Abstract
Lower risk myelodysplastic syndromes (MDS), defined as MDS with a Revised International Prognostic Scoring System score ≤3.5 points, will remain a challenging entity in 2018. Supportive care continues to be the linchpin of treatment, although the options to reduce transfusion needs are broadening. To achieve red blood cell transfusion independence in non-del(5q) patients, erythropoiesis-stimulating agents remain a mainstay of therapy as long as endogenous erythropoietin levels are <500 U/L (and preferably <200 U/L). Experimental strategies for patients ineligible for erythropoiesis-stimulating agents or relapsing after gaining transfusion independence include immunosuppressive agents, transforming growth factor β inhibitors, and lenalidomide. All these alternatives have shown reasonable response rates in selected patient populations with lower risk MDS. Patients with del(5q) disease can derive long-term benefit from lenalidomide, and some patients remain transfusion free for extended periods even after discontinuation of the drug. In rare cases in which thrombocytopenia is the main clinical problem leading to clinically significant bleeding events, thrombopoietin receptor analogues may alleviate bleeding, increase platelet counts, and rarely lead to trilineage responses. It seems prudent to use these drugs only in patients with confirmed bone marrow blast counts <5%. Allogeneic stem cell transplantation is reasonable for patients with high molecular risk of progression and those failing several lines of treatment with signs of progression toward higher-risk MDS.
Learning Objectives
Recognize both approved and nonapproved treatment options in lower risk myelodysplastic syndromes (MDS) and gain knowledge regarding their respective probabilities of success
Develop the ability to identify suitable MDS subpopulations for distinct treatment approaches
Identify possible novel therapies in MDS
Introduction
Establishing a universally accepted algorithm for the treatment of myelodysplastic syndromes (MDS) is impossible, which is fortunate because an algorithm in the strict sense means a set of rules that precisely defines a sequence of operations. However, in our day-to-day interaction with MDS patients, a precise definition of sequential therapies will always fall short of the clinical reality. Therefore, the goal of the present article was to provide “hands-on” advice for the treatment of patients experiencing lower risk MDS.
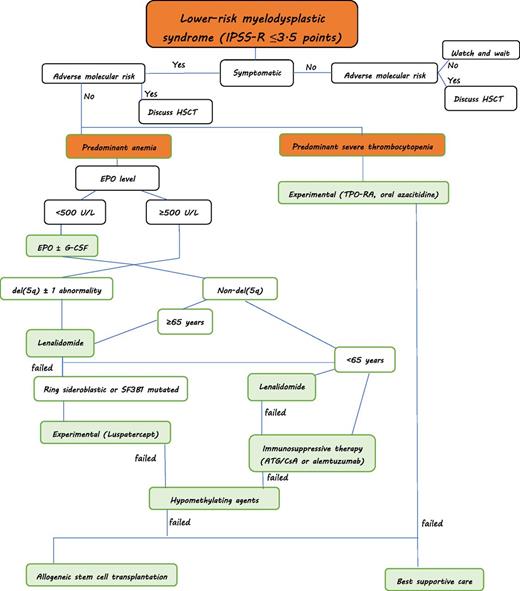
Treatment algorithm for myelodysplastic syndromes (personal approach): Predominant severe neutropenia is rare in lower-risk MDS. Supportive treatment with G-CSF and antibiotic/antifungal therapy is warranted if infections occur. HSCT, hematopoietic stem cell transplantation. ATG, antithymocyte globulin; CsA, ciclosporine A; EPO, erythropoietin; G-CSF, granulocyte-colony stimulating factor.
Treatment algorithm for myelodysplastic syndromes (personal approach): Predominant severe neutropenia is rare in lower-risk MDS. Supportive treatment with G-CSF and antibiotic/antifungal therapy is warranted if infections occur. HSCT, hematopoietic stem cell transplantation. ATG, antithymocyte globulin; CsA, ciclosporine A; EPO, erythropoietin; G-CSF, granulocyte-colony stimulating factor.
