Abstract
The introduction of new drugs with less severe toxicity profiles than those of conventional antimyeloma agents allowed the evaluation of continuous therapy compared with fixed duration therapy. In transplant-eligible patients, consolidation therapy with bortezomib or bortezomib-based regimens showed significant progression-free survival (PFS) benefit in cytogenetic standard-risk patients and to a lesser extent, high-risk patients. Continuous therapy with lenalidomide maintenance treatment after autologous stem cell transplantation resulted in a significant survival gain. In transplant noneligible patients, continuous lenalidomide-dexamethasone therapy improved survival over fixed duration melphalan-prednisone-thalidomide. The concept of prolonged treatment in elderly patients is supported by some other studies, but most of them revealed a gain in PFS only. Young patients with unfavorable prognosis show a greater willingness to accept long-term treatment, whereas the readiness to undergo such treatments and the benefits therefrom decline with increasing age and decreasing fitness, rendering fixed duration therapy a suitable option in elderly frail patients.
Learning Objectives
To understand the benefits and limitations of fixed duration and continuous therapy
To understand selection of treatment of patients with different characteristics such as age, cytogenetic risk, frailty, comorbidities, and other risk factors
Introduction
The introduction of novel drugs and treatment concepts resulted in a marked increase in survival of patients with multiple myeloma, but in spite of significant improvement, only a fraction of patients will remain in continuous remission and can be considered as operationally cured.1 The majority will relapse after variable periods of time after initiation of induction therapy. Given these facts, it becomes clear that deepening our understanding of the pathophysiology of the disease, including the pathways driving clonal evolution, progression, and resistance, is key for the development of more rational therapies.2 Because the outcome of research and the timelines of new advances are not predicable, we are left with optimizing treatment with the armamentarium available today. One of the options for gaining important improvements is to optimize treatment duration.
Historical development
Traditionally, treatment of multiple myeloma was administered for a defined number of cycles, because long-term treatment was limited because of accumulating toxicity, including secondary myelodysplastic syndrome and acute myeloid leukemia. Moreover, a comparison between long-term continuous therapy with intermittent melphalan dosing did not reveal a benefit compared with the former approach.3 Maintenance with corticosteroids revealed conflicting results in 2 independent studies,4,5 and steroids have not been accepted as single agents for long-term therapy. Interferon showed an increase in progression-free survival (PFS) in most and in overall survival in several studies. Two meta-analyses reported survival benefits of 4 and 6 months,6,7 respectively, but because of toxicity problems in a relevant proportion of patients, lack of predictive factors for clinical benefit without major toxicity and lastly, the introduction of new drugs limited its inclusion into clinical practice.
Continuous and fixed duration therapy
The classic definition of continuous therapy is the prolonged administration of the same induction regimen or a part of it as opposed to a fixed number of cycles (fixed duration therapy), and continuous therapy has been evaluated in several clinical situations in both transplant-eligible (TE) and transplant-noneligible (TNE) patients. In TE patients, continuous therapy refers to maintenance treatment after autologous transplantation. Adding a consolidation after transplantation extends the strategy of short treatment intervention by usually 2 to 4 months of additional chemotherapy and therefore, will also briefly be discussed. In TNE patients, different concepts of continuous therapy have been studied: one fulfilling the true meaning of continuous, with treatment administered until progression or intolerance; another applying a less stringent definition, with treatment given for prolonged but still limited time; and a third one using maintenance therapy after induction treatment.
Aims and challenges of fixed duration and continuous therapy
Myeloma therapy aims to control the “Darwinian nature” of multiple myeloma, where evolving myeloma subclones become increasingly more independent from their bone marrow environment and more resistant to antimyeloma therapy. Ideally, continuous therapy should deepen the response to the best possible category, namely minimal residual disease negative (MRDneg), and maintain results for as long as possible.
The heterogeneity of the molecular characteristics of myeloma clones among individual patients imposes major hurdles to success of a uniform myeloma treatment strategy. Therefore, novel myeloma therapies have preferentially improved survival in patients with standard-risk disease defined by molecular profiling or fluorescence in situ hybridization, whereas in those with high-risk disease, advances in therapy still lag behind. Given this complexity, it becomes clear that neither fixed duration nor continuous therapy will satisfy the modern paradigm of individualized therapy in every patient.
