Abstract
The introduction of high throughput sequencing (HTS) techniques greatly improved the knowledge of inherited thrombocytopenias (ITs) over the last few years. A total of 33 different forms caused by molecular defects affecting at least 32 genes have been identified; along with the discovery of new disease-causing genes, pathogenetic mechanisms of thrombocytopenia have been better elucidated. Although the clinical picture of ITs is heterogeneous, bleeding has been long considered the major clinical problem for patients with IT. Conversely, the current scenario indicates that patients with some of the most common ITs are at risk of developing additional disorders more dangerous than thrombocytopenia itself during life. In particular, MYH9 mutations result in congenital macrothrombocytopenia and predispose to kidney failure, hearing loss, and cataracts, MPL and MECOM mutations cause congenital thrombocytopenia evolving into bone marrow failure, whereas thrombocytopenias caused by RUNX1, ANKRD26, and ETV6 mutations are characterized by predisposition to hematological malignancies. Making a definite diagnosis of these forms is crucial to provide patients with the most appropriate treatment, follow-up, and counseling. In this review, the ITs known to date are discussed, with specific attention focused on clinical presentations and diagnostic criteria for ITs predisposing to additional illnesses. The currently available therapeutic options for the different forms of IT are illustrated.
Learning Objectives
Become familiar with the several inherited thrombocytopenias identified so far and recognize forms predisposing to other diseases (“predisposing forms”)
Understand the need to investigate the molecular basis of predisposing forms to arrange the most appropriate treatment and follow-up for each patient
Be updated on the newest treatments available for patients with the different forms of inherited thrombocytopenia
Introduction
The history of inherited thrombocytopenias (ITs) began in 1948 with the description of a patient suffering from a congenital bleeding disorder, which was called Bernard-Soulier syndrome (BSS). Almost 70 years later, advances in clinical and biomedical research led to the definition of a total of 33 different forms of IT caused by molecular defects affecting at least 32 genes. Some decades ago, a first major boost to the improvement of knowledge on ITs came from the widespread diffusion of automated cell counters; they greatly increased the identification of patients with thrombocytopenia, eventually leading to the recognition that the prevalence of ITs is much higher than previously thought, affecting at least 2.7:100 000 individuals, as estimated in the Italian population.1 The development and diffusion of genetic technologies definitively led to the explosion of knowledge on ITs. Sanger sequencing and linkage analysis have revealed the molecular bases of some ITs since 1990. Currently, Sanger sequencing is considered a low throughput and time-consuming approach that is mainly used in patients for whom a diagnostic hypothesis has been formulated based on clinical and laboratory investigation of their phenotypes. A number of high-throughput sequencing (HTS) technologies, formerly next-generation sequencing (NGS), have been recently developed and widely applied to human inherited diseases. NGS-targeted platforms allow the analysis of a predefined group of genes at once, thus making molecular diagnosis of inherited disorders quicker and less expensive. A targeted sequencing platform, covering 63 genes linked to bleeding and thrombotic disorders, showed 100% sensitivity in detecting causal variants previously identified by Sanger sequencing and allowed detection of the disease-causing gene in 90% of the patients who had not been previously investigated at the molecular level.2
A major improvement in knowledge of ITs came from the introduction of whole exome sequencing (WES) and whole genome sequencing (WGS). They have been adopted by several national and international consortia focused on the identification of the genes responsible for IT in patients who remained without a molecular diagnosis after the analysis of 1 or more candidate genes. PRKACG, GFI1b, STIM1, FYB, SLFN14, ETV6, DIAPH1, and SRC are examples of disease-causing genes detected by these HTS approaches.3-11
The findings obtained by both WES and WGS not only improved the definition of the phenotypes of several ITs but also inspired a number of functional studies on the role of molecules coded by the causative genes, whose function in platelet production was often previously unknown. This approach is expected to improve our comprehension of the physiologic mechanisms of megakaryopoiesis and platelet biogenesis.
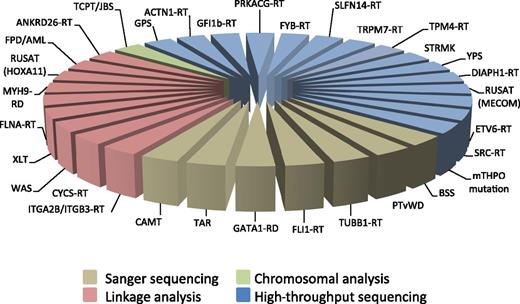
Figure 1 illustrates the different approaches used over the decades for discovering the molecular defects responsible for the known forms of IT.
Genetic approaches used for discovering the molecular defects responsible for the known forms of inherited thrombocytopenias. For the abbreviations, see Table 1. mTHPO mutation, inherited thrombocytopenia from a monoallelic THPO mutation.
Genetic approaches used for discovering the molecular defects responsible for the known forms of inherited thrombocytopenias. For the abbreviations, see Table 1. mTHPO mutation, inherited thrombocytopenia from a monoallelic THPO mutation.
Classification
The classifications of ITs that proved most effective for diagnostic purposes were those based on platelet size, which is greatly variable in the different forms,12 and the presence of further defects in addition to thrombocytopenia.13 Although useful, the effectiveness of the classification by inheritance is affected by the high frequency of sporadic cases due to de novo mutations in some disorders14 and the incomplete penetrance of the same mutations, which often make it difficult to recognize the inheritance pattern.15-17
On the basis of our more recent view on ITs, we propose a classification (Table 1),3-11,14-37 in which ITs are divided into 3 groups: forms characterized by only the platelet defect; disorders in which the platelet phenotype associates with additional congenital defects (syndromic forms); and forms characterized by increased risk of acquiring additional diseases during life. Thus, this classification is useful for both diagnostic and prognostic purposes.
Main features of inherited thrombocytopenias classified according to their clinical picture
| Disease (abbreviation, OMIM entry) . | Inheritance . | Gene (locus) . | Thrombocytopenia*/platelet size . | Bleeding† . | Additional features to thrombocytopenia . |
|---|---|---|---|---|---|
| Forms with only thrombocytopenia | |||||
| Bernard-Soulier syndrome (bBSS, mBSS, 231200/153670) | |||||
| Biallelic18 | AR | GP1BA (17p13) GPIBB (22q11) | ++,+++/giant, large | S | Impaired platelet function. |
| Monoallelic15 | AD | GP9 (3q21) | +/large | A/Mi | |
| Gray platelet syndrome (GPS, 139090)3 | AR | NBEAL2 (3p21) | ++,+++/large | Mi/S | Impaired platelet function. Severely reduced content of α granules. Platelet count decreases over time. Development of progressive bone marrow fibrosis and splenomegaly. Elevated serum vitamin B12 levels. |
| ACTN1-related thrombocytopenia (ACTN1-RT, 615193)19 | AD | ACTN1 (14q24) | +/large | A/Mi | — |
| Platelet-type von Willebrand disease (PTvWD, 177820)20 | AD | GP1BA (17p13) | +,+++/ normal, slightly increased | A/Mi | Platelet count is normal in most patients but may decrease greatly under stressful conditions (pregnancy, surgery, and infection). |
| ITGA2B/ITGB3-related thrombocytopenia (ITGA2B/ITGB3-RT (187800)21 | AD | ITGA2B (17q21) | +,++/large | Mi/Mo | Impaired platelet function. |
| ITGB3 (17q21) | |||||
| TUBB1-related thrombocytopenia (TUBB1-RT, 613112)22 | AD | TUBB1 (20q13) | +/large | A/Mo | — |
| CYCS-related thrombocytopenia (CYCS-RT or THC4, 612004)23 | AD | CYCS (7p15) | +/ normal, slightly reduced | A | — |
| GFI1b-related thrombocytopenia (GFI1b-RT, 187900)5 | AD | GFI1B (9q34) | +,++/large | Mo/S | Impaired platelet function. Reduced α granules. Red cell anisocytosis. |
| PRKACG-related thrombocytopenia (PRKACG-RT, 616176)4 | AR | PRKACG (9q21) | +++/large | S | Impaired platelet function. |
| FYB-related thrombocytopenia (FYB-RT, na)7 | AR | FYB (5p13.1) | ++,+++/ normal, slightly reduced | Mi/Mo | — |
| SLFN14-related thrombocytopenia (SLFN14-RT, na)8 | AD | SLFN14 (17q12) | +,++/normal, large | Mi/S | Impaired platelet function. |
| FLI1-related thrombocytopenia (FLI1-RT, na)24 | AR | FLI1 (11p24.3) | ++/large | Mi/Mo | Impaired platelet function. Giant α granules. |
| Inherited thrombocytopenia from monoallelic THPO mutation (na, na)25 | AD | THPO (3q27.1) | +/ normal, slightly increased | A | — |
