Abstract
Most people with mantle cell lymphoma (MCL) present with diffuse adenopathy and benefit from early initiation of rituximab and high-dose cytarabine- or bendamustine-based therapies. Some patients, however, present with primarily nonnodal disease that can follow either an indolent or a rapidly progressive, treatment-resistant clinical course. Rarely, patients present with explosive disease that can be challenging to manage and often involves the central nervous system. New agents with improved therapeutic indices facilitate treatment while maintaining quality of life, but also present new complications at the time of treatment failure. Although uncommon presentations are not new to clinicians who treat MCL, the increasing clarity of underlying biology and prognostic implications may help us develop more specialized treatment strategies.
Learning Objectives
Identify patients with unique presentations of mantle cell lymphoma that may not benefit from standard therapies
Develop a treatment approach for patients with unique presentations of mantle cell lymphoma
Introduction
Over the past decade, several trials have demonstrated that many patients with mantle cell lymphoma (MCL) can be treated effectively in the frontline setting with rituximab and high-dose cytarabine- or bendamustine-based therapies.1-3 Recent guidelines rooted in evidence-based medicine provide a useful framework to help clinicians diagnose and manage most cases of MCL,4,5 but some patients have a disease biology or presentation that is underrepresented in prospective interventional trials (Table 1). As clinicians, our first challenge is to recognize these uncommon presentations. Our second and greater challenge is to determine whether standard, evidence-based recommendations should apply to every patient. It is becoming increasingly clear that the primary driver of long-term outcomes in MCL is not the choice of therapy but rather a combination of disease biology and patient-related factors. In confronting the challenges associated with management of uncommon presentations of MCL, it is important to appreciate that although we may have limited ability to change long-term outcomes, our decisions have significant implications with respect to how people with MCL experience life.
Uncommon presentations of MCL that may be underrepresented in prospective interventional clinical trials
| Type of MCL . | Presentation . |
|---|---|
| Indolent | May present with leukemic, nonnodal MCL and wild-type TP53 or low-tumor-burden–typical MCL with low Ki-67 expression, simple karyotype, normal LDH, and lack of B symptoms. There is no clear disadvantage to deferred therapy. |
| Leukemic, nonnodal | Presents with minimal adenopathy but splenomegaly and lymphocytosis, which is frequently IGHV mutated/SOX11-negative. Mutated TP53 predicts poor prognosis. |
| Super-high risk | Key predictive factors include an elevated MIPI score and mutated TP53. Other features typically include blastoid histology and high Ki-67 expression. Prognosis is poor despite intensive therapy. |
| High risk for CNS involvement | Blastoid histology and high Ki-67 expression are the most commonly reported risk factors. The role of prophylaxis is unclear. |
| Type of MCL . | Presentation . |
|---|---|
| Indolent | May present with leukemic, nonnodal MCL and wild-type TP53 or low-tumor-burden–typical MCL with low Ki-67 expression, simple karyotype, normal LDH, and lack of B symptoms. There is no clear disadvantage to deferred therapy. |
| Leukemic, nonnodal | Presents with minimal adenopathy but splenomegaly and lymphocytosis, which is frequently IGHV mutated/SOX11-negative. Mutated TP53 predicts poor prognosis. |
| Super-high risk | Key predictive factors include an elevated MIPI score and mutated TP53. Other features typically include blastoid histology and high Ki-67 expression. Prognosis is poor despite intensive therapy. |
| High risk for CNS involvement | Blastoid histology and high Ki-67 expression are the most commonly reported risk factors. The role of prophylaxis is unclear. |
LDH, lactate dehydrogenase.