Assessing the risk of MDS patients
For the purpose of this publication, the Revised International Prognostic Scoring System (IPSS-R)1 was used because it is universally accepted, based on the biggest patient database ever used in MDS, and has been validated by several independent groups. The lower risk MDS patients are defined as those having a risk of very low, low, or intermediate disease according to the IPSS-R with a score ≤3.5 points.2 Higher-risk patients would be those with intermediate risk and >3.5 points, high, or very high risk according to this scoring system. Undoubtedly, molecular aberrations can have a significant impact on the prognosis of patients with MDS. Mutations in TP53, EZH2, ETV6, RUNX1, and ASXL1 increase the risk, independent of the IPSS-R score.3,4 However, it remains unclear how to incorporate this knowledge into therapeutic decisions, given the low number of specific genotype-directed therapeutic options currently available to treat MDS.
Defining treatment goals in MDS
The goals in treating patients with MDS should be twofold: improving peripheral blood values (ie, increasing hemoglobin levels, reducing infectious episodes or bleeding), and ultimately, to alter the natural course of the disease. Whether this goal requires immediate treatment is a matter of debate. For example, in the Aza-001 trial (azacitidine versus best supportive care in patients with higher-risk MDS), the median time from diagnosis to treatment was ∼1 year.5 In lower risk MDS, treatment of patients with established del(5q) with lenalidomide is approved only after occurrence of transfusion dependency amid concerns that side effects could harm patients who are nontransfusion dependent. Conversely, early initiation of erythropoietin (EPO) treatment may increase response rates and duration of response.6
This goes to show that asymptomatic patients with lower risk MDS may present with various grades of cytopenias and still be managed according to a supportive care strategy if they do not display high-risk chromosomal or molecular abnormalities. These strategies include, for example, blood transfusions, iron chelation therapy, antibiotic and antifungal treatment during infections, and granulocyte colony–stimulating factor (G-CSF) support.
The lower risk patients with MDS can be subdivided according to certain morphologic, cytogenetic, and clinical characteristics, which can be used to direct treatment approaches. As such, patients with del(5q) would constitute one possible entity, patients with ring sideroblasts another. Patients mainly experiencing thrombocytopenia can be grouped into a third category. Hypoplastic MDS can be seen as another entity. Finally, a large cluster of patients will present with predominant anemia. Of course, several patients will present with overlapping features. Patients with complex karyotypic abnormalities will only rarely be part of lower risk patients with MDS when applying the IPSS-R. Figure 1 provides a possible approach to patients with lower-risk MDS.
Patients with predominant anemia
High-dose EPO remains the mainstay of therapy for lower risk MDS patients with anemia. To predict response to treatment with erythropoiesis-stimulating agents (ESAs), a prognostic tool has been developed by the Nordic MDS study group and is widely used.7 As a rule of thumb, patients with a low transfusion burden of <2 U per month and an EPO level <500 U/L are expected to have a response rate of 75%, compared with 25% if 1 of the 2 parameters is not met. If neither of the parameters is met, the response rate is minimal. A refined scoring system including EPO levels, serum ferritin level, and the IPSS-R has been proposed and allows prediction of response ranging between 20% and 85%.8 A recently conducted double-blind, placebo-controlled randomized study with epoietin α9 in patients with a high likelihood of response failed to recapitulate these positive predicted results. Erythroid response rate in the EPOANE3021 (An Efficacy Study for Epoetin Alfa in Anemic Patients With Myelodysplastic Syndromes) study according to the International Working Group (IWG) was 33% at 26 weeks and occurred exclusively in patients with EPO levels <200 U/L. A response review committee was asked to evaluate all the responses and clear them of notorious shortcomings induced by the IWG 2006 assessment.
Problems with assessing erythroid response in clinical studies
For example, in the above-mentioned EPOANE3021 study, a patient who responded with an increase in hemoglobin level to >12 g/dL had to withhold epoietin alfa dosage and was allowed to restart study medication only if the hemoglobin level dropped to <10 g/dL. In some patients, this scenario led to oscillating hemoglobin levels that never reached IWG criteria, as those would require a continuous hemoglobin increase of at least 8 weeks. The response review committee amended the erythroid response rate to 46%. Further data analysis allowed calculation of a predictive scoring system for erythroid responses in MDS (Table 1).