| TRPM7-related thrombocytopenia (TRPM7-RT)26 | AD | TRPM7 (15q21.2) | +,++/large | A/Mi | Aberrant distribution of granules, increased number and anarchic organization of microtubules. |
| Tropomyosin 4-related thrombocytopenia (TPM4-RT, na)17 | AD | TPM4 (19p13.1) | +/large | Mi | — |
| Forms with additional clinically relevant congenital defects/syndromic forms | |||||
| Wiskott-Aldrich syndrome (WAS, 301000)27 | XL | WAS (Xp11) | +++/ normal, slightly reduced | S | Severe immunodeficiency leading to early death. Eczema. Increased risk of malignancies and autoimmunity. |
| X-linked thrombocytopenia (XLT, 313900)28 | XL | WAS (Xp11) | ++,+++/ normal, slightly reduced | A/Mo | Mild immunodeficiency. Mild transient eczema. Increased risk of malignancies and autoimmunity. Nonsyndromic patients with only thrombocytopenia are described. |
| Paris-Trousseau thrombocytopenia (TCPT, 188025), Jacobsen syndrome (JBS, 147791)29 | AD | Deletions in 11q23 | +++/ normal, slightly increased | Mo/S | Growth retardation, cognitive impairment, facial and skull dysmorphisms, malformations of the cardiovascular system, CNS, gastrointestinal apparatus, kidney, and/or urinary tract; other malformations. Impaired platelet function. Giant α-granules and reduced dense granules. Thrombocytopenia may resolve over time. |
| FLNA-related thrombocytopenia (FLNA-RT, na)30 | XL | FLNA (Xq28) | ++/large | Mi/Mo | Periventricular nodular heterotopia (OMIM 300049). Nonsyndromic patients with only thrombocytopenia are described. |
| GATA1-related diseases: X-linked thrombocytopenia with thalassemia (XLTT, 314050), X-linked thrombocytopenia with dyserythropoietic anemia (XLTDA, 300367)31 | XL | GATA1 (Xp11) | +++/ normal, slightly increased | Mi/S | Hemolytic anemia with laboratory abnormalities resembling β-thalassemia, splenomegaly, and dyserythropoietic anemia. Congenital erythropoietic porphyria. |
| Thrombocytopenia-absent radius syndrome (TAR, 274000)32 | AR | RBM8A (1q21) | +++/ normal, slightly reduced | S | Bilateral radial aplasia +/− additional upper and lower limb abnormalities. Possible kidney, cardiac, and/or CNS malformations. Possible intolerance to cow’s milk that can be associated with exacerbation of thrombocytopenia. Reduced megakaryocytes in bone marrow. Platelet count may rise over time. |
| Stormorken syndrome (STRMK,185070)6 | AD | STIM1 (11p15) | ++,+++/na | Mi | Myopathy with tubular aggregates, congenital miosis, functional or anatomical asplenia, ichthyosis, headache, mild anemia, facial dysmorphisms, defects in physical growth, and cognitive impairment. |
| York platelet syndrome (YPS, na)33 | AD | STIM1 (11p15) | ++,+++/normal | A | Myopathy, platelet ultrastructural abnormalities, such as moderately decreased α granules, increased vacuoles, and giant electron dense and targeted-like bodies. |
| Forms with increased risk of acquiring additional illnesses/predisposing forms | |||||
| MYH9-related disease (MYH9-RD (na)14 | AD | MYH9 (22q12) | +,+++/giant, large | A/Mi | Possible development of sensorineural deafness, nephropathy, and/or cataract. Half of the patients present with elevated liver enzymes without developing liver dysfunction. |
| DIAPH1-related thrombocytopenia (DIAPH1-RT, na)10 | AD | DIAPH1 (5q31.3) | +,+++/large | A | Risk of sensorineural deafness during infancy. Possible mild transient leukopenia. |
| Congenital amegakaryocytic thrombocytopenia (CAMT, 604498)34 | AR | MPL (1p34.2) | +++/normal, slightly reduced | S | Reduced/absent megakaryocytes. Evolution to severe bone marrow aplasia in infancy in all patients. |
| Radioulnar synostosis with amegakaryocytic thrombocytopenia (RUSAT, 605432, 616738)35,36 | AD/AR | HOXA11 (7p15) or MECOM (3q26.2) | +++/ normal, slightly increased | S | Bilateral radio-ulnar synostosis +/− other malformations. Reduced/absent megakaryocytes in bone marrow. Possible progression to bone marrow aplasia. The hematological phenotype is more severe in the AR form because of MECOM mutations. |
| Familial platelet disorder with propensity to acute myelogenous leukemia (FPD/AML, 601399)37 | AD | RUNX1 (21q22) | ++/ normal, slightly increased | A/Mo | Over 40% of patients acquire acute myelogenous leukemia or myelodysplastic syndromes. Increased risk of T acute lymphoblastic leukemia. Impaired platelet function. |
| ANKRD26-related thrombocytopenia (ANKRD26-RT (or THC2, 188000)16 | AD | ANKRD26 (10p12) | ++,+++/ normal, slightly increased | A/Mi | About 8% of patients acquire myeloid malignancies. Some patients have increased levels of hemoglobin and/or leukocytes. Reduced α granules in some patients. |
| ETV6-related thrombocytopenia (ETV6-RT, na)9 | AD | ETV6 (12p13) | +,++/ normal, slightly increased | A/Mo | About 25% of patients acquire acute lymphoblastic leukemia and other hematological malignancies. |
| SRC-related thrombocytopenia (SRC-RT)11 | AD | SRC (20q11.23) | ++,+++/large | Mo/S | Congenital facial dysmorphism, juvenile myelofibrosis and splenomegaly, severe osteoporosis, premature edentulism. Platelets hypogranular or agranular. Abundant vacuoles. |
| Disease (abbreviation, OMIM entry) . | Inheritance . | Gene (locus) . | Thrombocytopenia*/platelet size . | Bleeding† . | Additional features to thrombocytopenia . |
|---|---|---|---|---|---|
| Forms with only thrombocytopenia | |||||
| Bernard-Soulier syndrome (bBSS, mBSS, 231200/153670) | |||||
| Biallelic18 | AR | GP1BA (17p13) GPIBB (22q11) | ++,+++/giant, large | S | Impaired platelet function. |
| Monoallelic15 | AD | GP9 (3q21) | +/large | A/Mi | |
| Gray platelet syndrome (GPS, 139090)3 | AR | NBEAL2 (3p21) | ++,+++/large | Mi/S | Impaired platelet function. Severely reduced content of α granules. Platelet count decreases over time. Development of progressive bone marrow fibrosis and splenomegaly. Elevated serum vitamin B12 levels. |
| ACTN1-related thrombocytopenia (ACTN1-RT, 615193)19 | AD | ACTN1 (14q24) | +/large | A/Mi | — |
| Platelet-type von Willebrand disease (PTvWD, 177820)20 | AD | GP1BA (17p13) | +,+++/ normal, slightly increased | A/Mi | Platelet count is normal in most patients but may decrease greatly under stressful conditions (pregnancy, surgery, and infection). |
| ITGA2B/ITGB3-related thrombocytopenia (ITGA2B/ITGB3-RT (187800)21 | AD | ITGA2B (17q21) | +,++/large | Mi/Mo | Impaired platelet function. |
| ITGB3 (17q21) | |||||
| TUBB1-related thrombocytopenia (TUBB1-RT, 613112)22 | AD | TUBB1 (20q13) | +/large | A/Mo | — |
| CYCS-related thrombocytopenia (CYCS-RT or THC4, 612004)23 | AD | CYCS (7p15) | +/ normal, slightly reduced | A | — |
| GFI1b-related thrombocytopenia (GFI1b-RT, 187900)5 | AD | GFI1B (9q34) | +,++/large | Mo/S | Impaired platelet function. Reduced α granules. Red cell anisocytosis. |
| PRKACG-related thrombocytopenia (PRKACG-RT, 616176)4 | AR | PRKACG (9q21) | +++/large | S | Impaired platelet function. |
| FYB-related thrombocytopenia (FYB-RT, na)7 | AR | FYB (5p13.1) | ++,+++/ normal, slightly reduced | Mi/Mo | — |
| SLFN14-related thrombocytopenia (SLFN14-RT, na)8 | AD | SLFN14 (17q12) | +,++/normal, large | Mi/S | Impaired platelet function. |
| FLI1-related thrombocytopenia (FLI1-RT, na)24 | AR | FLI1 (11p24.3) | ++/large | Mi/Mo | Impaired platelet function. Giant α granules. |
| Inherited thrombocytopenia from monoallelic THPO mutation (na, na)25 | AD | THPO (3q27.1) | +/ normal, slightly increased | A | — |
| TRPM7-related thrombocytopenia (TRPM7-RT)26 | AD | TRPM7 (15q21.2) | +,++/large | A/Mi | Aberrant distribution of granules, increased number and anarchic organization of microtubules. |
| Tropomyosin 4-related thrombocytopenia (TPM4-RT, na)17 | AD | TPM4 (19p13.1) | +/large | Mi | — |
| Forms with additional clinically relevant congenital defects/syndromic forms | |||||
| Wiskott-Aldrich syndrome (WAS, 301000)27 | XL | WAS (Xp11) | +++/ normal, slightly reduced | S | Severe immunodeficiency leading to early death. Eczema. Increased risk of malignancies and autoimmunity. |
| X-linked thrombocytopenia (XLT, 313900)28 | XL | WAS (Xp11) | ++,+++/ normal, slightly reduced | A/Mo | Mild immunodeficiency. Mild transient eczema. Increased risk of malignancies and autoimmunity. Nonsyndromic patients with only thrombocytopenia are described. |
| Paris-Trousseau thrombocytopenia (TCPT, 188025), Jacobsen syndrome (JBS, 147791)29 | AD | Deletions in 11q23 | +++/ normal, slightly increased | Mo/S | Growth retardation, cognitive impairment, facial and skull dysmorphisms, malformations of the cardiovascular system, CNS, gastrointestinal apparatus, kidney, and/or urinary tract; other malformations. Impaired platelet function. Giant α-granules and reduced dense granules. Thrombocytopenia may resolve over time. |
| FLNA-related thrombocytopenia (FLNA-RT, na)30 | XL | FLNA (Xq28) | ++/large | Mi/Mo | Periventricular nodular heterotopia (OMIM 300049). Nonsyndromic patients with only thrombocytopenia are described. |