Some patients with MCL may defer initial therapy
Most patients with MCL begin treatment shortly after diagnosis. The reasons for starting treatment are varied but familiar to physicians who treat indolent lymphomas (eg, ongoing or impending disease-related symptoms, progressive or bulky adenopathy, cytopenias, and/or organ dysfunction). For the ∼10% to 30% of patients who do not have clear indications for treatment, several studies have demonstrated there is no disadvantage to deferring initial therapy.6-9 On average, patients who defer initial therapy wait approximately 1 year before starting treatment, and it could be argued that these patients do not really benefit from an initial period of observation other than having time to research treatment options. For many patients, some of whom might be observed for a decade or more, there is clear value in deferring onset of treatment-related adverse effects while waiting for new options to become available.
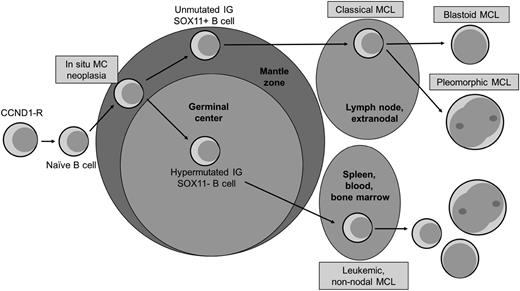
Patients with more indolent variants of MCL tend to present in 2 distinct ways (Figure 1). One group tends to have a leukemic, nonnodal MCL with splenomegaly (the so-called chronic lymphocytic leukemia [CLL]-like presentation), whereas the other presents with diffuse adenopathy, low tumor burden, and a slow rate of progression. The first group is characterized by a mutated immunoglobulin heavy chain variable region (IGHV), a κ light chain predominance, a simple karyotype, and a lack of SOX11 expression; it may also be mistaken for CLL, particularly in cases where cyclin D1 staining is negative and fluorescence in situ hybridization for the t(11,14) is not done.10 Patients who present with leukemic, nonnodal MCL are frequently excluded from clinical trials, given the lack of measurable lymphadenopathy and challenges associated with response assessment. The second group has a more typical MCL pathobiology, a simple karyotype, and low Ki-67 expression.10 Because of interlaboratory variability in reporting Ki-67 expression, it is difficult to recommend a threshold above which indolent behavior is unlikely, although 30% is frequently used. Interestingly, the Mantle Cell International Prognostic Index (MIPI) has not been especially helpful in identifying indolent presentations, perhaps because age and white blood cell count can drive up the MIPI score without reflecting underlying indolent presentation. In retrospective series, the absence of tumor bulk, blastoid histology, elevated lactate dehydrogenase level, Ki-67 index >30%, and B symptoms was associated with indolent presentation,6,9 making indolent presentation a diagnosis of exclusion.
Model of progression of mantle cell lymphoma. Adapted with permission from Swerdlow et al.44 Original illustration by Patrick Lane.
Model of progression of mantle cell lymphoma. Adapted with permission from Swerdlow et al.44 Original illustration by Patrick Lane.
It is worth noting that although retrospective series have found no clear negative impact of deferred therapy on patient survival, it is feasible that early initiation of appropriate therapy could improve outcomes. Without prospective identification and study of these presentations, our ability to practice truly evidence-based medicine remains limited.
Meanwhile, clinicians should discuss the pros and cons of observation with patients who do not have clear indications to start therapy at the time of diagnosis. When possible, these patients should be enrolled in clinical registries such as the Lymphoma Epidemiology Outcomes study (NCT02736357).
Some patients with leukemic nonnodal MCL do not have indolent disease
Despite its reputation as an indolent disease, many leukemic, nonnodal MCLs also harbor mutations in the TP53 gene, and akin to TP53-mutated CLL/small lymphocytic leukemia, these patients appear to have a disease that is less responsive to standard chemotherapy. In 1 series of IGHV-mutated SOX11-negative treated with chemotherapy, 14 patients with a wild-type TP53 had a 5-year overall survival (OS) of 92% compared with 36% among the 7 patients with a TP53 mutation (P = .003).11,12 In another series, TP53 mutations occurred at a similar frequency (∼30%) regardless of SOX11 status and were more prevalent in patients with 17p anomalies, but they were also found in cases without 17p alteration.13 Together, these series suggest that TP53 aberrations are common in MCL cases that might otherwise be assumed to have an indolent course, and that these mutations are associated with a poor outcome.