Predictive scoring model for erythroid responses in lower-risk IPSS MDS patients with EPO levels <500 U/L and low transfusion burden from the randomized EPOANE trial
| Predictive score . | Transfusion requirement/8 wk . | EPO level, U/L . |
|---|---|---|
| 67% | 0 | <200 |
| 25% | >0, ≤4 | <200 |
| 0% | >0, ≤4 | >200 |
| Predictive score . | Transfusion requirement/8 wk . | EPO level, U/L . |
|---|---|---|
| 67% | 0 | <200 |
| 25% | >0, ≤4 | <200 |
| 0% | >0, ≤4 | >200 |
In day-to-day practice, the initial dosage of 30 000 to 40 000 U per week of EPO can be increased to 60 000 to 80 000 U per week if there is no acceptable response after 4 weeks. Also, the addition of 300 µg of G-CSF per week may enhance responses and seems especially useful in patients with refractory anemia with ringed sideroblasts,10 although the incremental benefit has been debated.11 If darbepoietin is used, the dosage ranges between 150 and 300 µg per week to 500 µg every 3 weeks. Although there have been no prospective trials, matched-pair analyses suggest that ESA + G-CSF do not increase the transformation rate to acute myeloid leukemia (AML).12
Patients refractory to ESA
Lower risk patients with MDS without del(5q) failing to respond to EPO have a number of treatment options, but few of those are currently approved. In the United States and some other countries, supportive care with iron chelation and hypomethylating agents might be used in EPO-relapsed or refractory disease, whereas in Europe, only supportive care with iron chelation is approved in this situation. Lenalidomide has been evaluated as a treatment for patients ineligible or refractory to ESA in the lower risk MDS non-del(5q) setting. In an international, double-blind, randomized, placebo-controlled trial, lenalidomide was compared with placebo in transfusion-dependent lower risk patients with MDS at a dosage of 10 mg for 21 consecutive days every 4 weeks or 5 mg if creatinine clearance was 40 to 60 mL/min. Red blood cell transfusion independence (RBC-TI) >8 weeks was reported in 26.9% of patients, and 17.5% remained transfusion independent for >6 months.13 Further analysis of the response rate showed that patients with baseline EPO levels <100 U/L who had previously received EPO treatment had the highest response rate of 42.5% RBC-TI >8 weeks.14 Patients with EPO levels of 100 to 200 U/L, 200 to 500 U/L, and >500 U/L had RBC-TI of 33.3%, 23.3%, and 15.5%, respectively. Twelve long-term responders >52 weeks were recently reported. Their duration of response lasted between 400 and 1400 days. Seventy-five percent of these patients had molecular abnormalities in the spliceosome genes. However, a gene signature predicting response could not be found. Severe neutropenia or thrombocytopenia occurred in 61% and 35% of patients and was manageable with G-CSF and dose interruptions. Hence, until further analysis of the data, it remains a challenge to decide who could be treated with lenalidomide if a del(5q) chromosomal abnormality is lacking. The best option will be to treat patients with low EPO levels with spliceosome abnormalities, although this treatment is not approved and there seems to be no activity to file the data for approval.
Immunosuppressive treatment
Immunosuppressive treatment has fallen out of favor lately, although several trials have shown promising results with different immunosuppressive approaches. Treatment similar to that used in aplastic anemia has been shown to be effective in subgroups of patients with MDS. In 1 trial, 45 patients were treated with horse antithymocyte globulin at a dose of 15 mg/kg body weight for 5 days and oral cyclosporine for 180 days, and compared with 43 patients receiving best supportive care.15 Notably, EPO was an accepted treatment in the best supportive care arm of the study. Median age was 62 and 65 years, respectively, and 29% of patients receiving immunosuppression had a hematologic response compared with 9% receiving best supportive care. Responses occurred not only in hypoplastic bone marrow patients with MDS but throughout all cellularities.