| GATA1-related diseases: X-linked thrombocytopenia with thalassemia (XLTT, 314050), X-linked thrombocytopenia with dyserythropoietic anemia (XLTDA, 300367)31 | XL | GATA1 (Xp11) | +++/ normal, slightly increased | Mi/S | Hemolytic anemia with laboratory abnormalities resembling β-thalassemia, splenomegaly, and dyserythropoietic anemia. Congenital erythropoietic porphyria. |
| Thrombocytopenia-absent radius syndrome (TAR, 274000)32 | AR | RBM8A (1q21) | +++/ normal, slightly reduced | S | Bilateral radial aplasia +/− additional upper and lower limb abnormalities. Possible kidney, cardiac, and/or CNS malformations. Possible intolerance to cow’s milk that can be associated with exacerbation of thrombocytopenia. Reduced megakaryocytes in bone marrow. Platelet count may rise over time. |
| Stormorken syndrome (STRMK,185070)6 | AD | STIM1 (11p15) | ++,+++/na | Mi | Myopathy with tubular aggregates, congenital miosis, functional or anatomical asplenia, ichthyosis, headache, mild anemia, facial dysmorphisms, defects in physical growth, and cognitive impairment. |
| York platelet syndrome (YPS, na)33 | AD | STIM1 (11p15) | ++,+++/normal | A | Myopathy, platelet ultrastructural abnormalities, such as moderately decreased α granules, increased vacuoles, and giant electron dense and targeted-like bodies. |
| Forms with increased risk of acquiring additional illnesses/predisposing forms | |||||
| MYH9-related disease (MYH9-RD (na)14 | AD | MYH9 (22q12) | +,+++/giant, large | A/Mi | Possible development of sensorineural deafness, nephropathy, and/or cataract. Half of the patients present with elevated liver enzymes without developing liver dysfunction. |
| DIAPH1-related thrombocytopenia (DIAPH1-RT, na)10 | AD | DIAPH1 (5q31.3) | +,+++/large | A | Risk of sensorineural deafness during infancy. Possible mild transient leukopenia. |
| Congenital amegakaryocytic thrombocytopenia (CAMT, 604498)34 | AR | MPL (1p34.2) | +++/normal, slightly reduced | S | Reduced/absent megakaryocytes. Evolution to severe bone marrow aplasia in infancy in all patients. |
| Radioulnar synostosis with amegakaryocytic thrombocytopenia (RUSAT, 605432, 616738)35,36 | AD/AR | HOXA11 (7p15) or MECOM (3q26.2) | +++/ normal, slightly increased | S | Bilateral radio-ulnar synostosis +/− other malformations. Reduced/absent megakaryocytes in bone marrow. Possible progression to bone marrow aplasia. The hematological phenotype is more severe in the AR form because of MECOM mutations. |
| Familial platelet disorder with propensity to acute myelogenous leukemia (FPD/AML, 601399)37 | AD | RUNX1 (21q22) | ++/ normal, slightly increased | A/Mo | Over 40% of patients acquire acute myelogenous leukemia or myelodysplastic syndromes. Increased risk of T acute lymphoblastic leukemia. Impaired platelet function. |
| ANKRD26-related thrombocytopenia (ANKRD26-RT (or THC2, 188000)16 | AD | ANKRD26 (10p12) | ++,+++/ normal, slightly increased | A/Mi | About 8% of patients acquire myeloid malignancies. Some patients have increased levels of hemoglobin and/or leukocytes. Reduced α granules in some patients. |
| ETV6-related thrombocytopenia (ETV6-RT, na)9 | AD | ETV6 (12p13) | +,++/ normal, slightly increased | A/Mo | About 25% of patients acquire acute lymphoblastic leukemia and other hematological malignancies. |
| SRC-related thrombocytopenia (SRC-RT)11 | AD | SRC (20q11.23) | ++,+++/large | Mo/S | Congenital facial dysmorphism, juvenile myelofibrosis and splenomegaly, severe osteoporosis, premature edentulism. Platelets hypogranular or agranular. Abundant vacuoles. |
AD, autosomal dominant; AR, autosomal recessive; CNS, central nervous system; na, not available; OMIM, online Mendelian inheritance in man; vWD, von Willebrand disease; XL, X-linked.
Degrees of thrombocytopenia: + indicates > 100 × 109 platelets/L; ++, 50 × 109 to 100 × 109 platelets/L; +++, < 50 × 109 platelets/L.
Severity of bleeding tendency in most patients reported for each form: A indicates absent; Mi, mild; Mo, moderate; and S, severe.
Pathogenesis
In most ITs, the low platelet count derives from defects in 1 or more step(s) of the complex process of platelet biogenesis,38 whereas in a few disorders, thrombocytopenia results from reduced platelet lifespan. Table 24,5,7-11,17,19,20,22,24,26,27,29,30,32,35,36,39-45 summarizes the main pathogenetic mechanisms of ITs.
Main pathogenetic mechanisms of inherited thrombocytopenias
| Disease . | Gene . | Function of the defective gene/pathogenetic mechanisms of thrombocytopenia . |
|---|---|---|
| Defects in megakaryocyte differentiation | ||
| Congenital amegakaryocytic thrombocytopenia39 | MPL | MPL encodes for the receptor for THPO. MPL biallelic mutations abrogate THPO-MPL signaling, which is essential for the commitment differentiation of multipotent hematopoietic stem cells to MKs since birth and the self-renewal and maintenance of the multipotent stem cell compartment in postnatal hematopoiesis. |
| Thrombocytopenia-absent radius syndrome32 | RBM8A | RBM8A encodes for Y14, a component of the ubiquitous exon-junction complex, which is involved in RNA processing. Thrombocytopenia-absent radius syndrome is caused by compound inheritance of a low-frequency noncoding SNP and a rare null allele in RBM8A that significantly reduces Y14 expression. It has been hypothesized that Y14 deficiency affects MK differentiation by impairing the signaling downstream MPL. |
| Radioulnar synostosis with amegakaryocytic thrombocytopenia36 | HOXA11 | HOXA11 encodes for a member of the Homeobox family of DNA-binding proteins involved in the regulation of early hematopoiesis. Mutation of HOXA11 responsible for RUSAT affects MK differentiation in vitro. |
| MECOM | MECOM encodes the oncoprotein EVI1, a transcription factor involved in homeostasis of the hematopoietic stem cell compartment and MK differentiation. Missense pathogenetic variants of EVI1 may reduce its interaction with DNA and/or other transcription factors. | |
| Defects in megakaryocyte maturation | ||
| Familial platelet disorder with propensity to acute myelogenous leukemia40 | RUNX1 (AML1, CBFA2) | RUNX1 encodes for the DNA-binding α subunit of the CBF transcription complex, which transactivates multiple hematopoiesis-specific genes. Pathogenetic variants induce profound defects in MK maturation and platelet functional abnormalities deriving from dysregulated expression of several genes involved in MK development. Moreover, they affect the clonogenic potential and self-renewal capacities of the hematopoietic myeloid progenitors. |
| ANKRD26-related thrombocytopenia41 | ANKRD26 | ANKRD26 is downregulated during MK maturation by binding of RUNX1 and FLI1 to the 5′ UTR of the gene. Pathogenetic mutations abolish this binding, resulting in ANKRD26 overexpression in MKs, which, in turn, induces unbalanced activation of kinases downstream the MPL receptor, especially the MAPK/ERK 1/2 pathway. This mechanism induces altered MK maturation and reduced proplatelet extension. |
| Paris-Trousseau thrombocytopenia/Jacobsen syndrome29 | Deletions in 11q23 | Contiguous gene deletion syndrome. Different sizes and breakpoints of deletions are responsible for heterogeneity of the clinical picture. Thrombocytopenia derives from the deletion of FLI1, a transcription factor that promotes platelet production by transactivation of several genes associated with MK development, such as MPL, GP6, GP9, GP1BA, ITGA2B, and PF4. FLI1 haploinsufficiency results in altered MK maturation and dysmorphic and dysfunctional platelets. |
| FLI1-related thrombocytopenia24 | FLI1 | A homozygous missense mutation of FLI1 (see above) results in impaired DNA binding of the transcription factor and causes a phenotype similar to that of Paris-Trousseau thrombocytopenia. |
| ETV6-related thrombocytopenia9 | ETV6 | ETV6 encodes an ETS transcription factor that was initially identified as a tumor suppressor. ETV6 is involved in the balance between proliferation and differentiation of early hematopoietic progenitors and in late MK development. Mutations cause thrombocytopenia by affecting MK maturation. |
| GATA1-related diseases42 | GATA1 | GATA1 is a transcription factor regulating several genes with a key role in megakaryopoiesis, as GP1BA, GP1BB, ITGA2B, PF4, MPL, and NFE2, and in erythropoiesis, as HBB, ALAS1, and BCL2L1. Mutations affect MK maturation, resulting in thrombocytopenia and the production of dysmorphic and dysfunctional platelets. |
| GFI1b-related thrombocytopenia5 | GFI1B | GFI1B is a transcription factor regulating homeostasis of hematopoietic stem cells and development of the MK and erythroid lineages. Mutations cause defective MK maturation with defective expression of several platelet proteins, α granule deficiency, and variable alterations of platelet function. |