Although several groups have evaluated the prognostic impact of TP53 mutations in typical MCL treated in the context of prospective, interventional clinical trials, prospectively collected data in TP53-mutated nonnodal MCL are lacking, owing to the rarity of the presentation and the exclusion of nonnodal cases from interventional trials in MCL. Therefore, although there is a general impression that standard regimens may not provide satisfactory results, there is little guidance regarding how best to treat these patients. In the absence of prospective data, the National Comprehensive Cancer Network guidelines are limited to the suggestion that these patients be treated similarly to those with nodal disease in need of therapy. Others clinicians are suspicious that these patients should be treated similarly to TP53-mutated CLL/small lymphocytic leukemia, where nonchemotherapy approaches are clearly superior. When feasible, these patients should be enrolled in clinical trials based on novel strategies (eg, lenalidomide or ibrutinib), as well as prospective registries. Outside of clinical trials, there is little to no guidance based on evidence for clinicians and patients, but off-label use of newer agents may be reasonable under certain circumstances. Younger patients may benefit from early referral to a transplant center to initiate a discussion of allogeneic stem cell transplantation in the setting of progressive disease.
Some patients with MCL have poor outcomes despite intensive induction/consolidation regimens
Several prospective studies have conclusively demonstrated that younger, fit patients treated with a rituximab and high-dose cytarabine–containing induction followed by autologous stem cell transplantation experience an average time to progression exceeding 7 years.1,2 Despite this remarkable improvement over historical standards, it appears that a subset of patients who receive intensive therapy do not share in the success. Most clinicians will recognize these patients as those with blastoid histology, but recent research may allow us to better identify this subset.
In an analysis of patients treated in the European MCL Network Younger and Elderly trials,14 3 key variables known to be associated with prognosis were evaluated: growth pattern (ie, mantle zone, nodular, and diffuse), morphology (ie, classical, small cell, pleomorphic, or blastic), and Ki-67 expression. One caveat to their analysis was that the Ki-67 index was counted by a single pathologist blinded for outcome, but published guidelines regarding Ki-67 are available.15 In a multivariate analysis that included the 3 variables and the MIPI score, Ki-67 index >30% but not growth pattern or morphology retained its prognostic significance (hazard ratio of 1.44 for OS and 1.34 for progression-free survival [PFS]). The prognostic effect of the combination of MIPI score and level of Ki-67 expression was significant in the European MCL Network Younger and Elderly trials (Table 2).14 Among the 6% of patients with a high combined MIPI and Ki-67 expression score treated in the European MCL Network Younger trial, the median OS was ∼2 years. Investigators from the Nordic Lymphoma Group evaluated the combination of Ki-67 expression and MIPI in a long-term evaluation of the Second Nordic Mantle Cell Lymphoma trial and found them to be similarly prognostic.16 In a subsequent analysis of the mutational status of ATM, CCND1, TP53, KMT2D, NOTCH1, NOTCH2, WHSC1, and BIRC3 among 73 patients treated on the Second Nordic Mantle Cell Lymphoma Trial (MCL2) or the Third Nordic Mantle Cell Lymphoma Trial (MCL3), investigators found that ∼10% of patients had TP53 mutations and that these mutations (especially missense mutations) were associated with worse OS and PFS in a multivariate analysis that included Ki-67 expression.17 In preliminary results from the Italian MCL0208 trial, investigators again found that ∼10% of analyzed patient samples harbored a mutation in TP53 and that such mutations were associated with a 2-year PFS of only 45% compared with 83% among patients with wild-type TP53 and KMT2D genes.18
The proportion of patients, PFS, and OS, according to the MIPI-c from the European MCL Network Younger and Elderly Trials
| Original MIPI . | Ki-67 index, % . | MIPI-c score . | Percentage . | Median PFS, y . | Median OS, y . |
|---|---|---|---|---|---|
| Low risk | <30 | Low risk | 32 | 7.4 | Not reached |
| >30 | Low-intermediate risk | 34 | 4.4 | Not reached | |
| Intermediate risk | <30 | ||||
| >30 | High-intermediate risk | 23 | 2.0 | 4.3 | |
| High risk | <30 | ||||
| >30 | High risk | 11 | 1 | 1.5 |
| Original MIPI . | Ki-67 index, % . | MIPI-c score . | Percentage . | Median PFS, y . | Median OS, y . |
|---|---|---|---|---|---|
| Low risk | <30 | Low risk | 32 | 7.4 | Not reached |
| >30 | Low-intermediate risk | 34 | 4.4 | Not reached | |
| Intermediate risk | <30 | ||||
| >30 | High-intermediate risk | 23 | 2.0 | 4.3 | |
| High risk | <30 | ||||
| >30 | High risk | 11 | 1 | 1.5 |
Adapted from Hoster et al14 with additional data provided by Dr. Hoster.