Desired characteristics of drugs suited for continuous therapy
The ideal drug or drug combination should be orally administrable, be well tolerated, exert a direct antimyeloma effect, and result in favorable modulation of bone marrow stroma compartments. Drugs that inhibit osteoclast activity and angiogenesis, induce T-cell and osteoblast activity, and suppress myeloid-derived and other suppressor cells seem best suited for continuous therapy. Table 1 lists the relevant features of drugs presently used for long-term therapy.
Drugs presently used for prolonged therapy, including comments on their tolerance profile, activity in high-risk disease, effect on stroma compartments, synergistic activity, and route of administration
| Antimyeloma drug . | Factors potentially limiting feasibility of continuous therapy . | Activity in high-risk disease . | Impact on stroma . | Synergistic activity with . | Route of administration . |
|---|---|---|---|---|---|
| Dexamethasone | Hormonal side effects, psychological side effects | Limited evidence | Immunosuppressive | IMiDs and PIs | Oral |
| Thalidomide | Neuropathy | Reduces PFS and OS | Negative (shorter PFS after previous thalidomide exposure) | Dexamethasone | Oral |
| Lenalidomide | Second primary malignancies, hematological side effects | Some but less active than PI | Enhances several immune functions | PIs, dexamethasone, and MoAb | Oral |
| Bortezomib | Neuropathy, hematological and GI side effects, reactivation of latent viral infections | Yes | Inhibition of osteoclasts | IMiDs, dexamethasone, and MoAb | SC |
| Carfilzomib | Cardiac events, hematological side effects | Yes | Data n.a. but a class effect likely | IMiDs, dexamethasone, and MoAb | IV |
| Ixazomib | Few hematological and other side effects | Yes | Data n.a. but a class effect likely | IMiDs, dexamethasone and MoAb | Oral |
| Elotuzumab | Uncommon, usually grade 1 and 2 infusion reactions | Yes | Activates ADCC | IMiDs and PIs | IV |
| Daratumumab | Infusion reactions in ∼50% | Yes | Activates several immune functions | IMiDs and PIs | IV |
| Antimyeloma drug . | Factors potentially limiting feasibility of continuous therapy . | Activity in high-risk disease . | Impact on stroma . | Synergistic activity with . | Route of administration . |
|---|---|---|---|---|---|
| Dexamethasone | Hormonal side effects, psychological side effects | Limited evidence | Immunosuppressive | IMiDs and PIs | Oral |
| Thalidomide | Neuropathy | Reduces PFS and OS | Negative (shorter PFS after previous thalidomide exposure) | Dexamethasone | Oral |
| Lenalidomide | Second primary malignancies, hematological side effects | Some but less active than PI | Enhances several immune functions | PIs, dexamethasone, and MoAb | Oral |
| Bortezomib | Neuropathy, hematological and GI side effects, reactivation of latent viral infections | Yes | Inhibition of osteoclasts | IMiDs, dexamethasone, and MoAb | SC |
| Carfilzomib | Cardiac events, hematological side effects | Yes | Data n.a. but a class effect likely | IMiDs, dexamethasone, and MoAb | IV |
| Ixazomib | Few hematological and other side effects | Yes | Data n.a. but a class effect likely | IMiDs, dexamethasone and MoAb | Oral |
| Elotuzumab | Uncommon, usually grade 1 and 2 infusion reactions | Yes | Activates ADCC | IMiDs and PIs | IV |
| Daratumumab | Infusion reactions in ∼50% | Yes | Activates several immune functions | IMiDs and PIs | IV |
ADCC, antibody-dependent cytotoxicity; GI, gastrointestinal; IMiD, immunomodulatory agent; MoAB, monoclonal antibodies; n.a, not available; PI, proteasome inhibitor; SC, subcutaneous administration.
Patient characteristics, their willingness to accept continuous therapy, and benefit of therapy
Patient characteristics, including age, fitness and comorbidities, disease biology, and disease manifestations, are the major factors determining the selection of appropriate therapy. Willingness to accept continuous therapy is a prerequisite for prolonged treatment and depends on various factors, such as age, social situation, and aggressiveness of the disease.8 Younger patients with aggressive disease and good social relations usually are significantly more willing to accept the burden of prolonged therapy compared with single elderly patients, whose readiness to undergo lengthy therapy usually is quite limited (Figure 1).
Patient and disease characteristics, their willingness to accept long-term treatment, and expected outcome. High-risk: t(4;14), t(14;16), t(14;20) del 17p, nonhyperdiploidy, and ampl1q21. Yes+ denotes benefit, and Yes+++ denotes greater benefit.