| Gray platelet syndrome43 | NBEAL2 | NBEAL2 encodes neurobeachin-like protein 2, which is involved in protein-protein interactions, membrane dynamics, and vesicle trafficking. Mutations result in defective MK maturation, severe deficiency of platelet α granules, and variable defects of platelet function. |
| SLFN14-related thrombocytopenia8 | SLFN14 | The role of SLFN14 protein is poorly known. Possible role as an endoribonuclease, regulating rRNA, and ribosome-associated mRNA cleavage. Mutations induce reduced SLFN14 expression in platelets. Patients’ platelets have defective dense granule formation and ATP secretion and heterogeneous functional defects. Some observations suggested that thrombocytopenia derives from reduced MK maturation and proplatelet formation. |
| FYB-related thrombocytopenia7 | FYB | The FYB protein (ADAP) is a candidate linker between cell membrane activation signals and intracellular events regulating actin organization. Because of immature MKs in bone marrow and activated platelets in blood, it has been hypothesized that thrombocytopenia derives from defective maturation of MKs and clearance of activated platelets. |
| SRC-related thrombocytopenia11 | SRC | SRC encodes for a tyrosine kinase, with a key role in several MK and platelet signaling pathways. Thrombocytopenia is caused by a gain-of-function missense mutation, resulting in a constitutive kinase activation, which causes increased overall tyrosine phosphorylation. Some observations suggest that thrombocytopenia derives from defective MK maturation and reduced proplatelet formation. |
| Defects in platelet release | ||
| MYH9-related disease43 | MYH9 | The MYH9 gene encodes for the heavy chain of non-muscle myosin IIA, a cytoplasmic myosin involved in processes requiring the generation of a chemomechanical force by the actin cytoskeleton. Mutations cause macrothrombocytopenia by inducing multiple defects of proplatelet formation and possible premature, ectopic release of platelets within the bone marrow. |
| ACTN1-related thrombocytopenia19 | ACTN1 | The ACTN1 protein (α1 actinin) stabilizes the actin cytoskeleton by crosslinking actin filaments in bundles. Mutations cause macrothrombocytopenia by inducing multiple defects of proplatelet formation. |
| FLNA-related thrombocytopenia30 | FLNA | The FLNA protein (filamin A) stabilizes the actin cytoskeleton and connects it to the plasma membrane. In MKs, filamin A binds the intracytoplasmatic domain of GPIbα to the actin filament network. It has been suggested that mutations result in defective proplatelet formation. |
| Bernard-Soulier syndrome | ||
| Biallelic43 | GP1BA GPIBB GP9 | These genes encode for components of the GPIb-IX-V complex in platelets and MKs. Mutations result in defective expression of the complex in the plasma membrane and cause thrombocytopenia by hampering proplatelet formation. |
| Monoallelic43 | ||
| ITGA2B/ITGB3-related thrombocytopenia44 | ITGA2B ITGB3 | ITGA2B and ITGB3 encode for the components of the GPIIb-IIIa complex. Mutations responsible for macrothrombocytopenia cause constitutive activation of the complex, which affects actin cytoskeleton reorganization. Proplatelet formation and proplatelet conversion to platelets are defective. |
| TUBB1-related thrombocytopenia22 | TUBB1 | The TUBB1 protein (β1 tubulin) is a component of microtubules expressed only in mature MKs and platelets. Mutations disrupt microtubule assembly and result in defective proplatelet formation. |
| TRPM7-related thrombocytopenia26 | TRPM7 | TRPM7 is a cation channel regulating calcium and magnesium homeostasis and a kinase that modulates the activity of non-muscle myosin IIA. Mutations induce macrothrombocytopenia deriving from altered acto-myosin cytoskeletal reorganization, which, in turn, impairs proplatelet formation. |
| TPM4-related thrombocytopenia17 | TPM4 | Tropomyosins are cytoskeletal proteins that regulate multiple functions of the acto-myosin cytoskeleton. TPM4 encodes 2 tropomyosin isoforms that are abundantly expressed in MKs and platelets. Mutations cause TPM4 insufficiency, which induces macrothrombocytopenia by altering actin cytoskeleton reorganization and eventually proplatelet formation. |
| CYCS-related thrombocytopenia45 | CYCS | CYCS encodes cytochrome c, a ubiquitous mitochondrial protein involved in mitochondrial respiration and initiation of the intrinsic pathway of apoptosis. Data suggest that mutations cause thrombocytopenia by inducing ectopic, premature release of platelets by a proplatelet-independent mechanism. |
| DIAPH1-related thrombocytopenia10 | DIAPH1 | DIAPH1 encodes a conserved member of the formin protein family, which mediates ρ GTPase-dependent assembly of filamentous actin and microtubule regulation during cytoskeletal remodeling. Mutation causes constitutive activation of the protein, which affects proplatelet formation by inducing abnormalities of cytoskeletal and microtubule function. |
| PRKACG-related thrombocytopenia4 | PRKACG | PRKACG encodes the γ isoform of the catalytic subunit of cAMP-dependent protein kinase A. Mutations affect proplatelet formation and platelet activation. |
| Shortened platelet survival | ||
| Platelet-type von Willebrand disease20 | GP1BA | The gene GP1BA encodes GPIbα, whose extracellular domain binds to vWF. The disorder derives from gain-of-function mutations that increase the affinity of GPIbα for von Willebrand factor. Reduced platelet survival derives from platelet clumping within the circulation. |
| Wiskott-Aldrich syndrome27 | WAS | The WAS protein plays a key role in the polymerization of actin in hematopoietic cells. Mutations result in increased clearance of platelets by the reticuloendothelial system and premature, ectopic platelet release of platelets in the bone marrow. The observation that splenectomy normalizes or significantly increases platelet counts in patients with WAS/XLT suggests that increased platelet clearance is the key mechanism of thrombocytopenia. |
| X-linked thrombocytopenia27 | ||
| Unknown defect | ||
| Stormorken syndrome/York platelet syndrome6,35 | STIM1 | STIM1 encodes for a protein of the endoplasmic reticulum that regulates Ca2+ influx to cells through the Ca2+ release-activated Ca2+ channels of the plasma membrane in many cell types. The causative variants are gain-of-function monoallelic mutations, resulting in constitutive Ca2+ influx from the extracellular space to the cytoplasm. Platelets have several in vitro defects of aggregation, activation, and δ granule secretion. |
| Disease . | Gene . | Function of the defective gene/pathogenetic mechanisms of thrombocytopenia . |
|---|---|---|
| Defects in megakaryocyte differentiation | ||
| Congenital amegakaryocytic thrombocytopenia39 | MPL | MPL encodes for the receptor for THPO. MPL biallelic mutations abrogate THPO-MPL signaling, which is essential for the commitment differentiation of multipotent hematopoietic stem cells to MKs since birth and the self-renewal and maintenance of the multipotent stem cell compartment in postnatal hematopoiesis. |
| Thrombocytopenia-absent radius syndrome32 | RBM8A | RBM8A encodes for Y14, a component of the ubiquitous exon-junction complex, which is involved in RNA processing. Thrombocytopenia-absent radius syndrome is caused by compound inheritance of a low-frequency noncoding SNP and a rare null allele in RBM8A that significantly reduces Y14 expression. It has been hypothesized that Y14 deficiency affects MK differentiation by impairing the signaling downstream MPL. |
| Radioulnar synostosis with amegakaryocytic thrombocytopenia36 | HOXA11 | HOXA11 encodes for a member of the Homeobox family of DNA-binding proteins involved in the regulation of early hematopoiesis. Mutation of HOXA11 responsible for RUSAT affects MK differentiation in vitro. |
| MECOM | MECOM encodes the oncoprotein EVI1, a transcription factor involved in homeostasis of the hematopoietic stem cell compartment and MK differentiation. Missense pathogenetic variants of EVI1 may reduce its interaction with DNA and/or other transcription factors. | |
| Defects in megakaryocyte maturation | ||
| Familial platelet disorder with propensity to acute myelogenous leukemia40 | RUNX1 (AML1, CBFA2) | RUNX1 encodes for the DNA-binding α subunit of the CBF transcription complex, which transactivates multiple hematopoiesis-specific genes. Pathogenetic variants induce profound defects in MK maturation and platelet functional abnormalities deriving from dysregulated expression of several genes involved in MK development. Moreover, they affect the clonogenic potential and self-renewal capacities of the hematopoietic myeloid progenitors. |
