Abstract
Waldenström macroglobulinemia (WM) is a rare lymphoma with 1000 to 1500 new patients diagnosed per year in the United States. Patients with WM can experience prolonged survival times, which seem to have increased in the last decade, but relapse is inevitable. The identification of recurrent mutations in the MYD88 and CXCR4 genes has opened avenues of research to better understand and treat patients with WM. These developments are giving way to personalized treatment approaches for these patients, focusing on increasing depth and duration of response alongside lower toxicity rates. In the present document, we review the diagnostic differential, the clinical manifestations, and the pathological and genomic features of patients with WM. We also discuss the safety and efficacy data of alkylating agents, proteasome inhibitors, monoclonal antibodies, and Bruton tyrosine kinase inhibitors in patients with WM. Finally, we propose a genomically driven algorithm for the treatment of WM. The future of therapies for WM appears bright and hopeful, but we should be mindful of the cost-effectiveness and long-term toxicity of novel agents.
Learning Objectives
To understand recent advances on the biology of Waldenström macroglobulinemia
To review available and investigational agents for the treatment of patients with Waldenström macroglobulinemia
To propose a genomically driven algorithm for the management of patients with Waldenström macroglobulinemia
Introduction
Waldenström macroglobulinemia (WM) is a rare subtype of non-Hodgkin lymphoma, representing about 1% of all cases of non-Hodgkin lymphoma.1 According to the Surveillance, Epidemiology, and End Results database, approximately 1000 to 1500 new cases of WM are diagnosed every year in the United States.2 Although the survival in a large proportion of patients with WM can be measured in decades,3,4 WM remains incurable with current treatment options. Alkylating agents, nucleoside analogs, monoclonal antibodies, proteasome inhibitors, and Bruton tyrosine kinase (BTK) inhibitors can be used to treat patients with symptomatic WM.5 In the present article, we review the diagnostic criteria, clinicopathological features, and treatment options for WM in the light of recent developments on the genomic profiling of WM. We also provide our approach to the treatment of WM, acknowledging that the recommendations provided are based on the limited available prospective and retrospective data, as well as our clinical experience.
Diagnostic considerations
The differential diagnosis of WM includes immunoglobulin M (IgM) monoclonal gammopathy of undetermined significance; other IgM-secreting lymphomas, especially marginal zone lymphoma (MZL); and the rare IgM multiple myeloma (MM). The diagnosis of WM is established by the presence of lymphoplasmacytic lymphoma in the bone marrow and an IgM monoclonal paraproteinemia of any size. The diagnosis of WM can be further secured by detecting the MYD88 L265P gene mutation, either by allele-specific polymerase chain reaction or next-generation sequencing techniques. The MYD88 mutation can be identified in >90% of patients with WM, with a rate of wild-type MYD88 of <10%.6 About 5% of lymphoplasmacytic lymphoma cases can secrete immunoglobulin G or immunoglobulin A, free light chains, be biclonal, or be nonsecretory. MYD88 L265 mutations have also been identified in these cases, but they are not considered WM. The absence of MYD88 L265P gene mutations does not rule out WM, and a few WM patients can have non-L265P MYD88 mutations.7 IgM monoclonal gammopathy of undetermined significance typically does not show an abnormal lymphoplasmacytic infiltrate in the bone marrow.8 MZL can secrete IgM and can carry the MYD88 L265P gene mutation.6 MZL should be suspected if lymphadenopathy and/or splenomegaly is a prominent clinical feature. In the bone marrow biopsy, the presence of mast cells can help direct our suspicion toward WM instead of MZL.9 Finally, IgM MM cells typically express cyclin D1, and t(11;14) can be identified by fluorescence in situ hybridization cytogenetic studies.10 In addition, IgM MM can present with lytic bone lesions and renal dysfunction, which are rare in WM. MYD88 mutations have not been identified in IgM MM.