Patient and disease characteristics, their willingness to accept long-term treatment, and expected outcome. High-risk: t(4;14), t(14;16), t(14;20) del 17p, nonhyperdiploidy, and ampl1q21. Yes+ denotes benefit, and Yes+++ denotes greater benefit.
Clinical evidence in TE patients
Consolidation therapy
The first proof of principle for beneficial outcome of consolidation therapy came from Ladetto et al,9 who administered 4 cycles of bortezomib-thalidomide-dexamethasone (VTD) to patients who had achieved at least very good partial response (VGPR) after autologous stem cell transplant (ASCT). In addition, they applied real-time quantitative polymerase chain reaction with a sensitivity of 5 × 106 to capture patients achieving a deep response in the sense of MRDneg. Consolidation with VTD induced an additional depletion of 4.14 natural logarithms of tumor burden. The complete response (CR) rate increased from 15% before to 49% after consolidation therapy, and the rate of MRDneg increased from 3% to 19%. The survival rate after a median follow-up of 8 years was 72% for MRDneg patients compared with 42% in the MRDpos group (P < .041).10 Reappearance of MRDpos heralded relapse; the median time from change from an MRDneg to an MRDpos status to start of salvage therapy was 9 months. These results paved the way for MRDneg to become a new, important end point in clinical practice and clinical research.
Bortezomib single agent.
Mellqvist et al11 compared the results of consolidation with 20 IV injections of bortezomib given over 21 weeks with no consolidation. Consolidation therapy improved the depth of response and PFS, but a benefit was mainly seen in patients with less than VGPR after ASCT. Overall survival rate was 80% at 3 years in both groups.
Einsele et al12 compared 4 cycles of IV bortezomib with no additional therapy in 2 studies: one with an age limit of ≤60 years and the other with patients 60 to 75 years old. Consolidation therapy resulted in a higher rate of greater than or equal to VGPR and increased PFS, but overall survival was similar in both groups. A Cox multivariate analysis showed greater benefit of consolidation in patients with less than VGPR and a similar benefit in those with or without adverse cytogenetics.
A smaller study by Sezer et al13 on 104 patients who had achieved greater than or equal to partial response (PR) after single or double ASCT compared 4 cycles of bortezomib consolidation with observation. Numerically, bortezomib consolidation resulted in greater depth of response and longer PFS, but none of the differences were statistical significant; likewise, only a trend toward prolonged OS was noted. Toxicity of consolidation was similar in these 3 studies. Significantly more overall polyneuropathy and grade 3 polyneuropathy were observed with IV bortezomib consolidation. Other more frequent complications were diarrhea, nausea, and vomiting. Up to 15% of patients discontinued consolidation therapy because of toxicities.
VTD.
Cavo et al14 compared consolidation with 2 cycles VTD with 2 cycles of thalidomide-dexamethasone (TD) consolidation therapy in 476 patients who received 3 cycles of the same regimen as induction therapy followed by tandem transplantation. This design limits a clear comparison between both consolidation regimens. After consolidation, CR and CR/near CR (nCR) rates were significantly higher for VTD-treated patients. After a median follow-up of 30.4 months from start of consolidation, the PFS rate at 3 years was significantly longer for the VTD group, but no difference in overall survival was noted. Grade 2 or 3 peripheral neuropathy was more frequent with VTD compared with the TD consolidation (8.1% vs 2.4%).
The Intergroupe Francophone du Myélome (IFM) group retrospectively compared 121 patients who underwent 3 cycles of VTD induction followed by 2 cycles of the same regimen as consolidation after ASCT with 96 patients matched by baseline characteristics who underwent only VTD induction and ASCT without consolidation.15 Consolidation therapy resulted in a significantly higher CR rate (52% vs 30%) compared with the no consolidation group.
Revlimid, bortezomib, and dexamethasone.
The EMN02/HO98 Trial evaluated the impact of 2 cycles of Velcade-Revlimid-dexamethasone (VRD) consolidation after induction with 4 cycles Velcade-cyclophosphamide-dexamethasone (VCD) followed by either 1 or 2 ASCT or by 4 cycles Velcade-melphalan-prednisone (VMP)16 in 903 patients. After a follow-up period of 25 months, a significant increase in the depth of response was noted in the consolidation group, independent of prior induction therapy. The PFS rate at 3 years increased from 60% to 65%. Consolidation did not improve PFS in patients with high-risk cytogenetics and overall survival. Toxicity from VRD was limited with 5% Common Terminology Criteria for Adverse Events (CTCAE) grade 4, mainly hematologic.