| ANKRD26-related thrombocytopenia41 | ANKRD26 | ANKRD26 is downregulated during MK maturation by binding of RUNX1 and FLI1 to the 5′ UTR of the gene. Pathogenetic mutations abolish this binding, resulting in ANKRD26 overexpression in MKs, which, in turn, induces unbalanced activation of kinases downstream the MPL receptor, especially the MAPK/ERK 1/2 pathway. This mechanism induces altered MK maturation and reduced proplatelet extension. |
| Paris-Trousseau thrombocytopenia/Jacobsen syndrome29 | Deletions in 11q23 | Contiguous gene deletion syndrome. Different sizes and breakpoints of deletions are responsible for heterogeneity of the clinical picture. Thrombocytopenia derives from the deletion of FLI1, a transcription factor that promotes platelet production by transactivation of several genes associated with MK development, such as MPL, GP6, GP9, GP1BA, ITGA2B, and PF4. FLI1 haploinsufficiency results in altered MK maturation and dysmorphic and dysfunctional platelets. |
| FLI1-related thrombocytopenia24 | FLI1 | A homozygous missense mutation of FLI1 (see above) results in impaired DNA binding of the transcription factor and causes a phenotype similar to that of Paris-Trousseau thrombocytopenia. |
| ETV6-related thrombocytopenia9 | ETV6 | ETV6 encodes an ETS transcription factor that was initially identified as a tumor suppressor. ETV6 is involved in the balance between proliferation and differentiation of early hematopoietic progenitors and in late MK development. Mutations cause thrombocytopenia by affecting MK maturation. |
| GATA1-related diseases42 | GATA1 | GATA1 is a transcription factor regulating several genes with a key role in megakaryopoiesis, as GP1BA, GP1BB, ITGA2B, PF4, MPL, and NFE2, and in erythropoiesis, as HBB, ALAS1, and BCL2L1. Mutations affect MK maturation, resulting in thrombocytopenia and the production of dysmorphic and dysfunctional platelets. |
| GFI1b-related thrombocytopenia5 | GFI1B | GFI1B is a transcription factor regulating homeostasis of hematopoietic stem cells and development of the MK and erythroid lineages. Mutations cause defective MK maturation with defective expression of several platelet proteins, α granule deficiency, and variable alterations of platelet function. |
| Gray platelet syndrome43 | NBEAL2 | NBEAL2 encodes neurobeachin-like protein 2, which is involved in protein-protein interactions, membrane dynamics, and vesicle trafficking. Mutations result in defective MK maturation, severe deficiency of platelet α granules, and variable defects of platelet function. |
| SLFN14-related thrombocytopenia8 | SLFN14 | The role of SLFN14 protein is poorly known. Possible role as an endoribonuclease, regulating rRNA, and ribosome-associated mRNA cleavage. Mutations induce reduced SLFN14 expression in platelets. Patients’ platelets have defective dense granule formation and ATP secretion and heterogeneous functional defects. Some observations suggested that thrombocytopenia derives from reduced MK maturation and proplatelet formation. |
| FYB-related thrombocytopenia7 | FYB | The FYB protein (ADAP) is a candidate linker between cell membrane activation signals and intracellular events regulating actin organization. Because of immature MKs in bone marrow and activated platelets in blood, it has been hypothesized that thrombocytopenia derives from defective maturation of MKs and clearance of activated platelets. |
| SRC-related thrombocytopenia11 | SRC | SRC encodes for a tyrosine kinase, with a key role in several MK and platelet signaling pathways. Thrombocytopenia is caused by a gain-of-function missense mutation, resulting in a constitutive kinase activation, which causes increased overall tyrosine phosphorylation. Some observations suggest that thrombocytopenia derives from defective MK maturation and reduced proplatelet formation. |
| Defects in platelet release | ||
| MYH9-related disease43 | MYH9 | The MYH9 gene encodes for the heavy chain of non-muscle myosin IIA, a cytoplasmic myosin involved in processes requiring the generation of a chemomechanical force by the actin cytoskeleton. Mutations cause macrothrombocytopenia by inducing multiple defects of proplatelet formation and possible premature, ectopic release of platelets within the bone marrow. |
| ACTN1-related thrombocytopenia19 | ACTN1 | The ACTN1 protein (α1 actinin) stabilizes the actin cytoskeleton by crosslinking actin filaments in bundles. Mutations cause macrothrombocytopenia by inducing multiple defects of proplatelet formation. |
| FLNA-related thrombocytopenia30 | FLNA | The FLNA protein (filamin A) stabilizes the actin cytoskeleton and connects it to the plasma membrane. In MKs, filamin A binds the intracytoplasmatic domain of GPIbα to the actin filament network. It has been suggested that mutations result in defective proplatelet formation. |
| Bernard-Soulier syndrome | ||
| Biallelic43 | GP1BA GPIBB GP9 | These genes encode for components of the GPIb-IX-V complex in platelets and MKs. Mutations result in defective expression of the complex in the plasma membrane and cause thrombocytopenia by hampering proplatelet formation. |
| Monoallelic43 | ||
| ITGA2B/ITGB3-related thrombocytopenia44 | ITGA2B ITGB3 | ITGA2B and ITGB3 encode for the components of the GPIIb-IIIa complex. Mutations responsible for macrothrombocytopenia cause constitutive activation of the complex, which affects actin cytoskeleton reorganization. Proplatelet formation and proplatelet conversion to platelets are defective. |
| TUBB1-related thrombocytopenia22 | TUBB1 | The TUBB1 protein (β1 tubulin) is a component of microtubules expressed only in mature MKs and platelets. Mutations disrupt microtubule assembly and result in defective proplatelet formation. |
| TRPM7-related thrombocytopenia26 | TRPM7 | TRPM7 is a cation channel regulating calcium and magnesium homeostasis and a kinase that modulates the activity of non-muscle myosin IIA. Mutations induce macrothrombocytopenia deriving from altered acto-myosin cytoskeletal reorganization, which, in turn, impairs proplatelet formation. |
| TPM4-related thrombocytopenia17 | TPM4 | Tropomyosins are cytoskeletal proteins that regulate multiple functions of the acto-myosin cytoskeleton. TPM4 encodes 2 tropomyosin isoforms that are abundantly expressed in MKs and platelets. Mutations cause TPM4 insufficiency, which induces macrothrombocytopenia by altering actin cytoskeleton reorganization and eventually proplatelet formation. |
| CYCS-related thrombocytopenia45 | CYCS | CYCS encodes cytochrome c, a ubiquitous mitochondrial protein involved in mitochondrial respiration and initiation of the intrinsic pathway of apoptosis. Data suggest that mutations cause thrombocytopenia by inducing ectopic, premature release of platelets by a proplatelet-independent mechanism. |
| DIAPH1-related thrombocytopenia10 | DIAPH1 | DIAPH1 encodes a conserved member of the formin protein family, which mediates ρ GTPase-dependent assembly of filamentous actin and microtubule regulation during cytoskeletal remodeling. Mutation causes constitutive activation of the protein, which affects proplatelet formation by inducing abnormalities of cytoskeletal and microtubule function. |
| PRKACG-related thrombocytopenia4 | PRKACG | PRKACG encodes the γ isoform of the catalytic subunit of cAMP-dependent protein kinase A. Mutations affect proplatelet formation and platelet activation. |
| Shortened platelet survival | ||
| Platelet-type von Willebrand disease20 | GP1BA | The gene GP1BA encodes GPIbα, whose extracellular domain binds to vWF. The disorder derives from gain-of-function mutations that increase the affinity of GPIbα for von Willebrand factor. Reduced platelet survival derives from platelet clumping within the circulation. |
| Wiskott-Aldrich syndrome27 | WAS | The WAS protein plays a key role in the polymerization of actin in hematopoietic cells. Mutations result in increased clearance of platelets by the reticuloendothelial system and premature, ectopic platelet release of platelets in the bone marrow. The observation that splenectomy normalizes or significantly increases platelet counts in patients with WAS/XLT suggests that increased platelet clearance is the key mechanism of thrombocytopenia. |
| X-linked thrombocytopenia27 | ||
| Unknown defect | ||
| Stormorken syndrome/York platelet syndrome6,35 | STIM1 | STIM1 encodes for a protein of the endoplasmic reticulum that regulates Ca2+ influx to cells through the Ca2+ release-activated Ca2+ channels of the plasma membrane in many cell types. The causative variants are gain-of-function monoallelic mutations, resulting in constitutive Ca2+ influx from the extracellular space to the cytoplasm. Platelets have several in vitro defects of aggregation, activation, and δ granule secretion. |
CBF, core binding factor; ERK, extracellular signal-regulated kinase; Mk, megakaryocyte. Additional abbreviations are explained in Table 1.