Clinical features and indications for treatment
In about half of the patients, the diagnosis of WM is made incidentally, because patients can be asymptomatic. In asymptomatic patients, watchful waiting is the appropriate course of action. In patients with symptomatic disease, treatment is indicated to improve the patient’s quality of life. The most common clinical manifestation of WM is anemia, which can be seen in 75% of patients with symptomatic WM. Anemia can be due to bone marrow replacement by WM, hemolysis, and iron-deficiency anemia.11 Other common causes of anemia should also be sought and evaluated appropriately. These include iron, cobalamin, or folate deficiency and renal or thyroid dysfunction. Peripheral neuropathy is seen in about 20% of patients with WM. IgM-mediated neuropathy typically is distal, is sensory, and has demyelinating features, although in some cases axonal processes can be associated.12 Anti–myelin-associated glycoprotein and anti-GM1 antibodies can be identified in about half of patients with demyelinating disease. Other common causes of neuropathy should also be investigated, including diabetes, thyroid dysfunction, amyloidosis, HIV infection, and Lyme disease.11,12 Symptomatic hyperviscosity due to high serum IgM levels affects 15% of patients with WM.13 Symptomatic hyperviscosity should be suspected in patients with serum IgM >3000 mg/dL. Symptoms include recurrent nosebleeds, headaches, blurred vision, or mentation changes without other potential explanation. Funduscopic examination should be pursued to identify patients with silent hyperviscosity, which can be manifested by engorgement, increased tortuosity or sausaging of retinal vessels, or retinal hemorrhages. In patients with signs and/or symptoms of hyperviscosity, plasmapheresis should be started promptly as a temporary control measure, which should be followed by more definitive therapy. Other less common clinical features of WM include thrombocytopenia, neutropenia, symptomatic lymphadenopathy or splenomegaly, cryoglobulinemia, amyloidosis, or extramedullary involvement.11 Extramedullary manifestations of WM involvement include renal dysfunction, malignant pleural effusions, and central nervous system involvement.14-17 In all cases, ruling out other causes for the presenting symptoms is strongly recommended, as well as histological confirmation of WM in extramedullary sites. Current recommendations for initiation of treatment are shown in Table 1. Rarely, WM can transform into the more aggressive diffuse large B-cell lymphoma.18,19 In these cases, the approach should mimic the standard diagnostic and treatment algorithm for patients with de novo diffuse large B-cell lymphoma.
Current criteria for initiation of treatment in patients with WM
| Hemoglobin ≤10 g/dL on basis of disease |
| Platelet count <100 K/μL on basis of disease |
| Constitutional symptoms in setting of disease progression |
| Symptomatic hyperviscosity |
| Moderate/severe peripheral neuropathy |
| Symptomatic extramedullary disease (lymphadenopathy, hepatosplenomegaly, renal involvement, pleural effusions, Bing-Neel syndrome, etc) |
| Symptomatic cryoglobulins, cold agglutinins, amyloidosis |
| Hemoglobin ≤10 g/dL on basis of disease |
| Platelet count <100 K/μL on basis of disease |
| Constitutional symptoms in setting of disease progression |
| Symptomatic hyperviscosity |
| Moderate/severe peripheral neuropathy |
| Symptomatic extramedullary disease (lymphadenopathy, hepatosplenomegaly, renal involvement, pleural effusions, Bing-Neel syndrome, etc) |
| Symptomatic cryoglobulins, cold agglutinins, amyloidosis |
Pathological and genomic features
The underlying malignant process in WM includes clonally related B cells, lymphoplasmacytic cells, and plasma cells. The B-cell compartment expresses bright CD20, CD19, and CD22 and does not express CD5, CD10, and CD23.8 The plasma cell compartment express plasma cell markers such as CD38 and CD138. Both B-cell and plasma cell compartments are restricted for the same light chain, and in MYD88 L265P mutated cases, all compartments carry the MYD88 L265P gene mutation.6 A common cytogenetic abnormality associated with WM is del6q, which is observed in up to half of the patients.20 However, it does not have a diagnostic or prognostic value. Other less common cytogenetic abnormalities, such as del13q, del11q, and trisomy 4, do not appear to have clinical significance.20
