1, open label; stage 2, randomized, placebo controlled | NCT03151304 |
| CPI-0610 | BET inhibition | Phase 1 | MDS excludes low or very low-risk disease, MDS/MPN, AML, myelofibrosis | NCT02158858 |
| RO6870810/TEN-010 | BET inhibition | Phase 1 | MDS-HMA failure, AML-R/R | NCT02308761 |
| GSK2879552* | LSD1 inhibition | Phase 2 | MDS-HMA failure; single-agent cohort or combination with azacitidine cohort | NCT02929498 |
| Tranylcypromine | LSD1 inhibition | Phase 1 | +ATRA in MDS-R/R and AML-R/R | NCT02273102 |
| Phase 1/2 | +ATRA and L-DAC in MDS-HMA failure and AML-R/R | NCT02717884 | ||
| Pevonedistat azacitidine† | NAE inhibition | Phase 2 | MDS or CMML HMA naive with high or very high-risk and/or excess blasts | NCT02610777 |
| DNMT inhibition | Randomized, open label |
| Agents . | Mechanism of action . | Phase of development . | Comments . | Reference . |
|---|---|---|---|---|
| ASTX727 | DNMT inhibition | Phase 1/2 | Intermediate 2/high-risk MDS-HMA failure in phase 1, HMA-naive in phase 2 | NCT02103478 |
| Guadecitabine (SGI-110) | DNMT inhibition | Phase 3 | Intermediate 2/high-risk MDS-HMA failure | NCT02907359 |
| Pracinostat azacitidine | HDAC inhibition DNMT inhibition | Phase 2 | MDS-HMA naive; high or very high-risk; stage 1, open label; stage 2, randomized, placebo controlled | NCT03151304 |
| CPI-0610 | BET inhibition | Phase 1 | MDS excludes low or very low-risk disease, MDS/MPN, AML, myelofibrosis | NCT02158858 |
| RO6870810/TEN-010 | BET inhibition | Phase 1 | MDS-HMA failure, AML-R/R | NCT02308761 |
| GSK2879552* | LSD1 inhibition | Phase 2 | MDS-HMA failure; single-agent cohort or combination with azacitidine cohort | NCT02929498 |
| Tranylcypromine | LSD1 inhibition | Phase 1 | +ATRA in MDS-R/R and AML-R/R | NCT02273102 |
| Phase 1/2 | +ATRA and L-DAC in MDS-HMA failure and AML-R/R | NCT02717884 | ||
| Pevonedistat azacitidine† | NAE inhibition | Phase 2 | MDS or CMML HMA naive with high or very high-risk and/or excess blasts | NCT02610777 |
| DNMT inhibition | Randomized, open label |
L-DAC, low-dose cytarabine; R/R, relapsed and/or refractory.
*Not yet recruiting at time of manuscript submission.
†Accrual is complete.
Oral azanucleosides
In the last few years we have witnessed the introduction of oral azanucleosides into clinical trials. These agents may improve patient convenience, eliminate injection site reactions, and facilitate chronic administration, including alternative dosing schedules, designed to lead to a more sustained cellular exposure.
Oral azacitidine (CC-486) was initially studied in an open-label pilot trial. The agent was demonstrated to have 17% bioavailability, when compared with historical experience with parenteral azacitidine, after single-dose administration at 60 or 80 mg.13 A subsequent dose-finding study conducted in patients with MDS, chronic myelomonocytic leukemia (CMML), and AML, evaluating a 7 consecutive day oral administration schedule, established the maximum tolerated dose (MTD) of CC-486 as 480 mg daily for 7 days, with cycles being repeated every 28 days. Diarrhea was the dose-limiting toxicity. The most common adverse events (AEs) included gastrointestinal toxicities, febrile neutropenia, and fatigue. ORR in patients with MDS or CMML, and without prior HMA exposure, was 73% (11 of 15 responded, including 6 CR and 5 HI). ORR in those who had received prior therapy was 35%.14 Extended dosing schedules of CC-486, 300 mg daily for 14 or 21 days, were investigated in lower-risk MDS.15 According to the results of this early-phase trial, CC-486 is now being investigated in a phase 3 trial (NCT01566695) in IPSS lower-risk MDS, with red cell transfusion dependency and thrombocytopenia. A recent analysis that focused on the experience of CC-486 across trials in patients who were previously exposed to HMA therapy showed that of 20 patients who had received 6 or more cycles of prior HMA therapy, 7 (35%) responded.16 Thus, there is an ongoing effort evaluating CC-486 in the HMA failure space, in combination with other novel approaches (Table 2).