The key pathogenetic mechanism of some ITs is defective differentiation of hematopoietic stem cells (HSCs) into megakaryocytes (MKs), resulting in the absence or severe reduction in the number of bone marrow MKs. The clinical picture of congenital amegakaryocytic thrombocytopenia (CAMT) reflects the nonredundant roles of the MPL receptor in MK differentiation since birth and in the maintenance of the stem cell compartment in postnatal hematopoiesis. Modeling of hematopoiesis of CAMT by using patient-derived induced pluripotent stem cells showed a failure in the transition of multipotent toward MK progenitors as well as defective self-replication and survival of multipotent stem cells; these phenotypes were rescued by transduction of a functional MPL.39
In 11 forms of IT, the main pathogenetic mechanism is altered MK maturation and therefore the production of a normal or increased number of immature, dysmorphic, and dysfunctional MKs. Of note, 6 of these disorders are caused by mutations of transcription factors with a key role in megakaryopoiesis, ie, RUNX1, FLI1, GATA1, GFI1b, and ETV6 (Table 2). Each of these transcription factors regulates, as activator or repressor, the expression of numerous genes; thus, the ITs caused by their defects are characterized by the concurrent alterations of multiple mechanisms of MK and platelet development. For instance, RUNX1 directly transactivates genes encoding for other transcription factors involved in MK maturation (NF-E2), components of the MK cytoskeleton (MYH9, MYL9, MYH10), proteins implicated in α and dense granule development (PF4, PLDN), and members of MK/platelet signaling pathways (ANKRD26, MPL, PRKCQ, ALOX12, PCTP).42 Some studies identified the links between the MK abnormalities observed in patients with familiar platelet disorder (FPD) with predisposition to acute myeloid leukemia (AML) and altered expression of specific RUNX1 transcriptional targets. Defective MK polyploidization is explained by dysregulation of MYH10 and impaired proplatelet formation by reduced MYH9 and MYL9 expression,46 whereas downregulation of PLDN contributes to defective dense granule development.47 The erythrocyte abnormalities typical of the disorders caused by GATA1 and GFI1b mutations indicate the essential role of these transcription factors in also controlling red cell production. Moreover, the predisposition to hematological neoplasms of patients with the ITs caused by RUNX1 and ETV6 mutations highlights how these pathogenetic variants also disrupt the homeostasis of myeloid and multipotent progenitors, respectively.
In a third group of ITs, the differentiation and maturation of MKs are substantially preserved, and thrombocytopenia derives from alterations of the extension and release of proplatelets from mature MKs and/or the conversion of proplatelets to platelets within the bloodstream. Most of these disorders are macrothrombocytopenias caused by mutations of genes encoding for components of the actomyosin cytoskeleton or microtubule system, such as MYH9, ACTN1, FLNA, TPM4, TRPM7, or TUBB1. This scenario reflects the key role of the mechanical forces generated by the cytoskeleton and microtubules in driving the processes of remodeling, protrusion, and fission of the MK cytoplasm, leading to the release of platelets.48 Defective proplatelet formation and platelet release are the key pathogenetic mechanisms also of the macrothrombocytopenias that are caused by mutations of genes for the major MK membrane glycoprotein (GP) complexes GPIb/IX/V and GPIIb/IIIa, ie, biallelic and monoallelic BSS and ITGA2B/ITGB3-related thrombocytopenia (ITGA2B/ITGB3-RT). Macrothrombocytopenia results from disruption of the interactions of these membrane integrins with the actomyosin cytoskeleton, which are essential for preserving MK cytoskeletal structure and reorganization.43 For instance, mutations responsible for ITGA2B/ITGB3-RT are gain-of-function variants resulting in constitutive, inappropriate activation of the GPIIb/IIIa. Thrombocytopenia of this disorder is explained by defects in proplatelet formation and conversion of proplatelets into platelets. Recent observations showed how these defects actually derive from altered remodeling of the actin cytoskeleton, which, in turn, is caused by hyper-activation of the outside-in signaling downstream of GPIIb/IIIa.44
Clinical picture
Bleeding diathesis
The presence and severity of bleeding tendency is greatly variable among patients with IT. Some patients present with no bleeding at all, whereas others may have hemorrhages only during hemostatic challenges. A minority of patients presents with spontaneous bleeding and may even develop life-threatening bleeding episodes. Bleeding is muco-cutaneous and consists of petechiae, ecchymoses, epistaxis, menorrhagia, and gastrointestinal hemorrhages. The degree of bleeding tendency correlates with platelet count in those ITs that are not associated with significant platelet dysfunction.14 Conversely, bleeding tendency is usually more severe than expected on the basis of platelet count in those forms associated with alteration of platelet function. For instance, the degree of bleeding diathesis is independent of platelet count in patients with biallelic BSS, suggesting that the impaired interaction between platelets and the subendothelium due to the GPIb/IX/V defect is the major determinant of bleeding.18 Clinically relevant defects of platelet function are variably present in patients with Gray platelet syndrome (GPS), GFI1b-related thrombocytopenia, SLFN14-related thrombocytopenia, PRKACG-related thrombocytopenia, ITGA2B/ITGB3-RT, Paris-Trousseau thrombocytopenia/Jacobsen syndrome, FPD/AML, and Wiskott-Aldrich syndrome (WAS) (Table 1).
The degree of thrombocytopenia remains stable during life in the large majority of ITs. However, platelet count tends to increase over time in thrombocytopenia-absent radius syndrome and in some patients with Paris-Trousseau thrombocytopenia/Jacobsen syndrome.29 Conversely, thrombocytopenia worsens over the years in GPS,3 whereas in patients with platelet-type von Willebrand disease, the platelet count may be variable and typically decreases under stressful conditions.20
Syndromic forms
In some patients, the molecular defects responsible for thrombocytopenia induce complex syndromic phenotypes. Congenital defects associated with thrombocytopenia in these ITs include skeletal deformity, cognitive impairment, malformations of the central nervous or cardiovascular system, and immunodeficiency. The abnormalities typical of the single syndromic forms are listed in Table 1.
Predisposing forms
The increasing number of ITs identified over the last few years let us understand that bleeding is no longer the only clinical risk for patients with IT. In fact, the development of hematological malignancies, bone marrow aplasia, juvenile myelofibrosis, or end-stage renal disease can be far more dangerous for the patient’s life than hemorrhages. The main predisposing forms are briefly discussed below.
MYH9-RD
With more than 300 families reported, MYH9-related disease (MYH9-RD) is the most prevalent IT worldwide. It is caused by monoallelic mutations in MYH9, the gene for the heavy chain of non-muscle myosin IIA (NMMHC-IIA). All patients present at birth with giant platelets and thrombocytopenia, which may result in bleeding tendency of different degrees. In some cases, macrothrombocytopenia remains the only manifestation of the disease throughout life; however, about one-third of patients with MYH9-RD develop proteinuric nephropathy, often leading to end-stage renal disease. Moreover, most individuals with MYH9-RD acquire sensorineural hearing loss, ranging from a mild defect to profound deafness, and about 20% of patients develop presenile cataracts.14,49 The investigation of a wide series of consecutive patients identified genotype-phenotype correlations that allow the prediction of the evolution of the disease in about 85% of MYH9-RD cases. In general, mutations affecting the N-terminal head domain of NMMHC-IIA are associated with a worse prognosis than those in the C-terminal tail domain. Moreover, a recent study showed how different mutations within the same NMMHC-IIA domain, and even hitting the same residue, may be associated with a significantly different risk of extra-hematological manifestations, thus providing a more accurate prognostic model.14
CAMT and radioulnar synostosis with amegakaryocytic thrombocytopenia
Due to biallelic mutations in MPL, the gene for the receptor of thrombopoietin (THPO), CAMT presents at birth as isolated hypomegakaryocytic thrombocytopenia without other phenotypic abnormalities. Patients with CAMT develop further cytopenias during childhood until progression to generalized bone marrow aplasia.34
Radioulnar synostosis with amegakaryocytic thrombocytopenia is a genetically heterogeneous disorder that can be caused by mutations in HOXA11 or MECOM. Development of bone marrow aplasia has been reported in both variants of the disease, even if patients with MECOM mutations seems to have a more severe evolution.36
FPD/AML, ANKRD26-RT, and ETV6-RT
These 3 autosomal-dominant disorders, with a quite different prevalence among ITs (3%, 18%, and 5%, respectively), share several clinical features. All of them present with isolated, non-syndromic thrombocytopenia, which is usually mild to moderate, with some patients having a platelet count above the lower limit of the normal range.16,37 Unlike the majority of ITs characterized by variable degrees of platelet macrocytosis, platelet size is normal; morphology of blood cells is also preserved. Bleeding is often absent or mild, especially in patients with ANKRD26-related thrombocytopenia (ANKRD26-RT) or ETV6-related thrombocytopenia (ETV6-RT). Patients with FPD/AML may present with variable degrees of bleeding tendency due to heterogeneous abnormalities of platelet function.37 Finally, but most importantly, another common feature of these conditions is the increased risk of hematological malignancies. Myelodysplastic syndromes and AML are reported in about 40% of patients with FPD/AML and 8% of patients with ANKRD26-RT. Of the 73 patients with ETV6-RT described so far, 17 (23%) had hematological malignancies: 7 patients developed acute lymphoblastic leukemia, whereas the remaining 10 had AML, myelodysplastic syndromes, multiple myeloma, or polycythemia vera.9,50 Of note, the “2016 revision of the World Health Organization classification of myeloid neoplasms and acute leukemia” included the myeloid malignancies arising from germline mutations in ANKRD26, RUNX1, and ETV6 in a new category defined as “Myeloid neoplasms with germline predisposition and pre-existing platelet disorders.”51
SRC-Related Thrombocytopenia
A dominant gain-of-function mutation of SRC has been recently described as causative of IT in a large pedigree. Platelets are dysmorphic and highly variable in size, with paucity of α granules. Five out of 9 affected individuals developed early-onset myelofibrosis, with hypercellular bone marrow and trilineage dysplasia. With the limitation of the observation of a single family, this IT should be regarded as a possible cause of juvenile myelofibrosis.11
Diagnosis
There are 3 main questions when dealing with the diagnosis of ITs: how to recognize that the thrombocytopenia we are investigating is a genetic form; how to reach a molecular diagnosis of a supposed IT; and which are the forms that require a molecular diagnosis. An effective diagnostic pathway cannot disregard the scrupulous collection of personal and family history, careful physical examination, and analysis of peripheral blood smears.
