Abstract
A 70-year-old man with relapsed/refractory chronic lymphocytic leukemia has multiple comorbidities including atrial fibrillation (on warfarin for anticoagulation), irritable bowel syndrome, and chronic renal insufficiency. Two years ago, he received bendamustine and rituximab as first-line therapy for chronic lymphocytic leukemia and achieved partial response, but now has relapsed. Fluorescence in situ hybridization cytogenetics reveals deletion 17p. Which novel agent would you recommend for this patient?
Learning Objectives
To understand the key toxicities of the three recently FDA-approved small molecule novel agents for the treatment of CLL
To learn how to individualize treatment plans based on the distinct safety profiles of these novel agents
Introduction
Since February 2014, the US Food and Drug Administration has approved 3 small molecule novel agents for the treatment of chronic lymphocytic leukemia (CLL): ibrutinib, idelalisib, and venetoclax. Each agent has a unique toxicity profile that can lead to unique clinical challenges. Further complicating the decision-making process, the treatment indications for these agents overlap substantially. The patient described in the abstract has relapsed/refractory CLL, deletion 17p, and multiple comorbidities, making him potentially eligible for therapy with any of these three small molecules, but also posing a dilemma as to which agent would best balance efficacy and safety.
Understanding the safety profiles of novel agent therapies in CLL is an important factor in making treatment recommendations. First, CLL is a disease of older adults. Particularly in this patient population, balancing quality of life and treatment efficacy is critical. Second, these novel agents typically require continuous therapy, which raises concerns for long-term toxicity. Consequences of long-term therapy may remain unclear for many years and unknown until mature results from large-scale trials become available. Third, growing evidence supports that dose adherence affects durability of response. As little as 8 consecutive days of ibrutinib interruption was associated with inferior progression-free survival in 1 study.1 Early recognition of toxicity and utilization of supportive care can maximize adherence to novel agents. Table 1 summarizes adverse events reported from 10 selected prospective trials using novel agents either alone or in combination with anti-CD20 monoclonal antibody therapy in CLL.
Summary of key adverse events related to targeted agents studied in CLL
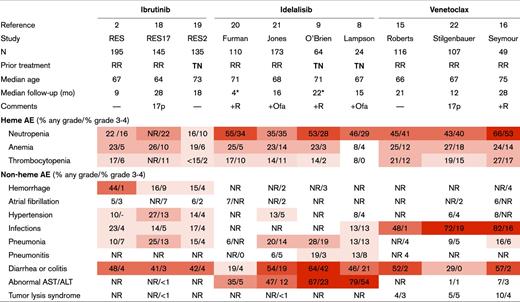
Darker red indicates a higher incidence of any grade AE. 17p, patients with deletion 17p; AE, adverse event; TN, treatment-naïve; RR, relapsed/refractory disease; +Ofa, combination therapy with ofatumumab; +R, combination therapy with rituximab; NR, not reported.
Median time on study treatment. Median follow-up was not reported.
Toxicities specific to ibrutinib
Bruton tyrosine kinase (BTK) inhibition by ibrutinib increases the risk of bleeding, leading to decreased platelet aggregation and adhesion. Most of the bleeding events observed in clinical trials were limited to grade 1 or 2. Fatal bleeding, such as intracranial hemorrhage, occurred in up to 6% in early studies and less frequently in later studies.2 US prescribing information recommends withholding ibrutinib for 3 to 7 days before and after surgical procedures. Patients on warfarin were excluded from most trials of ibrutinib, but safety data with novel oral anticoagulants and low-molecular-weight heparins provide reassurance that anticoagulation is not a contraindication to ibrutinib therapy. Major bleeding was uncommon (2%) in a study of 115 ibrutinib-treated patients on concomitant anticoagulants or antiplatelet agents.3
Based on a pooled analysis of 4 randomized trials for ibrutinib, the incidence of new or recurrent atrial fibrillation (afib) is 3.3 per 100 person-years.4 The impact of new or recurrent episodes of afib is usually clinically significant because it often requires the initiation of anticoagulation, thereby increasing the bleeding risk on ibrutinib. New or symptomatic afib in patients taking ibrutinib should prompt consideration of seeking expert consultation with a cardiologist. Further evaluation to rule out ischemia and structural causes of arrhythmia may be warranted. Ibrutinib is typically held until the need for cardioversion and anticoagulation is clarified. The mechanism of afib is thought to be related to inhibition of BTK and TEC kinase expressed in cardiac tissue.5 BTK inhibitors with more specific BTK inhibition and less TEC inhibition are now in clinical development. Acalabrutinib is one such second-generation BTK inhibitor and was tested in 61 CLL patients in a phase 1-2 study.6 None of the patients in this study developed afib in the median follow-up of 14 months.