Combination therapy with immune checkpoint inhibitors
| Agents . | Mechanism of action . | Phase of development . | Comments . | Reference . |
|---|---|---|---|---|
| Nivolumab ipilimumab azacitidine | Immune checkpoint inhibitors plus DNMT inhibition | Phase 2 | Cohorts include single-agent immune checkpoint inhibitors and combination with azacitidine; MDS-HMA failure and naive | NCT02530463 |
| Nivolumab azacitidine others* | Immune checkpoint inhibitors plus DNMT inhibition | Phase 2/3 | Randomized phase 2, multiple experimental group study, selected exptal group in phase 2 proceeds to phase 3, azacitidine is the control group in both phase 2 and 3 | NCT03092674 |
| Durvalumab CC-486 | Immune checkpoint inhibitors plus DNMT inhibition | Phase 2 | MDS-HMA failure | NCT02281084 |
| Durvalumab azacitidine | Immune checkpoint inhibitors plus DNMT inhibition | Phase 2 | Randomized phase 2, MDS, HMA naive-high or very high risk or intermediate risk with excess blasts or poor risk karyotype; AML ≥ 65 y old | NCT02775903 |
| Pembrolizumab azacitidine* | Immune checkpoint inhibitors plus DNMT inhibition | Phase 2 | MDS-HMA failure and naive cohorts, intermediate 1 or higher risk | NCT03094637 |
| Nivolumab lirilumab azacitidine | Anti-KIR MAB plus immune checkpoint inhibitors + DNMT inhibition | Phase 2 | Lirilumab + nivolumab in lower-risk MDS lirilumab + nivolumab + azacitidine in intermediate-2/high-risk MDS, HMA naive | NCT02599649 |
| Atezolizumab azacitidine | Immune checkpoint inhibitors plus DNMT inhibition | Phase 1 | Single-agent immune checkpoint inhibitors or combination with azacitidine. MDS-HMA failure and HMA-naive cohorts | NCT02508870 |
| Atezolizumab guadecitabine | Immune checkpoint inhibitors plus DNMT inhibition | Phase 1/2 | MDS-HMA failure, intermediate-1 or higher-risk | NCT02935361 |
| Ipilimumab decitabine | Immune checkpoint inhibitors plus DNMT inhibition | Phase 1 | MDS-HMA failure and with excess blasts or MDS-relapsed after allo-HCT, AML R/R, or ≥75 y old; allo-HCT naive and allo-HCT failure cohorts | NCT02890329 |
| Ipilimumab entinostat | Immune checkpoint inhibitors plus HDAC inhibition | Phase 1b | MDS-HMA failure | NCT02936752 |
| Agents . | Mechanism of action . | Phase of development . | Comments . | Reference . |
|---|---|---|---|---|
| Nivolumab ipilimumab azacitidine | Immune checkpoint inhibitors plus DNMT inhibition | Phase 2 | Cohorts include single-agent immune checkpoint inhibitors and combination with azacitidine; MDS-HMA failure and naive | NCT02530463 |
| Nivolumab azacitidine others* | Immune checkpoint inhibitors plus DNMT inhibition | Phase 2/3 | Randomized phase 2, multiple experimental group study, selected exptal group in phase 2 proceeds to phase 3, azacitidine is the control group in both phase 2 and 3 | NCT03092674 |
| Durvalumab CC-486 | Immune checkpoint inhibitors plus DNMT inhibition | Phase 2 | MDS-HMA failure | NCT02281084 |
| Durvalumab azacitidine | Immune checkpoint inhibitors plus DNMT inhibition | Phase 2 | Randomized phase 2, MDS, HMA naive-high or very high risk or intermediate risk with excess blasts or poor risk karyotype; AML ≥ 65 y old | NCT02775903 |
| Pembrolizumab azacitidine* | Immune checkpoint inhibitors plus DNMT inhibition | Phase 2 | MDS-HMA failure and naive cohorts, intermediate 1 or higher risk | NCT03094637 |
| Nivolumab lirilumab azacitidine | Anti-KIR MAB plus immune checkpoint inhibitors + DNMT inhibition | Phase 2 | Lirilumab + nivolumab in lower-risk MDS lirilumab + nivolumab + azacitidine in intermediate-2/high-risk MDS, HMA naive | NCT02599649 |
| Atezolizumab azacitidine | Immune checkpoint inhibitors plus DNMT inhibition | Phase 1 | Single-agent immune checkpoint inhibitors or combination with azacitidine. MDS-HMA failure and HMA-naive cohorts | NCT02508870 |
| Atezolizumab guadecitabine | Immune checkpoint inhibitors plus DNMT inhibition | Phase 1/2 | MDS-HMA failure, intermediate-1 or higher-risk | NCT02935361 |
| Ipilimumab decitabine | Immune checkpoint inhibitors plus DNMT inhibition | Phase 1 | MDS-HMA failure and with excess blasts or MDS-relapsed after allo-HCT, AML R/R, or ≥75 y old; allo-HCT naive and allo-HCT failure cohorts | NCT02890329 |
| Ipilimumab entinostat | Immune checkpoint inhibitors plus HDAC inhibition | Phase 1b | MDS-HMA failure | NCT02936752 |
KIR, killer cell immunoglobulin-like receptor; MAB, monoclonal antibody.
*Not yet recruiting at time of manuscript submission.
A major hurdle in the clinical development of oral azanucleosides is the fact that both azacitidine and decitabine are rapidly cleared by cytidine deaminase present in the gut and the liver, thus limiting their bioavailability. ASTX727, a novel formulation of oral decitabine paired with an oral cytidine deaminase inhibitor-E7727, is being studied in MDS, with a view to improving the bioavailability of the oral decitabine. The results of a first-in-human phase 1 dose escalation trial of ASTX727 demonstrated that the combination of the cytidine deaminase inhibitor E7727 and oral decitabine, administered concurrently, successfully emulated the pharmacokinetic profile of intravenous (IV) decitabine. ASTX727 exhibited similar area under the curve parameters and a similar safety profile to IV decitabine, given at the standard dose and schedule.17 The most common AEs were hematologic, including grade 3 or greater thrombocytopenia, neutropenia, and febrile neutropenia. No significant gastrointestinal-related AEs were reported. Preliminary report of efficacy revealed a number of responses, including 5 CR and 5 HI (n = 43). Four additional patients experienced a marrow CR. These results occurred in a patient population in which almost half had received prior HMA.