How to recognize that the thrombocytopenia we are investigating is a hereditary form
A diagnosis of IT may be straightforward when an informative family history is available or the low platelet count has been documented since birth. However, recognition of the genetic origin of thrombocytopenia is often difficult, as demonstrated by the fact that many patients with IT are misdiagnosed with acquired thrombocytopenias. For instance, the recent investigation of a cohort of 181 women with ITs showed that 31% of them were misdiagnosed with ITP; for this reason, 25% received 1 or more lines of undue immunosuppressive therapy, and 8% underwent unnecessary splenectomy.52
The genetic origin of a thrombocytopenia may not appear immediately evident because of several reasons: (1) many ITs are recessive forms (Table 1); (2) a number of patients carry de novo mutations, as documented in MYH9-RD, in which up to 40% of subjects have sporadic forms due to de novo mutational events14 ; (3) the penetrance of the causative mutation may be incomplete, as reported in monoallelic BSS, ANKRD26-RT, tropomyosin 4–related thrombocytopenia, and FPD/AML16-18,37 ; and (4) thrombocytopenia is often discovered only in adulthood because of the mild or absent bleeding tendency.53 Some helpful suggestions may be derived from the basic examination of patients. A lifelong history of bleeding, a bleeding tendency more severe than expected based on the platelet count, the presence of manifestations typically associated with thrombocytopenia in syndromic forms, and the finding of giant or dysmorphic platelets at the peripheral blood smear examination make the diagnosis of a genetic form more likely. For all the critical aspects discussed above, we recommend consideration of an inherited form whenever the acquired origin of thrombocytopenia is not obvious.
How to reach a molecular diagnosis of a supposed IT
The recent introduction of HTS technologies made it possible to manage the diagnostic workup of ITs either by the traditional tiered approach or by the quicker single-step approach. Until a few years ago, once the genetic nature of a thrombocytopenia was defined, a series of laboratory tests (in vitro platelet aggregation, flow cytometry for platelet surface GPs, examination of peripheral blood smear, and immunofluorescence assay for MYH9 protein aggregates in neutrophils) were performed to identify the candidate gene/genes to be sequenced. This approach was the mainstay of the diagnostic algorithm proposed in 2003 by the Italian Platelet Study Group and subsequently validated in different centers and progressively updated.13,54
Nowadays, targeted NGS platforms can be efficiently applied to search for the causative gene.2 Because the molecular basis of the disease remains unknown in a relevant proportion of patients with IT, despite the application of extensive targeted HTS studies, WES or WGS may be required. However, a positive finding is not always possible. In fact, a number of changes in many genes are often found and distinguishing the pathogenetic variants from nonpathogenetic variants may require complex functional studies.55
Although at the moment, the diagnostic workup is also largely dependent on local availability in terms of technical skills, expertise, and funds, it is likely that targeted NGS platforms will soon become the routine diagnostic test for ITs.
Which ITs require a molecular diagnosis
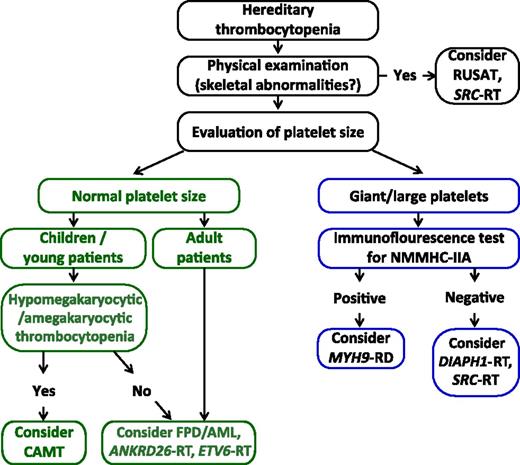
Despite the increasing accessibility to NGS, a rational and cost-effective diagnostic approach to ITs is requested. However, we suggest that a definitive diagnosis must always be sought after, at least in subjects in whom a predisposing form is suspected (Table 1). Before doing this, patients with a possible diagnosis of FPD/AML, ANKRD26-RT, and ETV6-RT should be exhaustively informed about the impossibility to predict whether and when they will possibly develop a hematological neoplasm and that no means are currently available to prevent such an eventuality. Figure 2 proposes a diagnostic algorithm for ITs predisposing to additional illnesses based on the evaluation of a few basic patients’ clinical features.
Diagnostic algorithm for hereditary thrombocytopenias predisposing to additional illnesses based on the evaluation of a few basic patients’ clinical features. Abbreviations are explained in Table 1.
Diagnostic algorithm for hereditary thrombocytopenias predisposing to additional illnesses based on the evaluation of a few basic patients’ clinical features. Abbreviations are explained in Table 1.
After excluding a syndromic IT by physical examination, evaluation of platelet size on peripheral blood smears can guide the diagnostic workup.12,14 In patients with large platelets, the suspicion for MYH9-RD should be raised independently of the presence of additional clinical features of the disease.14 In patients with normal MYH9 distribution in neutrophils, DIAPH1-related thrombocytopenia and SRC-related thrombocytopenia should be considered. In children and young individuals with thrombocytopenia and normal-sized platelets, with or without anemia and/or neutropenia, a bone marrow examination should be performed to search for CAMT; very high serum thrombopoietin levels will support this diagnostic hypothesis while not being sufficient. In children in whom CAMT is excluded and in adults with normal platelet size, FPD/AML, ANKRD26-RT, and ETV6-RT should be considered, especially if family history is consistent with autosomal dominant transmission. Molecular analysis is required to confirm the diagnosis and provide patients with personalized management, counseling, and follow-up.
Follow-up
A close follow-up is essential for patients with predisposing forms, although medical attention must be provided to all patients with IT in case of bleeding or for its prevention during hemostatic challenges. Follow-up of patients with MYH9-RD should be tailored on the basis of the specific NMMHC-IIA mutation and should include periodic measurements of proteinuria, which is the earliest sign of kidney involvement.14 In fact, patients with early-stage kidney disease may benefit from treatment of reducing proteinuria.56 Periodic evaluation of renal function and the search for sensorineural hearing loss and cataract are also recommended.
In patients with a diagnosis of FPD/AML, ANKRD26-RT, and ETV6-RT, a baseline bone marrow examination, including cytogenetic analysis, should be considered. Thereafter, patients should be monitored by performing a complete blood count and peripheral blood smear examination once a year. In the case of development of laboratory data (ie, appearance of leukocytosis, abnormal white blood cell differential, unexplained anemia, and worsening of thrombocytopenia) and/or symptoms suggestive of hematological malignancies, patients should be further investigated by bone marrow examination and other appropriate studies.
In patients with a predisposition to hematological neoplasms, HLA typing and the search for a compatible donor for hematopoietic stem cell transplantation (HSCT) before the possible occurrence of an overt neoplasm may be discussed with each single patient. Of note, the family members should be investigated at the molecular level to avoid the use of a relative affected by the same IT as the donor for HSCT.16,57
Therapy
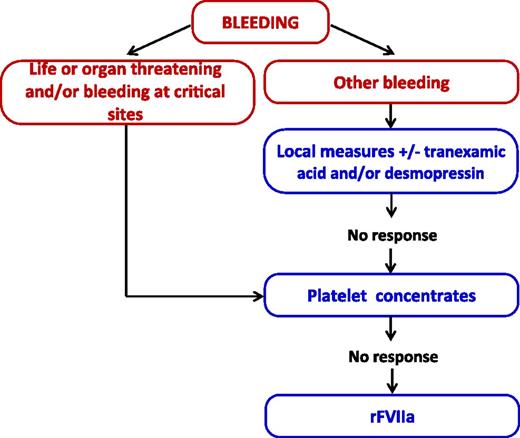
Most patients with ITs have no or mild spontaneous bleeding and require medical surveillance and sometimes prophylactic intervention only on the occasion of hemostatic challenges, such as surgery, other invasive procedures, or deliveries. Treatment of hemorrhages, which are usually mucocutaneous, is mainly based on local measures and platelet transfusions. Some agents for improving hemostasis are also widely used in clinical practice and recommended by available guidelines,58 even if evidence on their clinical effectiveness is mostly anecdotal. Table 3 recapitulates the currently available measures for the treatment of ITs, and Figure 3 outlines the approach we use for the management of bleeding episodes.