A prospective nonrandomized phase 2 trial of intravenous alemtuzumab in patients with intermediate-1 or intermediate-2 IPSS MDS showed high response rates for patients whose age plus number of months of transfusion dependence for red blood cells was <72 if they were HLA-DR15–positive or 58 if they were HLA-DR15–negative.16 Patients received a test dose of 1 mg of alemtuzumab on day 1 and then 10 mg of alemtuzumab for 10 consecutive days. Responses occurred in 77% of patients labeled as intermediate-1 risk.
Iron chelation
Regular red blood cell transfusions will result in significant iron accumulation in lower risk patients with MDS. One packed red blood cell concentrate contains ∼250 mg of iron, which cannot be excreted by the human body. Once transferrin saturation exceeds 80%, nontransferrin-bound iron will appear in the patient’s blood.17 Reactive oxygen species result from free iron atoms reacting with different molecules of the cell membrane and with intracellular structures. They have been incriminated as a cause of hematopoietic insufficiency,18,19 increased DNA mutation rate,18 and reduced antiapoptotic signaling.20 This scenario in turn could trigger apoptosis of hematopoietic progenitors.21 However, in patients with MDS, it has never been convincingly shown that even severe iron overload shortens overall survival. No randomized trial has been reported to date comparing iron chelation therapy with best supportive care in patients with MDS. The evidence supporting the use of iron chelation in MDS is based on anecdotal reports or uncontrolled clinical trials.22 These reports have described improvements in peripheral blood cell counts, independence of blood transfusions, and improvements in quality of life.23 A matched-pair analysis from Düsseldorf comparing patients with MDS receiving iron chelation vs those without iron-depleting therapies found a significant overall survival benefit for the iron chelation population, albeit no improvement in AML transformation rate.24 Given that ferritin is the most widely available marker for measurement of iron storage in the body, many international societies have issued guidelines for the treatment of iron overload in patients with MDS.25,26 In line with those recommendations, I suggest starting deferasirox at 20 mg/kg body weight for moderately transfused patients (≤4 U/4 weeks) and 30 mg/kg body weight for heavily transfused patients(>4 U/4 weeks). Personally, I start at low doses of 5 mg/kg body weight per week and increase it slowly to the desired target level. At the beginning, weekly blood tests to check renal and hepatic function should be conducted. A 25% reduction in the glomerular filtration rate may occur during deferasirox treatment, which is reversible after interrupting treatment.27 Caution should be exercised in combination with other drugs that impair renal function. Other side effects such as diarrhea, rash, nausea, or vomiting are usually tolerated with dose adjustments and supportive care.
Deletion 5q
Patients with del(5q) MDS mainly present with macrocytic anemia, many have increased platelet counts, and some have ring sideroblastic disease coupled with SF3B1 and/or JAK2 mutations. Del(5q) can be an EPO-sensitive disease, although EPO levels in del(5q) patients tend to be higher than in non-del(5q) patients, and responses to ESA are usually shorter than in other MDS subtypes.28 Nonetheless, there is no reason to avoid starting a trial of EPO in a del(5q) patient with symptomatic anemia if pretreatment requirements are met, especially as the trials leading to registration of lenalidomide in del(5q) have been undertaken in patients in whom the majority had been pretreated with EPO. Lenalidomide is approved in the United States for treatment of patients with del(5q) MDS with low- and intermediate-1 risk according to the IPSS. This group may include patients with del(5q) plus 1 other chromosomal abnormality or very rare patients with isolated anemia who have ≥2 additional chromosomal aberrations. In Europe, approval has been granted for patients with IPSS low or intermediate-1 risk and isolated del(5q) only. However, in patients with low- or intermediate-1 risk IPSS, the presence of 1 additional cytogenetic abnormality or a bone marrow blast count of up to 10% does not result in inferior outcome. In fact, a large, nonrandomized trial of patients with isolated del(5q) with centrally confirmed low or intermediate-1 risk IPSS and a medullary blast count <5% treated with lenalidomide at a dosage of 10 mg for 21 consecutive days every 4 weeks was performed in Germany (LeMON5 trial); the results were recently reported.29 The investigators found a complete cytogenetic remission rate of 47% and a transfusion independence rate of 67%, well in line with the results of the Lenalidomide-MDS-003 study that led to the approval in the United States and included patients with additional chromosomal abnormalities and higher blast counts.30
Drug interruptions and drug holidays
Lenalidomide treatment is approved until disease progression occurs. However, some patients will discontinue treatment because of side effects or personal preference. Even after discontinuation, these patients might remain in long-term transfusion independence. In fact, the vast majority of patients who achieved complete hematologic and cytogenetic remission and continued lenalidomide for another 6 to 12 months have shown surprisingly long transfusion independence for >5 years.31,32 I personally have treated several del(5q) patients who relapsed after hematologic remission with a drug holiday of several months and reexposed them to the drug. The majority regained transfusion independence, albeit for a shorter duration. One important reason may be acquired TP53 mutations.