A phase 2 fixed-dose confirmation stage of the study has just been completed, in which patients with intermediate- or high-risk MDS were randomly assigned in a crossover design to receive the dose of ASTX727 (35 mg decitabine plus 100 mg E7227) selected from the dose-escalation phase of the study, versus IV decitabine at the standard dose and schedule. The phase 2 results confirm that the area under the curve of oral ASTX727 at this dose and schedule, as well as its effect on demethylation of repetitive (LINE-1) sequences, is similar to that observed with IV decitabine. Future plans for the development of this agent will likely involve further evaluation as an alternative to IV decitabine. Ideally, the clinical development of this agent and other oral azanucleosides should also include evaluation of alternative doses and schedules targeted to induce more sustained hypomethylation and lower myelosuppression when compared with parenteral azanucleoside therapy.
Rationally designed HMA formulations
Another strategy that has been employed to try to circumvent the rapid degradation of azanucleosides by cytidine deaminase is to develop a novel formulation that is chemically modified to be relatively resistant to deamination. Guadecitabine (SGI-110) is a novel dinucleotide of decitabine and deoxyguanosine, linked by a phosphodiester bond. Gradual cleavage of the phosphodiester bond is purported to lead to a slower release of the active decitabine moiety, thus prolonging cellular exposure to the drug. In a phase 1 study in patients with MDS and relapsed/refractory AML, myelosuppression was the dose-limiting toxicity. The MTD in MDS was established to be 90 mg/m2 administered daily (90 mg/m2/d) for 5 consecutive days. The biologically effective dose was significantly lower, and was determined to be 60 mg/m2/d for 5 consecutive days, based on the achievement of maximum DNA hypomethylation at this dose level.18 This dose/schedule (60 mg/m2/d) is now under further investigation in a variety of trials in AML, MDS, and CMML. In MDS or CMML, ongoing studies (Tables 1 and 2) have focused on the HMA failure space, evaluating single-agent guadecitabine in the phase 3 setting, or in combination with other novel agents in early-phase trials. These trials in the HMA failure setting are based on preliminary results of encouraging activity seen with guadecitabine in a phase 2 trial, including patients with MDS who have had prior exposure toHMAs.19 Other groups evaluating the agent in patients with disease that has relapsed after, or is refractory to, azacitidine therapy have reported more modest results.20
Preliminary evidence of promising activity in a phase 2 trial conducted exclusively in higher-risk previously untreated MDS or CMML was also recently reported.21 Almost half the subjects enrolled (45%) had a complex karyotype, with 38% having TP53 mutations. Preliminary results indicate promising activity in this setting, with an ORR of 61% (n = 36), including 28% with CR. Myelosuppression, requiring dose reduction, occurred in a third of patients. The most common nonhematologic AEs were grade1/2 nausea, fatigue, and dyspnea. These results suggest guadecitabine is worthy of further investigation in larger randomized trials in the frontline setting in MDS.
Histone deacetylase inhibition
Histone acetylation is a dynamic process, catalyzed by histone acetyltransferases, and is associated with an open chromatin structure and recruitment to chromatin of factors involved in transcriptional regulation, DNA repair, and DNA replication. Histone deacetylases (HDACs) remove acetyl groups from the lysine tails of histones and lead to transcriptional repression and a closed chromatin configuration. HDAC inhibitors (HDACis) were investigated on the premise that transcriptional de-repression associated with their use would result in upregulation of a variety of genes aberrantly silenced in cancer cells, including tumor suppressor genes.22 These agents have, however, been shown to affect both histone and nonhistone proteins, and have been associated with pleiotropic effects on various genes involved in cell cycle regulation, apoptosis, and angiogenesis. They have limited single-agent activity in myeloid malignancies.
HDACis have been investigated extensively in combination trials with DNMT inhibitors (DNMTis), based on the hypothesis that therapeutic targeting of 2 pathways of epigenetic silencing in myeloid neoplasia would be synergistic. This hypothesized synergy between DNMTis and HDACis has been repeatedly demonstrated in vitro,23 but has been challenging to duplicate in vivo. Early-phase trials combining HDACis and DNMTis confirmed the feasibility of these combinations and yielded encouraging24-26 results. This promise has, however, not yet been realized in the context of a series of randomized phase 2 trials in higher-risk MDS evaluating HDACi/DNMTi combinations6,27-29 vs single-agent DNMTi. A higher incidence of adverse events and/or early treatment discontinuation in the combination groups has been cited as an explanation for some of the recent disappointing results obtained in the context of these randomized trials.28,29 Pharmacodynamic antagonism has been cited as another potential explanation for failure of HDACi/DNMTi combinations to show benefit in the randomized setting. For example, the addition of entinostat did not translate into clinical benefit, and less demethylation was observed in the combination group in the E1905 Intergroup randomized phase 2 trial, evaluating the HDACi entinostat combined with azacitidine vs single-agent azacitidine.6 The possibility that this issue is schedule-dependent has been raised, with an overlapping schedule of administration of the HDACi and DNMTi leading to less incorporation of the azanucleoside into DNA, and consequently less hypomethylation. There is an ongoing randomized early-phase trial in AML (NCT01305499) designed to test this hypothesis by evaluating an overlapping vs a sequential schedule of administration of the azacitidine/entinostat combination. At this time, ongoing trials in MDS such as the azacitidine/pracinostat combination trial (Table 1) are focused on exploring alternative doses and/or schedules of HDACi/DNMTi combinations, with a view to improving tolerability and outcome. In addition, beyond HDACi/DNMTi trials, other combinations involving HDACi and other novel agents such as immune checkpoint inhibitors are now underway in MDS in the HMA failure space (Table 2). At the moment, the future of HDACi-containing regimens in MDS is uncertain, given the multiple negative randomized trials that have been conducted thus far. Ultimately, further development of this class of drugs in MDS is predicated on being able to develop more tolerable combinations that are amenable to chronic administration and on the ability to demonstrate a relative clinical advantage of the HDACi to these combination regimens.