MIPI-c, combined Mantle Cell International Prognostic Index.
The optimal method for evaluation of TP53 aberrations is not clear. Although immunohistochemistry for p53 and fluorescent in situ hybridization for TP53 are the most common tests used clinically, at least 1 series has suggested that TP53 mutation detected by polymerase chain reaction had prognostic significance, whereas the more common 17p deletions were clinically insignificant.19 These data highlight the profound differences that continue to separate the clinical world from the research world and will need to be addressed in prospective trials.
Finally, investigators from the Lymphoma/Leukemia Molecular Profiling Group have developed and validated a NanoString-based proliferation assay (NanoString Technologies, Seattle, WA) that appears to discriminate among 3 different risk groups independent of the MIPI score.20 These results are important because they suggest that clinicians can use readily available tools (eg, MIPI and the Ki-67 index) and increasingly available sequencing analyses to predict outcomes following intensive therapy. This knowledge may also spark an open discussion with patients before initiating therapy, because some may opt for alternative strategies when faced with the likelihood of a short remission despite several months of aggressive treatment.
Although we may now be equipped to identify high-risk biology, there are limited data to suggest that alternative strategies might provide better outcomes. In the phase 3 LYM-3002 trial, the addition of bortezomib to first-line chemotherapy least benefited the group with a high-risk MIPIb score (ie, a combination of MIPI score and level of Ki-67 expression).21 Similarly, a large retrospective French series reported that bortezomib did not overcome the poor prognostic risk of 17p deletion in patients with multiple myeloma.22 Long-term follow-up from the phase 2 EMERGE (MCL-002) study, which evaluated single-agent lenalidomide in patients refractory to bortezomib, found that level of Ki-67 expression retained its prognostic value.23 In the phase 2 PHILEMON (MCL6) study from the Nordic Lymphoma Group, which evaluated the combination of rituximab, lenalidomide, and ibrutinib in previously treated MCL, there did not appear to be any association between TP53 mutational status and PFS.24 Moreover, TP53 copy number variation did not appear to be associated with response to palbociclib plus ibrutinib in preliminary results from a phase 1 trial.25 However, blastoid histology was associated with significantly worse outcomes in patients with previously treated MCL who were enrolled in the phase 3 RAY (MCL3001) trial, which compared ibrutinib to temsirolimus.26 Until more data become available, it is highly recommended that such patients be referred for clinical trials that include combination therapy in which at least 1 drug is a novel agent. Although data with maintenance therapy specific to the high-risk subgroup are lacking, it may be reasonable to continue a chronic suppressive therapy while waiting for development of new options.
A retrospective analysis of Center for International Blood and Marrow Transplant Research data reported that among 50 patients who underwent allogeneic stem cell transplantation after 1 or 2 prior lines of therapy (35 were in complete remission and 15 had primary refractory disease at the time of transplantation), the nonrelapse mortality rate at 1 year was 25%, and the 5-year survival rate was 61%.27 It is not known what effect variables like MIPI, Ki-67 expression, or TP53 mutation status might have in this setting. Thus, it is difficult to recommend allotransplantation for all high-risk patients, but it may be worth considering in selected cases.