TP53 mutations and their importance in del(5q) MDS
TP53 mutations have been identified as negative prognosticators for patients with MDS with del(5q) disease.33,34 In fact, strong p53 expression in immunohistochemistry in ≥1% of bone marrow progenitor cells at baseline was observed in 35% (30 of 85) of patients treated with lenalidomide in a retrospective analysis of an international trial. It was significantly associated with higher AML risk (P = .0006), shorter overall survival (P = .0175), and a lower cytogenetic response rate (P = .009) but not with achievement or duration of 26-week transfusion independence response.34 In the LeMON5 trial cited earlier, 67 patients were prospectively analyzed for TP53 mutations.35 At study entry, 12% of patients had TP53 mutations with allele frequencies ranging from 16% to 52%; the clinical characteristics between patients with and without TP53 mutation were identical. Neither achievement of transfusion independence nor cytogenetic remission was significantly influenced by TP53 mutation, although it was numerically in favor of wild-type patients. However, overall survival of TP53-mutated patients was significantly shorter than that of wild-type patients. This consistently shown overall survival disadvantage should prompt evaluation of allogeneic transplantation strategies in patients with baseline TP53 mutations. It should not, however, prevent from treating patients with lenalidomide even if transplantation is scheduled, as the drug may still lead to increases in hemoglobin level, consecutive reduction in iron overload, reduction in clone size, and improvement in quality of life, all of which can improve transplantation outcomes. Furthermore, in the LeMon5 trial, 15 randomly selected patients with no detectable TP53 mutation at baseline underwent TP53 sequencing after a median of 12 months. Forty percent had evidence of TP53 mutations with an allele frequency of 6% to 31%. This subsequent emergence of TP53 mutation had no impact on any outcome measures. Therefore, the emergence of TP53 mutation during treatment with lenalidomide does not necessarily entail negative consequences for the patients, and further investigation regarding its impact is warranted.
Treatment of patients relapsing during lenalidomide therapy
The median duration of response to lenalidomide in del(5q) patients is expected to last ∼2 years.36 Once patients failed lenalidomide, median overall survival was 23 months in an international retrospective study of 392 patients.37 Those with loss of hematologic response had a 39-month median overall survival; patients with del(5q) MDS according to the World Health Organization 2016 definition did better (45 months). Del(5q) patients who never responded to lenalidomide and those with progression during lenalidomide treatment fared worst, with median overall survival times of 17 and 11 months, respectively. Patients with complex cytogenetic abnormalities, including del(5q), had a median survival of 15 months after lenalidomide treatment. Any treatment resulted in increased overall survival (49 months) compared with best supportive care (P = .01). Treatment options of different MDS centers of excellence included allogeneic stem cell transplantation, hypomethylating agents, growth factors, conventional chemotherapy, and investigational therapies.