Lenalidomide-based combinations
Lenalidomide is FDA-approved for lower-risk MDS with deletion 5q [del(5q)] MDS who are transfusion dependent. The agent has more modest activity in non-del (5q) lower-risk MDS (reviewed extensively in an accompanying article by Giagounidis30 ). In higher-risk MDS, lenalidomide was combined with azacitidine in a phase 1/2 trial in 36 patients, with an ORR of 72%, including CR in 44% and HI in 28%.31 These promising results led to investigation of this combination in a larger group of patients with higher-risk MDS or CMML in a recent North American Intergroup trial, S1117. This was a randomized phase 2 trial in which 277 patients were randomly assigned in a 1:1:1 fashion to azacitidine combined with lenalidomide or with the HDACi vorinostat vs azacitidine monotherapy. The primary endpoint was response rate. ORR was similar, at 38% for azacitidine monotherapy, 49% for azacitidine plus lenalidomide, and 27% for azacitidine plus vorinostat. Response duration and overall survival (OS) were also similar across treatment groups. There was a higher incidence of dose modifications and reductions in the combination groups compared with azacitidine monotherapy, implying poorer tolerability of the combination regimens.28
In patients with CMML, the ORR was higher in the azacitidine plus lenalidomide group, with 68% (13 of 19 patients) responding vs 28% (5 of 18 patients) in the azacitidine monotherapy group.28 These results suggest the azacitidine plus lenalidomide combination may be beneficial in patients with CMML. The number of patients with CMML enrolled in the S1117 is too small, however, to be able to draw definitive conclusions. These results require validation in larger trials, focused on the CMML patient population.
Novel inhibitors of other posttranslational or posttranscriptional modifications
Epigenomic dysregulation is a critical aspect of MDS pathogenesis.32 Beyond targeting DNA methylation and HDAC recruitment in MDS, however, there has been an increasing focus, in recent times, on the clinical investigation of other inhibitors of posttranslational or posttranscriptional modifications that have the potential to affect the expression of key genes and pathways that are important in malignant myeloid transformation.
NEDD8-activating enzyme inhibition
Pevonedistat is a NEDD8-activating enzyme (NAE) small molecule inhibitor. NAE regulates neddylation, which is a process by which Cullin-RING E3 ubiquitin ligases (CRLs) are activated and involves conjugation of the ubiquitin-like protein NEDD8 to the Cullin protein scaffold. Activation of CRLs is, in turn, critical for proteasome-mediated protein degradation and proteasomal destruction of CRL substrates. Pevonedistat forms a covalent adduct with NAE, which leads to impaired CRL activation and accumulation of downstream CRL-dependent substrates. Several of these substrates are relevant to pathogenesis of myeloid malignancies, including cell cycle regulation, DNA damage, and signal transduction pathways. Preclinical work in AML demonstrated activity in cell lines, primary patient material, and murine xenograft models of AML.33
In a phase 1 study of pevonedistat in relapsed refractory AML or MDS, modest single-agent clinical activity was observed: 17% ORR for schedule A (days 1, 3, and 5; n = 27) and 10% for schedule B (days 1, 4, 8, and 11; n = 19). A subsequent dose escalation trial was conducted investigating the combination of pevonedistat with azacitidine in treatment-naive AML. The MTD of the combination was pevonedistat 20 mg/m2 administered on days 1, 3, and 5 plus azacitidine 75 mg/m2 administered on days 1 to 5, 8, and 9 on 28-day cycles. Grade 3 hyperbilirubinemia and grade 4 aspartate aminotransferase (AST) were dose limiting. In the dose expansion phase of the study, of 55 patients enrolled, ORR was 60%, including 18 CR. Myelosuppression was common, with a febrile neutropenia rate of 25%. The median OS was 7 months, with survival tending to be longer in patients with lower blast burden below 30%.34 A randomized trial is required to assess the relative contribution of pevonedistat to the combination. This is ongoing in high-risk MDS and low blast count AML (Table 1). Accrual to this trial was recently complete, and the results are eagerly awaited and are likely to determine the future development of this agent in MDS.
Bromodomain inhibition
Bromodomain and extraterminal (BET) proteins are epigenetic readers that recognize acetylated lysine tails of histones, and thus areas of open chromatin structure or transcriptionally active sites. BET proteins possess conserved bromodomain modules that bind acetylated lysine tails and also interact with a number of other proteins and function as scaffolds for molecules involved in gene transcription. BET proteins have been implicated in various cancers including myeloid malignancies. Inhibition of BET proteins leads to a significant reduction of a number of genes in a cell- and context-specific-dependent manner.35 The first selective BET inhibitor, JQ1, was demonstrated to be active in vitro and in vivo in preclinical models of NUT midline carcinoma, a rare aggressive intrathoracic squamous cell carcinoma characterized by a rearrangement of the BET proteins BRD4 or BRD3, thus establishing proof of concept for the therapeutic targeting of BET proteins.36 In preclinical studies in AML, the use of JQ1 in AML cell lines and primary patient samples was associated with downregulation of MYC and MYC-driven gene signatures specific to the leukemia stem cell population.37,38 There are a number of clinical trials ongoing with BET inhibitors in various malignancies including MDS (Table 1). These trials are based largely on the potential promise of this class of drugs based on the experience in preclinical models. It is too early at this juncture, however, to make definitive statements about clinical activity (and tolerability) of BETi in myeloid malignancies, including MDS.