The identification of the MYD88 L265P gene mutation in >90% of patients has been a major development in WM, because it can have diagnostic, therapeutic, and prognostic implications.21-25 MYD88 is a toll-like receptor, which undergoes homodimerization upon activation. In WM, the mutated MYD88 is constitutively activated.26 The MYD88 homodimer serves as the base for assembly of the Myddosome, which includes IRAK1/4 and BTK, among others.27 The activation of IRAK1/4 and BTK can independently generate downstream activation of NF-κB, promoting cell survival and downregulating apoptosis. The MYD88 L265P mutation can be identified by allele-specific polymerase chain reaction techniques with a high sensitivity.28 Mutations in the CXCR4 gene, similar to the ones congenitally seen in patients with warts, hypogammaglobulinemia, infection, and myelokathexis syndrome, have been detected in 40% of patients with WM.29 In WM patients, mutated CXCR4 results in loss of regulatory serines, allowing the g-protein-coupled receptor to remain intact and CXCR4 not to internalize. The fully active CXCR4 signals through g-protein and β-arrestins, resulting in activation of phosphatidylinositol 3-kinase, AKT, mitogen-activated protein kinases, and extracellular signal-regulated kinase 1/2 and providing an alternative pathway for cellular activation and survival.27 More than 30 CXCR4 mutations have been identified and can be frameshift or nonsense. Given the heterogeneity of CXCR4 mutations, these are best detected by means of Sanger sequencing or next-generation sequencing techniques, which can represent an obstacle to wide clinical use in the community. In addition, a low burden of disease in the marrow can decrease the sensitivity of the test, increasing the rate of false-negative results. Clinically, patients with CXCR4 mutations seem to have lower rates of lymphadenopathy and higher levels of serum IgM. CXCR4 mutations, however, have not been associated with worse survival outcomes.21 Other less common mutations have been identified in ARID1A, CD79A/B, and TP53, and their clinical value is under investigation.27
Treatment options
In the frontline setting, there are prospective studies supporting the use of alkylating agents and proteasome inhibitors, both in combination with rituximab. In a subset analysis of a randomized study with about 20 patients in each arm, the combination of bendamustine and rituximab (benda-R) was associated with longer progression-free survival than standard rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone.30 The overall response rate (ORR) and complete response (CR) rate to benda-R have been reported in a retrospective study at 90% and 20%, respectively.31 Adverse events are manageable and include cytopenias, rash, nausea, and constipation. The combination of bortezomib, dexamethasone, and rituximab (BDR) or of bortezomib and rituximab has been evaluated in 3 separate single-arm studies involving more than 100 patients with untreated WM.32-34 The ORR and CR rate have ranged from 90% to 95% and 5% to 15%, respectively. The median progression-free survival (PFS) time has ranged from 40 to 60 months. The main adverse event is neuropathy, which can be reduced by using weekly dosing and subcutaneous administration of bortezomib. Prospective data also exist on the combination of carfilzomib, rituximab, and dexamethasone (CARD) and of cyclophosphamide, dexamethasone, and rituximab (CDR).35-37 CARD was associated with an ORR of 87% and a CR rate of 3% with a median PFS that was not reached at 15 months. Adverse events associated with CARD include hypogammaglobulinemia, hyperglycemia, and hyperlipasemia. CDR induced an ORR of 80% and a CR rate of 7% with a median PFS of 3 years. Adverse events associated with CDR include nausea and cytopenias.
Ibrutinib was approved by the US Food and Drug Administration to treat symptomatic WM patients regardless of previous exposure to therapy.38 In Europe, the use of ibrutinib was approved in the relapsed or refractory setting and as a first-line therapy in patients who are not candidates for chemoimmunotherapy. The frontline use of ibrutinib is also endorsed by the current guidelines of the National Comprehensive Cancer Network (NCCN).39 A single-arm study in previously untreated WM patients has completed accrual (NCT02604511). Two separate single-arm prospective studies including almost 100 patients have reported on the safety and efficacy of ibrutinib in relapsed or refractory WM.22,40 In the study by Treon et al,22 ibrutinib was administered as a single agent to 63 patients with symptomatic WM and was associated with an ORR of 91%. Ibrutinib was administered to patients as young as 44 years of age. No CR was observed. The median time to response was 4 weeks. Patients with CXCR4 mutations had a lower ORR at 86% versus 100% in patients without CXCR4 mutations. The rate of major response (at least partial response) was also lower in CXCR4 mutated patients than in CXCR4 wild-type patients (91% and 62%, respectively). The 2-year PFS rate was 69%. CXCR4 mutations did not seem to affect time to response or PFS, but follow-up was short. The study by Dimopoulos et al40 evaluated ibrutinib in 31 patients with relapsed or refractory WM who were refractory to rituximab. The ORR and major response rates were 90% and 71%, respectively. The ORR in CXCR4 mutated and wild-type patients was 88% and 100%, and the rates of major response were 82% and 71%, respectively. The 18-month PFS was 86%, or 94% and 86% in CXCR4 mutated and wild-type patients, respectively. Adverse events associated with ibrutinib include cytopenias, mucocutaneous bleeding, and atrial fibrillation. Acquired mutations in the BTK gene can promote resistance to ibrutinib in WM patients.41 Based on these studies, ibrutinib should be taken indefinitely until disease progression or unacceptable toxicity.