Currently available measures for treatment of inherited thrombocytopenias
| Treatment . | Indications . |
|---|---|
| Tools for stopping bleeding and covering hemostatic challenges | |
| Local measures (eg, nasal packing or cauterization, suturing) | Whenever possible, should be considered as the first-line treatment of bleeding unless it is life or organ threatening or occurs at functionally critical sites. |
| Platelet transfusions | Currently considered as the standard measure to stop bleeding or cover hemostatic challenges. Their use should be limited to clinical situations that cannot be managed otherwise, mainly because of the risk of HLA alloimmunization that can lead to refractoriness to subsequent platelet infusions. Use HLA-matched concentrates whenever possible. Should be considered as the first-line treatment of life- or organ-threatening bleeding or bleeding at critical sites. |
| Antifibrinolytic agents | Widely used in clinical practice to treat bleeding or cover hemostatic challenges (especially low-risk procedures). |
| Anecdotal evidence about clinical efficacy in ITs; use based on experts’ opinion. | |
| Desmopressin | Widely used in clinical practice to treat bleeding or cover hemostatic challenges (especially low-risk procedures). |
| Anecdotal evidence about clinical efficacy in ITs; use based on improvement of bleeding time in some forms. | |
| Recombinant factor VIIa | Limited clinical experience in ITs; it has been used to treat bleeding or cover surgery in few patients with bBSS (case reports). |
| Short-term eltrombopag | Effective in increasing platelet count in most patients with MYH9-RD (phase 2 trial). |
| It has been used to cover elective surgery in few patients with MYH9-RD or ANKRD26-RT (case reports). | |
| Tools for achieving long-term increase of platelet count | |
| Long-term eltrombopag | Tested in 8 patients with WAS/XLT (phase 2 trial). Five of them achieved a clinical response. No major side effects were recorded. |
| Splenectomy | Increases platelet count in patients with WAS/XLT but also increases the incidence of infections, without affecting overall survival. Splenectomy has no role in all the other forms of IT. |
| In XLT, the pros and cons of splenectomy should be assessed in each individual patient. | |
| In WAS, splenectomy should be avoided in candidates for HSCT because it increases the risk of major infections after HSCT. | |
| HSCT | Treatment of choice for WAS and CAMT. |
| Should be considered in selected patients affected with other ITs presenting with severe clinical manifestations and/or poor prognosis. | |
| It has been used in a few patients with XLT, RUSAT, bBSS, and thrombocytopenia-absent radius syndrome, with good outcomes. | |
| Gene therapy | Experimental option for patients affected by severe WAS who do not have a suitable donor for HSCT. |
| Treatment . | Indications . |
|---|---|
| Tools for stopping bleeding and covering hemostatic challenges | |
| Local measures (eg, nasal packing or cauterization, suturing) | Whenever possible, should be considered as the first-line treatment of bleeding unless it is life or organ threatening or occurs at functionally critical sites. |
| Platelet transfusions | Currently considered as the standard measure to stop bleeding or cover hemostatic challenges. Their use should be limited to clinical situations that cannot be managed otherwise, mainly because of the risk of HLA alloimmunization that can lead to refractoriness to subsequent platelet infusions. Use HLA-matched concentrates whenever possible. Should be considered as the first-line treatment of life- or organ-threatening bleeding or bleeding at critical sites. |
| Antifibrinolytic agents | Widely used in clinical practice to treat bleeding or cover hemostatic challenges (especially low-risk procedures). |
| Anecdotal evidence about clinical efficacy in ITs; use based on experts’ opinion. | |
| Desmopressin | Widely used in clinical practice to treat bleeding or cover hemostatic challenges (especially low-risk procedures). |
| Anecdotal evidence about clinical efficacy in ITs; use based on improvement of bleeding time in some forms. | |
| Recombinant factor VIIa | Limited clinical experience in ITs; it has been used to treat bleeding or cover surgery in few patients with bBSS (case reports). |
| Short-term eltrombopag | Effective in increasing platelet count in most patients with MYH9-RD (phase 2 trial). |
| It has been used to cover elective surgery in few patients with MYH9-RD or ANKRD26-RT (case reports). | |
| Tools for achieving long-term increase of platelet count | |
| Long-term eltrombopag | Tested in 8 patients with WAS/XLT (phase 2 trial). Five of them achieved a clinical response. No major side effects were recorded. |
| Splenectomy | Increases platelet count in patients with WAS/XLT but also increases the incidence of infections, without affecting overall survival. Splenectomy has no role in all the other forms of IT. |
| In XLT, the pros and cons of splenectomy should be assessed in each individual patient. | |
| In WAS, splenectomy should be avoided in candidates for HSCT because it increases the risk of major infections after HSCT. | |
| HSCT | Treatment of choice for WAS and CAMT. |
| Should be considered in selected patients affected with other ITs presenting with severe clinical manifestations and/or poor prognosis. | |
| It has been used in a few patients with XLT, RUSAT, bBSS, and thrombocytopenia-absent radius syndrome, with good outcomes. | |
| Gene therapy | Experimental option for patients affected by severe WAS who do not have a suitable donor for HSCT. |
Abbreviations are explained in Table 1.
Approach to the management of bleeding episodes in hereditary thrombocytopenia. rFVIIa, recombinant activated factor VII.
Approach to the management of bleeding episodes in hereditary thrombocytopenia. rFVIIa, recombinant activated factor VII.
When managing patients affected by ITs without significant platelet dysfunction, the indication for the use of prophylactic platelets prior to elective invasive procedures can be deduced from general guidelines for platelet transfusion.59,60 On the contrary, patients affected by ITs with clinically relevant platelet dysfunction may need prophylactic intervention even for platelet counts (< 50 × 109/L for delivery and < 68 × 109/L for surgery) higher than the thresholds recommended for “safe” surgery. Two recent studies provided a systematic assessment of the risk of bleeding associated with delivery and surgery,52,61 respectively, by the investigation of a large series of patients with IT. Patients’ previous history of bleeding and low platelet count were the major predictors of excessive bleeding both postpartum and after surgery. Age over 70 years and diagnosis of some ITs with concurrent platelet dysfunction, ie, biallelic BSS, FPD/AML, GPS, and ITGA2B/ITGB3-RT, were also associated with an increased risk of excessive surgical bleeding.61 Interestingly, in both studies, prophylactic platelet transfusion was not associated with a reduced incidence of peri-procedural hemorrhages; however, transfusions were given to patients with a higher bleeding risk, making it difficult to interpret these results. Indeed, the fact that the incidence of peri-procedural bleeding was not increased in the patients with a higher risk of bleeding suggests that prophylactic platelet transfusions were effective in reducing the rate of hemorrhages.
THPO-receptor agonists opened new prospects in the treatment of ITs because they provided a potential pharmacological option to increase platelet count of these patients for the first time. In a phase 2 clinical trial, eltrombopag was given for 3 to 6 weeks to 12 patients with MYH9-RD and a platelet count below 50 × 109/L. A total of 11 patients achieved significant platelet response and remission of spontaneous bleeding without relevant adverse events.62 On the basis of these findings, short-term eltrombopag was successfully used for preparing patients with MYH9-RD and severe thrombocytopenia for major surgery.63,64 Elective surgery prepared by short-term eltrombopag administration was also reported in 1 subject with ANKRD26-RT.65 More recently, a group from the United States investigated the effects of long-term administration of eltrombopag (22 weeks to 4 years) in 8 patients with WAS/X-linked thrombocytopenia and chronic or recurrent spontaneous bleeding. Five obtained a platelet response and durable remission or reduction of spontaneous bleeding and no major adverse events.66,67 Thus, eltrombopag represents an option for the control of bleeding in patients with WAS or X-linked thrombocytopenia who are not candidates to or waiting for HSCT. Differently from splenectomy (the other available option; see Table 3), eltrombopag is not expected to worsen the immunodeficiency typical of the diseases. Of note, the effect of long-term eltrombopag on the outcome of HSCT is still to be determined. Of course, THPO-receptor agonists may be proposed only in the forms of HT, in which platelet function is normal, or only mildly reduced, so that endogenous platelets can efficiently promote hemostasis.
HSCT is the treatment of choice in WAS and CAMT, and may be considered in selected patients affected with other ITs and presenting with severe clinical manifestations and/or poor prognosis.36,68,69 The median life expectancy of patients with WAS who are not transplanted is approximately 15 years, whereas HSCT can cure all the features of the disease. The 5-year overall survival of patients who underwent HSCT after the year 2000 was 100% for those transplanted from HLA-matched related siblings and 90% for HLA-matched unrelated or mismatched-related donor procedures.70,71 The outcome of HSCT remarkably improved over the last few years for both patients transplanted from mismatched family donors and those treated at age older than 5 years using an unrelated donor. Among the 63 patients with CAMT registered in the European Society for Blood and Marrow Transplantation database in the years 1987 to 2013, 30 were transplanted from matched family donors, 25 were transplanted from matched unrelated donors, and 8 were transplanted from mismatched donors. The 5-year overall survival was 77%, and transplant-related mortality was 12.6%, with no clear differences by age, year of transplant, HLA compatibility, or stem cell source.72 Gene therapy for correction of WASp protein deficiency represents an experimental option for patients affected by WAS who do not have a suitable donor for HSCT. In the first gene therapy clinical trial, treatment was complicated by severe vector-related genotoxicity.73 Two subsequent trials treated 11 patients using a second-generation lentiviral vector.67,74,75 Ten patients achieved good responses in terms of improvement of bleeding tendency and reconstitution of immune system functions, whereas 1 patient died from a preexisting infection. No evidence of treatment-related genotoxicity was found after a median follow-up of 30 months. Several trials of gene therapy in WAS are currently ongoing worldwide.
Summary
Over the last few years, the concept of IT has changed significantly. The major concern for some patients with IT is no longer bleeding but additional disorders that may develop during life and be hazardous for the patient’s life. A multidisciplinary approach may be required to reach the correct diagnosis of these predisposing forms and arrange the most appropriate treatment and follow-up.
Correspondence
Patrizia Noris, Department of Internal Medicine, Fondazione IRCCS Policlinico San Matteo, Piazzale Golgi, 27100 Pavia, Italy; e-mail: p.noris@smatteo.pv.it.
References
Competing Interests
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Author notes
Off-label drug use: Eltrombopag for increasing the platelet count in patients with inherited thrombocytopenias.