LSD1 inhibition
Overexpression of the mono and dimethyl lysine demethylase, LSD1 (also known as KDM1A) has been implicated in a variety of tumors including myeloid malignancies. LSD1 is important in maintaining embryonic stem cell pluripotency and regulates hematopoietic differentiation by keeping key differentiation genes and programs silenced. Inhibition of LSD1 or knockdown of the gene enhances differentiation. LSD1 inhibition sensitized non-APL AML to all trans-retinoic acid (ATRA), and this was associated with an increase in histone 3 lysine 4 dimethylation (H3K4me2), a marker of active transcription, and expression of myeloid differentiation genes. Furthermore, treatment with ATRA and a pharmacologic inhibitor of LSD1, tranylcypromine, resulted in a significant decrease in engraftment of primary AML cells in nonobese diabetic-severe combined immunodeficient mice, suggesting this combination may target leukemia-initiating cells.39 Other novel LSD1 inhibitors have demonstrated activity in preclinical studies in AML and MDS40 Clinical trials are in progress evaluating LSD1 inhibitors in combination with prodifferentiating agents such as ATRA or HMAs in previously treated patients with AML and MDS (Table 1). The results of these early-phase trials will determine the likelihood for future development of these combinations in larger groups of patients with higher-risk MDS.
Immune checkpoint inhibition
Allogeneic stem cell transplant validates immunotherapy as a viable therapeutic strategy in MDS, but its applicability has been limited by the older age of patients at presentation and attendant comorbidities. The success of immune checkpoint inhibitors in solid tumors and Hodgkin lymphoma has led to the rapid introduction of these agents into clinical trials in other settings. These agents are based on the premise that a wide variety of tumors upregulate molecules such as PD-1/PDL-1 and CTLA4, which serve under normal circumstances as “checkpoints” to recognize self and prevent autoimmunity.41 Cancer cells hijack these checkpoints as a means to evade the immune system. Preclinical studies in myeloid malignancies have demonstrated that blockade of the PD-1/PDL1 pathway overcomes immune evasion and prolongs survival in a murine model of AML.42 Single-agent ipilimumab therapy has been investigated in a phase 1 trial in patients who relapsed after an allogeneic stem cell transplant and was associated with a CR in all 4 patients with extramedullary AML and in 1 patient with MDS that had evolved to AML.43 These observations serve as proof of concept for immune checkpoint inhibition in AML/MDS in the postallogeneic stem cell transplant space.
Upregulation of PD-1 and PDL-1 expression has been demonstrated in primary MDS and AML cells obtained from patients undergoing hypomethylating agent therapy, and has been linked to resistance to these agents.44 Immune checkpoint inhibitor plus HMA combinations in MDS are based in part on the premise that HMAs may act as an immune sensitizer45 and augment the activity of immune checkpoint blockade in MDS by facilitating recognition of malignant cells by cytotoxic CD8+ T cells. Immune checkpoint blockade may also help overcome a potential mechanism of resistance to azanucleoside therapy. The preliminary experience thus far suggests limited activity when immune checkpoint inhibitors are used as single agents after HMA failure.46 There are several combination trials of immune checkpoint inhibitors plus HMAs or HDACis that are now ongoing in MDS, in both the HMA-naive and failure settings (Table 2). These trials are heterogeneous in design and patient population enrolled, and are largely predicated on the success of immune checkpoint inhibitors in solid tumors and Hodgkin lymphoma. Randomized trials will be required to decipher the relative contribution of immune checkpoint inhibition to these combinations.