Toxicities specific to idelalisib
The key toxicities with idelalisib are thought to be immune-mediated. Idelalisib targets the p110δ subunit of phosphoinositide 3-kinase, expressed in malignant B cells as well as regulatory T cells. On-target inactivation of regulatory T cells may unleash pro-inflammatory cells that can infiltrate essential organs and lead to diarrhea/colitis, hepatotoxicity, and pneumonitis.7 The rates of these toxicities are higher in previously untreated CLL patients compared with those with relapsed/refractory disease.8 Inflammatory side effects can limit the duration of idelalisib therapy and lead to high rates of idelalisib discontinuation for toxicity (27% to 45%).9
Diarrhea is the most common side effect of idelalisib, and can be divided into 2 types. Low-grade diarrhea typically presents early in the treatment course (generally in the first 2 months) and generally resolves with supportive care using dietary modifications and/or antimotility agents. Serious diarrhea (grade ≥3) occurs in up to 20% of patients, usually has a delayed onset (generally 7 months or later into treatment), and can be hemorrhagic. Biopsy of the affected colon characteristically shows lymphocytic infiltrates in crypts and epithelial mucosa that are predominantly CD8+ T cells.10 When managing diarrhea in patients on idelalisib, it is important to rule out infection, hold idelalisib for grade 3-4 events, and consider adding enteric or systemic steroids. The addition of steroids can shorten the duration of severe diarrhea from 1 month to 1 to 2 weeks.11 Idelalisib can be resumed at a lower dose when diarrhea improves to grade 1 or lower. However, retreatment is successful in only about one-half of the patients (58%).11 When selecting idelalisib for treatment of CLL, prescribers should carefully evaluate for a history of other gastrointestinal diseases.
Presentations of idelalisib hepatotoxicity can widely vary from asymptomatic elevation of transaminases (up to 67%, any grade) to high-grade events (up to 23%, grade 3-4 in relapsed/refractory patients). Because hepatotoxicity generally occurs within 12 weeks of initiating idelalisib, serum transaminases should be closely monitored during this time frame. Idelalisib should be held for grade 2 or higher increase of serum transaminases (>5× the upper limit of normal), and permanently stopped for a grade 4 event (>20× the upper limit of normal). Transaminase elevation is reversible in almost all cases with simple discontinuation of idelalisib. Steroids can be used in cases that do not improve quickly with holding the drug alone. In a study of idelalisib in previously untreated patients, all cases of transaminitis resolved after initiation of steroids.8 Rechallenge with a lower dose of idelalisib is usually well-tolerated even in patients who had grade ≥3 hepatotoxicity (92% are successfully rechallenged).9
Pneumonitis is a relatively uncommon adverse event (4%), which can be life-threatening in a small number of cases (<0.5%).11 Autopsy of 2 patients who died from idelalisib-induced pneumonitis in early studies revealed findings consistent with pulmonary hypersensitivity, suggesting pulmonary function can deteriorate rapidly and that rechallenging of idelalisib may be unsafe even after recovery. In practice, it is challenging to make a confirmatory diagnosis of drug-induced pneumonitis because its clinical and radiographic presentations overlap with respiratory infections. Patients with pulmonary symptoms, such as cough, dyspnea, hypoxia, interstitial infiltrates, and >5% decline in oxygen saturation, should raise a concern for pneumonitis and be evaluated further. It is important to rule out treatable, infectious causes of pulmonary infiltrates because the clinical implications of lung infection are drastically different from that of pneumonitis. Specifically, patients with pneumonia can be rechallenged with idelalisib after recovery, whereas symptomatic pneumonitis (grade ≥2) will usually require permanent discontinuation of idelalisib. The role of steroids for treatment of idelalisib-induced pneumonitis is unclear. Anecdotally, rapid resolution of symptoms with steroids was reported,12 whereas others achieved improvement with idelalisib interruption alone.9
Idelalisib may increase the risk of serious and fatal infections in CLL patients. In a randomized trial for idelalisib combined with conventional chemoimmunotherapy, the idelalisib arm had higher rates of grade ≥3 infections (39%) and treatment-related deaths (11%) than the placebo arm (25% and 7%, respectively).13 Pathogens were not limited to typical viruses and bacteria but also included atypical organisms related to opportunistic infections (ie, Pneumocystis jirovecii). Atypical respiratory infection is not sensitively captured with noninvasive tests; thus, bronchoscopy has an important role in this setting. Increased risk of infection in patients treated with idelalisib led to termination of several trials using idelalisib as first-line therapy in CLL.