Patients with predominant thrombocytopenia
The prevalence of severe thrombocytopenia in lower risk patients with MDS is <10%, but in some of those patients, thrombocytopenia may be the major source of complications. With the advent of thrombopoietin (TPO) receptor analogues, there has been an increasing interest in using these compounds to reduce bleeding tendency in MDS. The TPO receptor is expressed on megakaryocytes but also on earlier hematopoietic progenitors. Thus, a certain risk of stimulating blasts and increasing the transformation rate to AML is inherent to those drugs. Moreover, given the dysplastic nature of megakaryocytes in MDS, higher doses of thrombopoietin–receptor agonists (TPO-RA) need to be used than in immune thrombocytopenia. Eltrombopag, a small-molecule TPO-RA, had initially been studied in higher-risk patients with MDS owing to the fact that in vitro data did not show substantial stimulation of blasts38 and that the prevalence of thrombocytopenia in this patient population is higher. In lower risk MDS, the results of a randomized trial with eltrombopag were recently published.39 At dosages between 50 and 300 mg orally once daily, 47% responded in the eltrombopag arm versus 3% in the placebo arm. This outcome also translated into a lower number of clinically significant bleeding events (World Health Organization grade ≥2) with eltrombopag 14% compared with placebo (42%), albeit at the expense of more grade 3 to 4 side effects (46% vs 16%). For AML transformation, however, there was no difference between treatment arms (12% for eltrombopag and 16% for placebo; P = .81).
The TPO-RA romiplostim has been studied in a double-blind, randomized, placebo-controlled clinical trial, the final long-term results of which were presented earlier this year.40 Due to an unfortunate data monitoring committee decision, the trial was terminated early before recruitment of all patients had taken place, and robust conclusions cannot be drawn as to romiplostim’s definite role in this situation. When the data monitoring committee met, the data suggested that more AMLs had happened in the romiplostim arm of the study. However, a critical number of AML events that had already occurred in the placebo arm of the study had not yet been reported in the clinical report files, and therefore the statistical analysis was incomplete. In this study, romiplostim was used at initial doses of 750 µg per week and led to a 36% response rate for hematologic improvement of platelets. Moreover, some patients experienced 3-lineage responses, and in a small subset of patients, the platelet count rose to normal levels and has remained there ever since without further dosage of study drug. The overall survival rate and AML transformation rate were identical for romiplostim and placebo. Although AML transformations have occurred, the great majority were in patients with initial blast counts >5% in the bone marrow, both for romiplostim and placebo; 55% of AML events occurred in the group of patients with refractory anemia with excess blasts that comprised only 14% of the study population. Other clinically relevant side effects can be well managed and are of minor concern.41 Thus, it seems reasonably safe and feasible to administer both eltrombopag or romiplostim to patients with MDS with low bone marrow blast counts (ie, <5%) in case of severe thrombocytopenia, although both drugs are not approved in this indication.
TGF-β pathway inhibitors
Endogenous EPO and its action through the EPO receptor are critical for the survival, proliferation, and differentiation of early erythroid progenitors up to the level of proerythroblasts.37 However, later stages of erythropoiesis mature independently of EPO. In mammals, the transforming growth factor β (TGF-β) superfamily includes molecules that are potent myelosuppressive cytokines, some of which (eg, activin A) are implicated in the normal maturation of erythroid precursors. Activin receptor fusion proteins have been developed to reduce the inhibitory action of TGF-β molecules and thus improve late-stage hematopoiesis in vivo. In MDS, 2 drugs have entered clinical trials, and preliminary reports have been reported. Luspatercept (ACE-536) has shown promising activity with minimal toxicity in a phase 1 dose escalation study conducted in Germany. In patients with ring sideroblastic disease, the overall hematologic improvement rate according to IWG 2006 was 46%, with 36% becoming transfusion independent for at least 8 weeks.42 Two main factors seem to influence luspatercept activity: the baseline EPO level and the presence or absence of ring sideroblasts: For EPO levels ≤500 U/L, the hematologic improvement rate in ring sideroblastic disease (RS-positive patients) was 65%, and in ring sideroblast–negative patients (RS-negative), it was 43%. For EPO levels >500 U/L, hematologic improvement in RS-positive patients was 56%, and it was 9% for RS-negative patients. A phase 3, double-blind, randomized, placebo-controlled trial of luspatercept in transfusion-dependent lower risk patients with MDS has recently been fully enrolled (MEDALIST trial). Similar results have been reported with the parent compound, sotatercept, in a similar patient population.43 If the data are corroborated and the preferential response of ring sideroblastic anemias is confirmed, TGF-β–targeted treatment may become an important addition to the MDS treatment armamentarium.