Therapies targeting specific genotypic subsets
IDH1/2 inhibition
Mutations in isocitrate dehydrogenase enzymes (IDH1 and IDH2), are present in approximately 15% to 20% of patients with AML. In MDS, these mutations are less common, being present in approximately 6% of cases, with the incidence rising with leukemic transformation.47,48 Under physiologic conditions, IDH enzymes catalyze the conversion of isocitrate to α-ketoglutarate. IDH1/2 mutations confer a neomorphic enzymatic activity, resulting in isocitrate being converted to the oncometabolite R-2-hydroxyglutarate (2-HG). Elevated levels of 2-HG result in competitive inhibition of α-ketoglutarate-dependent enzymes including TET2 and Jumonji-C enzymatic activity, leading to DNA and histone hypermethylation, changes in chromatin configuration, and differentiation block.49 Small molecule inhibitors of mutant IDH1 or IDH2 bind to the catalytically active site, preventing conversion of α-ketoglutarate to 2-HG and resulting in progressive reversal of histone and DNA hypermethylation, cellular differentiation, and lowering of 2-HG to more physiologic levels.49 In a phase 1/2 study evaluating the IDH2 inhibitor (enasidenib; AG-221) in subjects with mutant IDH2 and advanced myeloid malignancies (Table 3), 16 patients with MDS were enrolled. Among the 15 patients with MDS evaluable for a response, several responses were observed, including 1 CR, 1 PR, and 4 HI. Two additional patients had a marrow CR. Half the patients enrolled had higher-risk disease, and approximately two-thirds had prior HMA exposure. Responses were seen in HMA-naive patients, as well as in patients with prior HMA exposure. Treatment was well-tolerated, with the most frequent adverse effects being unconjugated hyperbilirubinemia, pneumonia, and thrombocytopenia.50
Other novel targeted agents and kinase inhibitors
| Agents . | Mechanism of action . | Phase of development . | Comments . | Reference . |
|---|---|---|---|---|
| Enasidenib (AG-221)* | IDH2 inhibition | Phase 1/2 | IDH2 mutant advanced and/or high-risk AML, MDS-RAEB1/2, or high-risk or R/R | NCT01915498 |
| Ivosidenib (AG-120)* | IDH1 inhibition | Phase 1/2 | IDH1 R132 mutant advanced heme malignancy | NCT02074839 |
| H3B8800 | Splicing modulator | Phase 1 | MDS-HMA failure/intolerant, intermediate-2 or high-risk; AML-R/R/U; CMML previously treated | NCT02841540 |
| Venetoclax azacitidine | BCL2 inhibition DNMT inhibition | Phase 1 | MDS- HMA failure, intermediate-2 or high-risk, single-agent venetoclax and azacitidine+venetoclax combination cohorts | NCT02966782 |
| Venetoclax azacitidine | BCL2 inhibition DNMT inhibition | Phase 2 | MDS- HMA naive, intermediate-2/high-risk and > 5% blasts, randomized | NCT02942290 |
| Rigosertib | Multitargeted kinase inhibition | Phase 3 | HMA failure MDS-EB, includes RAEB-t; phase 3 vs physician’s choice (includes best supportive care; azacitidine or DEC use also permitted) | NCT02562443 |
| Ibrutinib azacitidine | BTK inhibition DNMT inhibition | Phase 1b | Intermediate or higher-risk MDS, HMA failure (dose escalation stage only), HMA naive included in both stages of study | NCT02553941 |
| Selumetinib azacitidine† | MEK inhibition DNMT inhibition | Phase 1 | Advanced myeloid malignancies including MDS-relapsed/refractory |
| Agents . | Mechanism of action . | Phase of development . | Comments . | Reference . |
|---|---|---|---|---|
| Enasidenib (AG-221)* | IDH2 inhibition | Phase 1/2 | IDH2 mutant advanced and/or high-risk AML, MDS-RAEB1/2, or high-risk or R/R | NCT01915498 |
| Ivosidenib (AG-120)* | IDH1 inhibition | Phase 1/2 | IDH1 R132 mutant advanced heme malignancy | NCT02074839 |
| H3B8800 | Splicing modulator | Phase 1 | MDS-HMA failure/intolerant, intermediate-2 or high-risk; AML-R/R/U; CMML previously treated | NCT02841540 |
| Venetoclax azacitidine | BCL2 inhibition DNMT inhibition | Phase 1 | MDS- HMA failure, intermediate-2 or high-risk, single-agent venetoclax and azacitidine+venetoclax combination cohorts | NCT02966782 |
| Venetoclax azacitidine | BCL2 inhibition DNMT inhibition | Phase 2 | MDS- HMA naive, intermediate-2/high-risk and > 5% blasts, randomized | NCT02942290 |
| Rigosertib | Multitargeted kinase inhibition | Phase 3 | HMA failure MDS-EB, includes RAEB-t; phase 3 vs physician’s choice (includes best supportive care; azacitidine or DEC use also permitted) | NCT02562443 |
| Ibrutinib azacitidine | BTK inhibition DNMT inhibition | Phase 1b | Intermediate or higher-risk MDS, HMA failure (dose escalation stage only), HMA naive included in both stages of study | NCT02553941 |
| Selumetinib azacitidine† | MEK inhibition DNMT inhibition | Phase 1 | Advanced myeloid malignancies including MDS-relapsed/refractory |
BTK, Bruton tyrosine kinase; DEC, decitabine; MEK, mitogen-activated protein/extracellular signal-regulated kinase; RAEB, refractory anemia with excess blasts.
*No longer recruiting.
†Not yet recruiting, clinical trials.org listing pending at the time of manuscript submission,
Results of the relapsed refractory AML cohort of 176 patients enrolled on this trial were recently published. ORR was 40% in this poor prognosis group with single-agent enasidenib.51 2-HG levels were uniformly significantly suppressed, independent of clinical responses observed. Evidence of hematopoietic differentiation was observed with persistence of mutant IDH2 in maturing myeloid elements.51,52 On the basis of its significant clinical activity in this setting, enasidenib has now been approved by the FDA for relapsed or refractory AML harboring an IDH2 mutation. Preliminary results documenting encouraging clinical activity with the use of the IDH1 inhibitor (AG-120; ivosidenib) in an early-phase trial (Table 3) have also been reported.53
These results suggest the promise of IDH inhibitors in IDH mutant myeloid neoplasms including MDS. Ongoing trials in AML include a phase 3 trial in advanced AML in the elderly, and early-phase trials in the treatment-naive setting in AML, in which enasidenib or ivosidenib are combined with standard chemotherapy or hypomethylating agent therapy. Additional trials with these targeted agents, specific to the MDS population, are yet to be launched. Given the significant single-agent activity of this class of drugs in AML and the preliminary reports of efficacy in MDS, these agents are worthy of further investigation in both the HMA failure and frontline settings in IDH1/2 mutant MDS.