Preliminary results of the Blood and Marrow Transplant Clinical Trials New York 0702.
The Stem Cell Transplant in Myeloma Incorporating Novel Agents (StaMINA) Trial failed to show a benefit of consolidation therapy.17 This study randomized 758 patients within 12 months of initiating induction therapy into 3 arms: (1) ASCT and consolidation with 4 cycles of VRD, (2) ASCT 2 times, and (3) ASCT only. Lenalidomide maintenance for 3 years was planned for all patients. After a median follow-up of 38 months, no differences in either PFS or overall survival (OS) were noted. This is the only trial that failed to show a benefit of consolidation therapy. Because patients could have received induction therapy for up to 1 year, it remains unresolved whether a prolonged induction therapy may, in fact, obviate the need of consolidation therapy.
Bortezomib, cyclophosphamide, and dexamethasone.
In the Myeloma XI Study, a “response adapted” approach was evaluated.18 Patients were initially treated with either cyclophosphamide-Revlimid-dexamethasone (CRD) or cyclophosphamide-thalidomide-dexamethasone (CTD) for 4 (TE) or 6 cycles (TNE) and to maximum response. A total of 581 patients with suboptimal response (minor response (MR)/PR) were randomized to either additional induction therapy with bortezomib, cyclophosphamide and dexamethasone (CVD), or no additional therapy. Additional therapy with CVD (median of 4 cycles, range of 1 to 8 cycles) resulted in upgrading of response from MR/PR to VGPR/CR by 41%, which was seen in both pathways and was not affected by the immunomodulatory agent received in the initial induction randomization. PFS was significantly improved with additional CVD therapy from a median of 24 to 30 months, which was largely caused by improvements in the TE pathway (55 vs 31 months), whereas in the TNE pathway, a statistically nonsignificant increase in PFS was noted after longer follow-up only (14 vs 20 months). CVD improved PFS in patients with cytogenetic high-risk disease. Treatment was well tolerated, and relevant grade 3/4 toxicities were neutropenia (7.1%), thrombocytopenia (7.5%), anemia (3.1%), and peripheral neuropathy (5.1%).
Which TE patients should receive consolidation therapy?
Consolidation therapy should be offered to patients with short induction therapy (4 to 6 cycles or fewer), suboptimal response after ASCT (less than VGPR), and standard- and high-risk cytogenetics, although for the latter group, conflicting results have been reported. The presently available data show a significant improvement in the following parameters: depth of response, rate of MRD negativity, and PFS. Until now, a survival benefit has not been observed.
Consolidation with single or multiple agents
Randomized comparisons between single-agent bortezomib, VCD, and VRD are not available, precluding a statistically reliable comparison between these treatments. Differences in the PFS and hazard ratios of individual trials are shown in Table 2.
Phase 2 and phase 3 trials evaluating the role of consolidation therapy after ASCT
| References . | Age (median), y; no. of patients . | Induction therapy . | Consolidation therapy . | Improvement in depth of response . | EFS or PFS* . | OS* . | Comments . | |
|---|---|---|---|---|---|---|---|---|
| Ladetto et al9 | 59 | VAD 4 cycles, single ASCT | VTD | CR: 15%-49% | MRDneg: 68 mo | OS at 8 y | Clear benefit of consolidation therapy and long-term MRD monitoring in MM patients | |
| Ferrero et al10 | N = 39 | MRDneg: 3%-19% | MRDpos: 23 mo (P < .001) | MRDneg: 72%; MRDpos: 42% (P < .041) | ||||
| Cavo et al14 | 57.4; N = 160 | VTD; double ASCT | VTD × 2 | CR: 60.6%; CR/nCR 73.1% | PFS at 3 y: 60%; PFS: median not reached | No difference in OS recorded | No difference in outcome in patients with and without high-risk cytogenetics | |