Toxicities specific to venetoclax
The B-cell leukemia/lymphoma-2 inhibitor venetoclax carries a risk of tumor lysis syndrome (TLS) during treatment initiation. A phase 1 study of venetoclax started with venetoclax dosed at 200 or 100 mg/day, and laboratory TLS was observed in the first 3 patients treated.14 Despite protocol modifications, there was a death from TLS in 1 patient and acute renal failure requiring dialysis in another patient early on during the phase 1 venetoclax study,15 with a second death from TLS observed early in a phase 1b study of venetoclax with rituximab.16 This led to incorporation of a 5-week ramp-up dosing schedule, starting from 20 mg/day followed by weekly intrapatient dose ramp-up to 400 mg/day. During the ramp-up phase, moderate or strong CYP3A inhibitors should be avoided because they interact with venetoclax. All patients receiving venetoclax should start TLS prophylaxis before initiating therapy. Patients with high tumor burden or reduced creatinine clearance (<80 mL/min) should receive a more intensive approach including oral and intravenous hydration, antihyperuricemics, hospitalization at the initiation of 20- and 50-mg doses, and frequent monitoring of blood chemistry. Patients with clinical TLS (acute renal insufficiency, symptomatic hypocalcemia, or cardiac dysrhythmia) should be managed intensively, with aggressive intravenous hydration, correction of abnormal electrolytes, rasburicase, and monitoring of cardiac function, neuromuscular irritability, and urine output.
B-cell leukemia/lymphoma-2 inhibition affects the survival of myeloid progenitors, leading to neutropenia. Absolute neutrophil counts frequently drop below 1000/μL in patients receiving venetoclax (40%-53%). Venetoclax should be held for febrile neutropenia and for grade 4 neutropenia that persists for more than 1 week despite growth factor support. However, dose interruption or reduction because of neutropenia was uncommon (4%-5%), and grade 3-4 infections were relatively rare.15 Trials have variably used prophylactic antibiotics, and there is no consensus for using antibiotics in these patients. Another common side effect of venetoclax is gastrointestinal toxicity, which is typically low grade but affects up to one-half of the patients on therapy. These events usually present as low-grade nausea and diarrhea that are self-limited.
Conclusions
There is no “right” or “wrong” answer to the question posted in the abstract, which is designed to highlight that novel agent treatment recommendations should be individualized for each CLL patient. Based on available literature, we cannot recommend 1 treatment over others for this particular patient; rather, treatment selection should take into account patient characteristics, values, and other factors such as health care coverage. A thorough understanding of the toxicity profile of each drug is crucial to educating patients and helping them to recognize and report new symptoms early. Because the key side effects of the novel agents are largely mutually exclusive, combination or sequencing of multiple agents may be able to achieve deep response (ie, minimal residual disease) without compromising safety and allow time-limited therapy instead of continuous therapy. Several clinical trials are already under way to evaluate such combinations. Moreover, novel agents with more selective target inhibition (eg, acalabrutinib for BTK,6 umbralisib (TGR-1202) for phosphoinositide 3-kinase δ17 ) are in late-stage clinical development and may have more favorable safety profiles compared with the first-generation compounds. Ongoing studies with these and other agents will address how to balance the efficacy and safety of novel agents to optimize treatment of patients with CLL.
Acknowledgments
I.E.A. is supported by the intramural program of the National Heart, Lung, and Blood Institute, National Institutes of Health.
Correspondence
Matthew S. Davids, Department of Medical Oncology, Dana-Farber Cancer Institute, 450 Brookline Ave, Boston, MA 02215; e-mail: matthew_davids@dfci.harvard.edu.
References
Competing Interests
Conflict-of-interest disclosure: I.E.A. declares no competing financial interests. M.S.D. is on the Board of Directors or an advisory committee for TG Therapeutics, Genentech, Pharmacyclics, Janssen, Abbvie, Gilead, and InCyte; has received research funding from TG Therapeutics, Genentech, Pharmacyclics, and Infinity and has consulted for Janssen, TG Therapeutics, Genentech, Astra-Zeneca, Merck, Celgene, Abbvie, and Pharmacyclics.
Author notes
Off-label drug use: None disclosed.