Splicing modulation
Spliceosome mutations are the most common mutations in MDS, occurring in more than 60% of patients.47 The mutations in MDS occur most commonly in SF3B1, SRSF2, and U2AF1. These mutations are always heterozygous and rarely co-occur with other spliceosome mutations. Heterozygous mutant mice have an MDS phenotype.54 Hemizygosity in mutant mice leads to hematopoietic failure and strong repression of key hematopoietic genes, suggesting dependency of the wild-type allele for hematopoiesis in mutant mice. This vulnerability is being exploited for therapeutic benefit by developing modulators of the splicing complex. These have demonstrated preferential inhibition of cell growth in spliceosome mutant cells when compared with their wild-type counterpart. In preclinical studies, a survival benefit has been observed in murine models of spliceosome mutant myeloid leukemia and in patient-derived xenograft models.55,56 There is now an ongoing phase 1 trial in myeloid malignancies including AML, MDS, and CMML, with the splicing modulator H3B-8800, an orally bioavailable modulator of the SF3B complex.55 Results from this early-phase trial are eagerly awaited. Given the widespread importance of splicing in normal physiology, evaluating the tolerability of splicing modulators is of significant importance and is an issue that is being monitored closely in the context of this early-phase trial.
Other novel targeted approaches
BCL2 inhibition
Overexpression of the antiapoptotic protein BCL2 has been associated with resistance to chemotherapy and maintenance and survival of leukemic stem cells. Venetoclax (ABT-199; GDC-0199) is a selective, orally bioavailable BH3 mimetic and potent BCL2 inhibitor with demonstrable activity in AML cell lines, primary patient samples, and murine xenograft models.57 In a phase 2 single-agent trial conducted in patients with high-risk relapsed/refractory AML or untreated and deemed unfit for intensive chemotherapy, ORR was 19%. BH3 profiling demonstrated on-target BCL2 inhibition.58 Median time to disease progression was short, 2.5 months, indicating that combination approaches will be necessary to have a meaningful effect with this agent.
Preclinical evidence of synergy of BCL2 inhibitors with HMAs has been demonstrated.59,60 Preliminary results from a phase1b early-phase trial combining venetoclax with azacitidine or decitabine in treatment-naive older adults, was associated with promising clinical activity with an ORR (CR/CRi) of 72% (16 of 22) patients. The combination was relatively well tolerated, with the most frequent serious AE being febrile neutropenia, occurring in 33% of patients. Myelosuppression was common and necessitated study drug interruption in 12 patients (55%).61 The combination of azacitidine and venetoclax is now being evaluated in higher-risk MDS in both the frontline HMA naive setting as well as HMA failure settings (Table 3). Overlapping myelosuppression of both these agents is a potential issue that dictates close attention to dose/schedule with regard to the ongoing investigation of this combination, especially in the MDS population.
Kinase inhibition
Approximately 15% of patients with MDS will have mutations in RAS or negative regulators of the pathway, resulting in activated kinase signaling.47 Rigosertib (ON 0910.Na) is a small molecule RAS mimetic that binds to the RAS binding domains of multiple RAS effectors and inhibits the RAS–RAF interaction, thus inhibiting downstream signaling intermediates. Rigosertib also inhibits related signaling pathways including the PI3K pathway and polo-like kinases.62 Encouraging results from early-phase trials, which were developed largely on the basis of the potential of rigosertib to target cell cycle regulatory molecules, including cyclin D1 in MDS,63,64 led to a more extensive evaluation of this agent. In a phase 3 trial of rigosertib vs best supportive care in the HMA failure setting, in patients with MDS and excess of blasts (defined as 5%-30% blasts), there was no improvement in the primary endpoint of OS. In a preplanned exploratory analysis, there was a trend toward an improvement in OS (median OS, 8.6 months vs 5.3 months; HR, 0.72; P = .06) in patients who were primary refractory to HMA in the rigosertib group vs those in the best supportive care group.65 There is currently an ongoing phase 3 trial of rigosertib vs physician’s choice in patients with MDS with excess blasts in the HMA failure setting (Table 3). The future of this agent in higher-risk MDS will likely be determined by the results of that trial. There are also other early-phase trials with other kinase inhibitors ongoing, or about to be launched, that include the MDS population (Table 3).
Gemtuzumab ozogamicin
Gemtuzumab ozogamicin (GO) is a recombinant humanized anti-CD33 monoclonal antibody conjugated to calicheamicin. HMA-GO-based combinations have been investigated in myeloid malignancies on the basis of the premise that HMAs facilitate maturation of AML blasts and increase CD33 expression, thus enhancing uptake of GO by these cells. HMAs also downregulate p-glycoprotein, potentially sensitizing cells to GO.66-68 A pilot trial combining azacitidine with GO in older adults with previously untreated AML included 3 patients with high-risk MDS and demonstrated promising results.67 A subsequent larger phase 2 effort within the SWOG intergroup evaluated the same combination in older adults older than 60 years with previously untreated AML.68 Patients were stratified into a good risk cohort (n = 83), defined as age 60 to 69 years or PS 0 to 1, or a poor risk cohort (n = 59), defined as age 70 years or older or performance status 2 or 3. The CR/CRi rate in the good-risk cohort was 44%, with a 30-day mortality of 7%. In the poor-risk cohort, the CR/CRi rate was 35%, with a 30-day mortality of 13%, and these results met prespecified criteria for success. Overall, there are limited data with regard to HMA-GO combinations specific to MDS, but the recent FDA approval of the agent in CD33+ AML is likely to lead to a resurgence in interest in evaluating this combination further in MDS.