Some patients with MCL are at high risk of central nervous system involvement
Although it is uncommon for patients with MCL to present with central nervous system (CNS) involvement at the time of diagnosis, like diffuse large B-cell lymphoma (DLBCL), progression in the CNS after primary therapy does occur.28-30 Compared with DLBCL, however, the time to CNS progression is a later event, typically occurring in ∼5% by 1 to 2 years after primary therapy and continuing to rise in incidence thereafter, and it is predominantly leptomeningeal disease as opposed to parenchymal lesions. Like DLBCL, more aggressive biology is associated with increased risk, with blastoid histology, or high Ki-67 expression being the most reproducible risk factors, and outcomes after diagnosis of CNS disease are poor, with average survivals reported as 4 to 8 months. Based on these data, it may be reasonable to screen for CNS involvement in high-risk patients and to consider prophylactic intrathecal therapy. It should be noted, however, that in retrospective series, primary therapy that included drugs with potential activity against CNS lymphoma, including rituximab, high-dose cytarabine, methotrexate, and autologous stem cell transplantation, there was no apparent protective effect. It is unclear whether rituximab maintenance, or novel agents with activity in CNS lymphomas, such as lenalidomide or ibrutinib, should be incorporated in patients at high risk. Phase 3 trials that have included 1 arm with these drugs might be best equipped to evaluate their effect on CNS relapse.
Information regarding management of MCL with CNS involvement is limited to small series where the heterogeneous approaches are reflective of differences in presentation, prior therapies, and comorbid conditions, including the frequent presence of systemic lymphoma in addition to CNS disease. Given the known sensitivity of MCL to high-dose cytarabine and methotrexate, younger or fitter patients might be managed with a combination of the 2 with or without the addition of thiotepa (eg, the MATRix regimen31 ), with the advantage over methotrexate alone being that the cytarabine may help treat systemic disease as well. Responding patients might be candidates for autologous or allogeneic stem cell transplantation, although there are few data to support these procedures. More commonly, patients will either have already received these drugs or will not be candidates for intensive approaches. Recently reported prospective clinical trials have demonstrated the activity of lenalidomide and ibrutinib in primary CNS lymphoma,32-35 and at least 1 retrospective series36 has suggested that ibrutinib can produce responses in patients with MCL and CNS disease. Given the low probability of durable remissions in patients with blastoid MCL, it may be appropriate to select better-tolerated treatments with the aim of maximizing quality of life.
Most patients with MCL have poor outcomes after ibrutinib failure
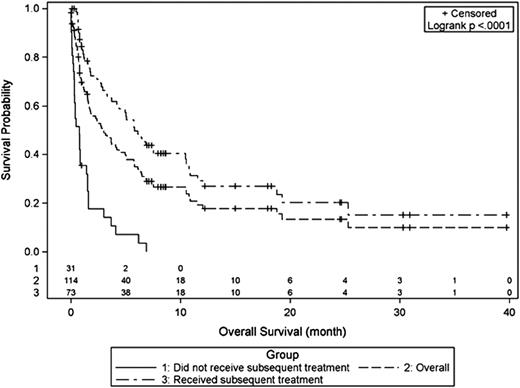
Multiple retrospective series have demonstrated that outcomes after ibrutinib failure are disappointing, with an average OS of ∼3 months (Figure 2).37-39 The interpretation of these data must take into account the characteristics of patients included in these series. One international, multicenter report included 114 patients, many of whom had a high-risk MIPI score, with a median of 3 prior therapies, a 50% response rate to ibrutinib, and a median duration of ibrutinib treatment of only 4.7 months.37 One-quarter of patients received no subsequent therapy and the survival of those who did was only 6 months, perhaps reflective of the fact that these patients were already resistant to other available agents before starting ibrutinib.37 Interestingly, a subsequent multicenter report included 97 patients with a median of 2 prior therapies, a response rate to ibrutinib of 63%, and a median response duration of 17 months, and again found a median OS of 2.5 months, with a median OS of 5 months among those who initially responded to ibrutinib.39 In the prospective, randomized phase 3 RAY (MCL3001) trial comparing ibrutinib with temsirolimus in 280 patients with previously treated MCL, a median of 2 prior therapies, and a median follow-up of 20 months, the median PFS in the ibrutinib arm was 14.6 months.26 With 44 patients undergoing post-ibrutinib therapy, the median PFS2 (ie, time from start of ibrutinib to progression or death after first post-ibrutinib therapy) was 19.1 months.26 Collectively, these data suggest that patients with fewer prior therapies and those who are responsive to ibrutinib can achieve meaningful responses to post-ibrutinib therapy, but it remains unclear why outcomes tend to be poor.