Any of the frontline options can be used in patients with relapsed disease. In some scenarios, the same treatment can be repeated in cases in which a PFS longer than 2 years was achieved the first time around. Other treatment options in relapsed or refractory WM patients include the nucleoside analogs fludarabine and cladribine,42-45 the immunomodulators thalidomide and lenalidomide,46,47 and the mammalian target of rapamycin inhibitor everolimus.48-50 However, the benefit/toxicity ratio is less favorable with these agents. Ofatumumab can be used in the occasional WM patient who develops rituximab intolerance.51,52 A prospective study evaluating the phosphatidylinositol 3-kinase inhibitor idelalisib in relapsed or refractory WM patients was stopped early due to severe liver toxicity.53
Several prospective studies, some of them including WM patients, support an improvement in PFS with maintenance rituximab in patients with low-grade lymphomas.54-57 A retrospective study evaluated the value of rituximab maintenance in WM patients after rituximab-containing regimens.58 The maintenance rituximab group had a longer median PFS than the patients who were placed on observation. Therefore, maintenance rituximab can be considered in selected cases, as supported by NCCN guidelines. We do not favor maintenance rituximab in patients who have not responded to chemoimmunotherapy, in patients with severe hypogammaglobulinemia, or in patients with rituximab intolerance. Randomized studies of maintenance rituximab versus observation in WM patients are ongoing (NCT00877214 and NCT01461928). The role of hematopoietic stem cell transplantation (SCT) has yet to be defined. Most data on the use of autologous SCT in WM derive from retrospective, registry-based studies without a comparator group.59,60 Autologous SCT can be considered in younger patients who have been treated with multiple lines of therapy or those patients with primary refractory disease who have been previously exposed to BTK inhibitors. Allogeneic SCT has been associated with some efficacy, although with a high rate of nonrelapse mortality, and should be evaluated properly in the setting of well-designed clinical trials.61 A consensus position on the role of SCT in WM is being discussed.
There are myriad novel agents undergoing clinical development in WM. The oral proteasome inhibitors ixazomib and oprozomib,62,63 the second-generation BTK inhibitors acalabrutinib and BGB-3111 (NCT02180724 and NCT03053440), the BCL2 inhibitor venetoclax (NCT02677324), the anti-CD38 monoclonal antibody daratumumab, and the anti-CXCR4 monoclonal antibody ulocuplumab are selected examples of upcoming potentially practice-changing interventions.
Genomically driven approach to treatment in WM
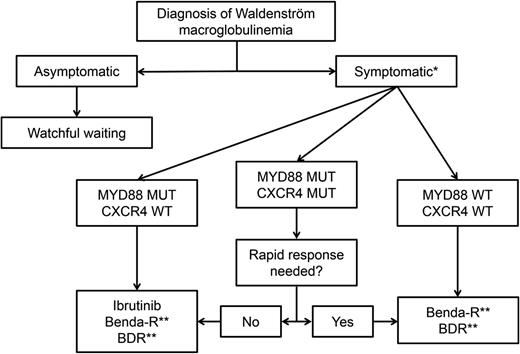
There are several treatment options for patients with symptomatic WM, and treatment selection can be daunting. Patients’ clinical and genomic features, preferences, and comorbidities, as well as the efficacy and toxicity profile of the regimen to be used, can guide the treatment. Therefore, the treatment of WM patients is highly personalized. See Figure 1 for a proposed algorithm.
Suggested genomic-driven treatment algorithm in patients with WM. *In patients with hyperviscosity, plasmapheresis should be instituted urgently. Single-agent rituximab can be considered in patients with a low burden of disease. Rituximab can induce a temporary but potentially significant IgM flare. **Maintenance therapy can be considered in patients who have responded to chemoimmunotherapy. MUT, mutated; WT, wild type.
Suggested genomic-driven treatment algorithm in patients with WM. *In patients with hyperviscosity, plasmapheresis should be instituted urgently. Single-agent rituximab can be considered in patients with a low burden of disease. Rituximab can induce a temporary but potentially significant IgM flare. **Maintenance therapy can be considered in patients who have responded to chemoimmunotherapy. MUT, mutated; WT, wild type.