Conclusion
The approval of the azanucleosides, azacitidine and decitabine, for the treatment of MDS several years ago undoubtedly represents a major advance for this group of disorders. Many gaps remain, however, and effective strategies are needed to overcome both primary and secondary resistance to HMA therapy. The plethora of new drugs available for investigation pose both unique opportunities and challenges with regard to how best to fill the existing gaps in our therapeutic armamentarium.
Myelosuppression is the major toxicity associated with HMA therapy and contributes to early discontinuation of therapy, especially in the context of the development of combination therapies with potentially overlapping toxicity. Approaches that focus on development of alternative doses and schedules of administration, focused on enhancing tolerability, deserve further investigation. Oral azanucleosides are particularly appealing in that regard. The ease and convenience of oral HMA therapy may lend itself to the development of alternative schedules of administration. For example, lower doses administered more frequently may minimize myelosuppression without sacrificing drug exposure or the ability to effect sustained hypomethylation. Focusing on the development of such approaches in the context of early-phase trials may increase the potential of these novel azanucleoside formulations, both as single agents and in combination with other novel agents, to overcome primary and/or secondary resistance to HMA therapy.
Many novel HMA-based combinations are being developed, both in the HMA-naive and HMA failure settings. Tables 1-3 highlight some of the selected combinations, with many more in development, as outlined on clinical trials.gov. A major challenge is figuring out optimal dose and scheduling of these combinations to enhance tolerability and maximize efficacy in a disease in which patients are used to chronic outpatient therapy. Beyond the development of optimal dose/schedules, early randomized trials are necessary to try to figure out the relative contribution of the novel agent from both a toxicity and efficacy standpoint. The HDACi/DNMTi combination trials have been instructive in this regard, where relatively large randomized phase 2 trials have been negative and there are now ongoing early-phase randomized trials focused on optimizing the doses and schedules of administration.
Finally, the clinical, genetic, epigenetic, and molecular heterogeneity47,69 of this group of diseases dictates careful analysis of emerging clinical trial results to see whether specific subsets would derive particular benefit. This approach is paying off in AML, where 4 agents have been approved by the FDA recently for distinct subsets of that disease. This underscores the fact that a “one size fits all” approach may no longer be appropriate in MDS. Targeted therapeutic approaches deserve a laser sharp focus, even in rare subsets such as the IDH1/2 mutant subsets, especially when there is already evidence of clinical activity in other myeloid neoplasms.
In this era, with the abundance of new agents and combinations, how do I approach the treatment of higher-risk MDS (Figure 2)? In the HMA-naive setting, I strongly favor HMA-based therapy, preferably on a clinical trial. I determine early on fitness and eligibility for transplant and advocate early referral for allogeneic stem cell transplant, especially if this is also aligned with the patient’s own personal goals. In the case of prior azanucleoside exposure and HMA failure (defined as progression, failure to respond, or relapse after 4-6 cycles of azacitidine or 4 cycles of decitabine therapy), given the dismal prognosis of this subset of patients, I strongly recommend enrollment on a clinical trial evaluating a novel agent or agents, including first-in-human early-phase trials. I do also employ mutational (molecular) profiling via next-generation sequencing–based methodology to help determine whether there is a targeted therapeutic approach worth pursuing, and available, within the context of a clinical trial. Ultimately, it is the hope that the rapid investigation of new agents or combinations in a randomized fashion early on, in the frontline setting, or the evaluation of agent or agents targeting rare genotypic subsets looking for a big effect signal, will lead us closer to the acquisition of more agents in the MDS therapeutic armamentarium that have the potential to significantly change the natural history of these diseases.
How I treat higher-risk MDS: I employ risk stratification by IPSS and IPSS-R. Higher-risk patients include intermediate-2 and high-risk by IPSS or high-/very high risk by IPSS-R. I favor azanucleoside-based therapy, preferably on a clinical trial, and strongly advocate early referral for allogeneic hematopoietic stem cell transplant (allo-HCT) in transplant-eligible individuals. In the case of prior azanucleoside exposure and HMA failure (defined as progression, failure to respond, or relapse after 4-6 cycles of azacitidine or 4 cycles of decitabine), I strongly recommend clinical trial enrollment.
How I treat higher-risk MDS: I employ risk stratification by IPSS and IPSS-R. Higher-risk patients include intermediate-2 and high-risk by IPSS or high-/very high risk by IPSS-R. I favor azanucleoside-based therapy, preferably on a clinical trial, and strongly advocate early referral for allogeneic hematopoietic stem cell transplant (allo-HCT) in transplant-eligible individuals. In the case of prior azanucleoside exposure and HMA failure (defined as progression, failure to respond, or relapse after 4-6 cycles of azacitidine or 4 cycles of decitabine), I strongly recommend clinical trial enrollment.
Correspondence
Olatoyosi Odenike, Department of Medicine, Section of Hematology/Oncology, The University of Chicago, 5841 S. Maryland Ave, MC 2115, Chicago, IL 60637; e-mail: todenike@medicine.bsd.uchicago.edu.
References
Competing Interests
Conflict-of-interest disclosure: The author is on the board of directors or an advisory committee for Baxalta, CTI BioPharma, Celgene, Incyte, Pfizer, Jazz Pharmaceuticals, and ABIM, and has received honoraria from AbbVie and Dava Oncology.
Author notes
Off-label drug use: I will be discussing investigational drugs in the treatment of MDS including novel formulations of azanuclesoides, novel epigenomic modulators, combinations involving immune checkpoint inhibitors, agents targeting specific genotypic subsets, kinase inhibitors, and BCL2 inhibitors.