Several groups have described potential mechanisms of resistance to ibrutinib,40,41 and it is likely that more will be identified. Ideally, knowledge of disease biology and the availability of new drugs will facilitate post-ibrutinib treatment selection, but, for now, clinicians must rely on experience and limited data to make the best possible decisions. Unfortunately, there is little evidence from existing series that any given treatment is more effective than any other regimen.37,39 A multicenter, retrospective analysis of lenalidomide after ibrutinib failure or intolerance suggested that approximately one-third of patients respond to lenalidomide or lenalidomide-based treatment. Although the median duration of lenalidomide was only 2 cycles, responses appeared to be durable, a feature also noted in other lenalidomide trials.42 A subsequent report suggested that the combination of dexamethasone, rituximab, lenalidomide, and bortezomib was active in this setting.43 Importantly, both series reported low rates of treatment-emergent toxicity, which is particularly relevant to a population being treated for mainly palliative purposes. Patients who have highly treatment-resistant disease (to both chemotherapy and ibrutinib) appear to have poor outcomes after ibrutinib failure, and clinicians need to carefully consider whether intensive therapy and its associated toxicity is consistent with a palliative approach. The same considerations come into play for older or frail individuals who may not tolerate aggressive approaches. Fitter patients may best be served by participating in a clinical trial, particularly those that include chimeric antigen receptor T cells. Similarly, patients at high risk of early ibrutinib failure may wish to consider allogeneic stem cell transplantation or chimeric antigen receptor T-cell trials while responding to ibrutinib.
Conclusion
The diversity of MCL biology is striking. Differences between IGHV-mutated, SOX11-negative nonnodal MCL; and IGHV-unmutated, SOX11-positive nodal MCL, as well as variation in proliferation rates and mutations in TP53 contribute to heterogeneous presentations and clinical courses. Standard therapies are improving our ability to balance attempts to extend survival with minimization of disease and treatment-related symptoms for most patients. However, patients with uncommon presentations continue to struggle with divergent treatment recommendations based on limited, often retrospective data. Advances in pathology and broader recognition of the heterogeneity will facilitate prospective study in observational and interventional clinical trials. Physicians should be cognizant of the clinical relevance of different presentations and engage in open discussions about the uncertainty that persists.
Correspondence
Peter Martin, Department of Medicine, Division of Hematology-Medical Oncology, Weill Cornell Medicine, 1305 York Ave, New York, NY 10009; e-mail: pem9019@med.cornell.edu.
References
Competing Interests
Conflict-of-interest disclosure: The author has consulted for Janssen, Celgene, Gilead, Bayer, Genentech, Verastem, Novartis, AstraZeneca, Janssen, KiTE, and Seattle Genetics.
Author notes
Off-label drug use: This presentation includes discussion of off-label use of lenalidomide and ibrutinib. Both drugs are approved for previously treated mantle cell lymphoma but will be discussed in the context of previously untreated disease.