For patients with symptomatic hyperviscosity, cryoglobulinemia, cold agglutinemia, and in some cases of neuropathy, plasmapheresis should be initiated promptly for a fast serum IgM reduction, but only to be followed by more definitive therapy.
Nearly all patients with WM seen at our center undergo MYD88 and CXCR4 genotyping. Patients can then be divided into 3 genomic groups: the MYD88-only group, the double-mutated (MYD88 and CXCR4) group, and the wild-type MYD88 group. This classification of patients has been shown to be prognostic, because wild-type MYD88 WM patients (5% to 10% of all cases) tend to have worse overall survival and PFS, as well as more superficial and shorter responses to ibrutinib, than patients in the other 2 groups.21,22,40 At this time, we are recommending that all patients with WM have their MYD88 mutational status tested, if possible. We do not recommend, however, routine CXCR4 mutational testing.
For the treatment of MYD88-only WM patients, ibrutinib is our drug of choice, if available, as long as there are no contraindications and it matches the patient’s preference. Although there are no comparative studies, the response rate to single-agent ibrutinib (∼90%) is similar to that of benda-R, BDR, and CDR with the convenience of oral administration and a manageable adverse event profile. The use of ibrutinib in the frontline and relapsed settings is supported by current NCCN guidelines. In patients in whom ibrutinib is not an ideal option, we use BDR or benda-R. We tend to recommend BDR for patients younger than 65 years to avoid the small risk of secondary leukemia associated with chemotherapy. In patients older than 65 years or in patients in whom neuropathy is of concern, we recommend benda-R. In older patients, we sometimes recommend lower doses of bendamustine and, in some cases, 4 instead of 6 cycles of induction. There are emerging data supporting that lower doses or fewer cycles of bendamustine have not been associated with lower response rates in WM patients.31 In patients responding to rituximab-containing regimens, we consider maintenance therapy if there is no evidence of symptomatic hypogammaglobulinemia, recurrent respiratory infections, or rituximab intolerance. Patients who received BDR can continue with BDR as maintenance if toxicity is not an issue. Patients who received benda-R can continue with maintenance rituximab as a single agent, if indicated.
CXCR4 mutations are not a contraindication for ibrutinib therapy. For double-mutant WM patients, ibrutinib remains our drug of choice, if available. However, we should take into consideration that the response to ibrutinib could be delayed in these patients. Therefore, if immediate response is needed, then BDR and benda-R are better options, with the same considerations as in MYD88-only patients. No data to date support that CXCR4 mutational status affects response to BDR or benda-R. CXCR4 mutations were associated with a longer time to response in 20 patients receiving an ixazomib-containing regimen.62
For MYD88 wild-type WM patients, BDR or benda-R is our treatment of choice. Ibrutinib is not the preferred option given the low rates of deep response, as well a shorter duration of response.22,40 In our practice, we evaluate for the presence of rare non-L265P MYD88 mutations using gene sequencing, because patients who carry these non-L265P mutations can respond to ibrutinib.7
Conclusion
WM is a rare and incurable disease. However, with an increased understanding of the underlying biology of WM, a genomically driven approach to therapy can be delineated. Although recurrent mutations in MYD88 and CXCR4 genes shape the landscape of WM, multiple other mutations have been identified, which represents not only a challenge but also an opportunity to develop personalized therapeutic strategies. In addition, with novel agents under clinical development, deeper and longer responses with lower toxicity rates are expected. We should be mindful, however, of the increasing cost of novel medications, as well as potential long-term safety issues. We remain hopeful that future cost-effective treatments will translate into further improvements in survival and quality of life for patients with WM.
Correspondence
Jorge J. Castillo, 450 Brookline Ave, Mayer 221, Boston, MA 02215; e-mail: jorgej_castillo@dfci.harvard.edu.
References
Competing Interests
Conflict-of-interest disclosure: J.J.C. has received research funds and honoraria from Abbvie, Gilead, Janssen, Millennium, and Pharmacyclics. S.P.T. has received research funds and honoraria from Janssen and Pharmacyclics.
Author notes
Off-label drug use: Cyclophosphamide, bendamustine, bortezomib, carfilzomib, ixazomib, rituximab, ofatumumab, thalidomide, lenalidomide, everolimus, and idelalisib are not approved drugs by the US Food and Drug Administration or European Medicines Agency for the treatment of WM.