Hypomethylating agents
Although azacitidine is only approved for higher-risk MDS in Europe, it is licensed for all subtypes of MDS in the United States. The efficacy of azacitidine in lower risk MDS has never formally been addressed in a phase 3 clinical trial and, therefore, only limited data are available to assess responses. The Nordic MDS study group published a prospective series of 30 patients who were EPO refractory and received 5 days of azacitidine treatment at the dose of 75 mg/m2 daily.44 Two patients died and 5 patients terminated the study early because of severe neutropenia and/or thrombocytopenia. Twenty percent of patients achieved transfusion independence. For those patients completing all prespecified treatment cycles, the response rate was slightly higher. An Italian study reported higher response rates in red blood cell transfusion-dependent patients who were EPO refractory, with overall response rates of 47% and 33% of transfusion independence.45 Similar responses were seen in an earlier trial of the Cancer and Leukemia Group B study group in the United States that reported 32% of hematologic improvement in lower risk patients treated with azacitidine. However, because side effects of azacitidine can be serious, this drug should only be used by experienced hematologists, and the patients should be informed thoroughly about the risks and benefits of such treatment.
Oral azacitidine is being tested in a phase 3 clinical trial of lower risk patients with MDS with RBC transfusion dependence and thrombocytopenia <75 000/µL (Quazar MDS 003 trial)46 after a phase 1 study reported clinical activity in lower risk patients with MDS.47 At a median number of 7 treatment cycles of 300-mg oral azacitidine once daily for 14 or 21 days every 28 days, the overall response rate including transfusion independence for red blood cells or platelets, partial or complete response or hematologic improvement, was 41% with the 21-day dosing. RBC transfusion independence was achieved in ∼35% of patients with tolerable toxicity.
Low-dose decitabine at a schedule of 20 mg/m2 IV for 3 consecutive days has been evaluated in a phase 2 study of lower risk patients with MDS.48 Eighty-one percent had intermediate-1 risk MDS according to IPSS. Twenty-nine percent of patients achieved a complete remission, and complete or partial cytogenetic response in patients with abnormal karyotype was noted in 63% (26% CR); 32% of transfusion-dependent patients at baseline achieved transfusion independence with acceptable side effects.
Hematopoietic stem cell transplantation
There is no evidence-based strategy to assign lower risk patients with MDS to hematopoietic stem cell transplantation. Patients with multiple molecular abnormalities have been shown to have significantly reduced progression-free and overall survival rates compared with unmutated patients.49 These patients, and those with TP53 mutations may be advised to be closely monitored, HLA typed, and referred to allogeneic stem cell transplantation. The problem is that adverse molecular data also predict adverse outcomes after allogeneic transplantation.
Conclusions
Although the armamentarium of treatment strategies is broadening in the field of lower risk MDS, we still have a long way to go until we can confidently state that we are able to significantly alter the natural course of disease in the majority of patients. However, the ability to consider different treatment approaches in certain clinical settings is encouraging and the emergence of molecular subgroups responding preferentially to certain drugs strikes an upbeat tone in the ever more complex field of MDS.
Correspondence
Aristoteles Giagounidis, Department of Hematology, Oncology and Palliative Care, Marien Hospital Düsseldorf, Rochusstr 2, 40479 Düsseldorf, Germany; e-mail: agiagounidis@web.de.
References
Competing Interests
Conflict-of-interest disclosure: The author has received honoraria from Novartis, Celgene, and Acceleron.
Author notes
Off-label drug use: Lenalidomide in non-del(5q), luspatercept in low-risk MDS, romiplostim and eltrombopag in thrmobocytopenic patients with MDS, and immunosuppressive treatment of MDS.