| 56.8; N = 161 | TD; double ASCT | TD × 2 | CR: 46.6% (P = .012); CR/nCR: 60.9% (P = .020) | PFS at 3 y: 48% (P = .042); PFS: 32 mo | ||||
| Mellqvist et al11 | 59.1; N = 187 | Cyclophosphamide/dexamethasone or high-dose dexamethasone in the majority of patients | 20 Doses of bortezomib given during 21 wk | VGPR: 71%; CR/nCR: 45% | 27 mo | OS at 3 y 80%, no difference between both groups | ||
| Control; 58.7; N = 183 | Single ASCT | None | VGPR: 57% (P < .01); CR/nCR 35 (P = .055) | 20 mo (P = .05) | ||||
| Stadtmauer et al17 | 57; N = 254 | Different regimes | VRD × 4 | n.a. | PFS at 38 mo; 57% | No difference in OS recorded | Largest randomized US transplant trial in MM, showed comparable PFS and OS | |
| N = 257 | Single ASCT | None | PFS at 38 mo; 52% | |||||
| Jackson et al18 | N = 292 | CTD/CTDa | CVD × 4 | From MR/PR to VGPR/CR: 41% | PFS: 30 mo | n.a. | Greater PFS benefit in TE patients | |
| N = 289 | CRD/CRDa | n.a. | PFS: 24 mo | |||||
| Sonneveld et al41 | N = 459 | VCD × 4 plus either VMP or single or double ASCT | RVD × 2 | PFS from R2: 65% | No difference | Benefit seen in patients with revised ISS 3 but not in patients with high-risk cytogenetics | ||
| N = 444 | PFS from R2: 60% | |||||||
| Einsele et al12 | 59 | VCD, VD, VAD, AD, idarubicin/dexamethasone | Four 35-d cycles of bortezomib | Greater than or equal to VGPR: 62% | PFS: 33.6 mo | Similar in both groups | Greater benefit in patients with greater than or equal to VGPR, similar effect in standard- and high-risk patients; 15% of patients discontinued TX because of AE | |
| N = 371 | Single ASCT | VGPR: 48% (P = .003) | PFS: 27.8 mo (P = .0058) | (P = .75) | ||||
| Sezer et al13 | 58.0; N = 51 | Bortezomib based in majority of patients | Four 35-d cycles of bortezomib | sCR/CR: 22%, greater than or equal to VGPR: 80% | 44.9 mo | 40.1 mo | No statistically significant differences | |
| Control: 57.0; N = 53 | Single or double ASCT | None | sCR/CR: 11%; greater than or equal to VGPR: 68% | 21.8 mo | 36.6 mo (P = .098) | |||
| References . | Age (median), y; no. of patients . | Induction therapy . | Consolidation therapy . | Improvement in depth of response . | EFS or PFS* . | OS* . | Comments . | |
|---|---|---|---|---|---|---|---|---|
| Ladetto et al9 | 59 | VAD 4 cycles, single ASCT | VTD | CR: 15%-49% | MRDneg: 68 mo | OS at 8 y | Clear benefit of consolidation therapy and long-term MRD monitoring in MM patients | |
| Ferrero et al10 | N = 39 | MRDneg: 3%-19% | MRDpos: 23 mo (P < .001) | MRDneg: 72%; MRDpos: 42% (P < .041) | ||||
| Cavo et al14 | 57.4; N = 160 | VTD; double ASCT | VTD × 2 | CR: 60.6%; CR/nCR 73.1% | PFS at 3 y: 60%; PFS: median not reached | No difference in OS recorded | No difference in outcome in patients with and without high-risk cytogenetics | |
| 56.8; N = 161 | TD; double ASCT | TD × 2 | CR: 46.6% (P = .012); CR/nCR: 60.9% (P = .020) | PFS at 3 y: 48% (P = .042); PFS: 32 mo | ||||
| Mellqvist et al11 | 59.1; N = 187 | Cyclophosphamide/dexamethasone or high-dose dexamethasone in the majority of patients | 20 Doses of bortezomib given during 21 wk | VGPR: 71%; CR/nCR: 45% | 27 mo | OS at 3 y 80%, no difference between both groups | ||
| Control; 58.7; N = 183 | Single ASCT | None | VGPR: 57% (P < .01); CR/nCR 35 (P = .055) | 20 mo (P = .05) | ||||
| Stadtmauer et al17 | 57; N = 254 | Different regimes | VRD × 4 | n.a. | PFS at 38 mo; 57% | No difference in OS recorded | Largest randomized US transplant trial in MM, showed comparable PFS and OS | |
| N = 257 | Single ASCT | None | PFS at 38 mo; 52% | |||||
| Jackson et al18 | N = 292 | CTD/CTDa | CVD × 4 | From MR/PR to VGPR/CR: 41% | PFS: 30 mo | n.a. | Greater PFS benefit in TE patients | |
| N = 289 | CRD/CRDa | n.a. | PFS: 24 mo | |||||
| Sonneveld et al41 | N = 459 | VCD × 4 plus either VMP or single or double ASCT | RVD × 2 | PFS from R2: 65% | No difference | Benefit seen in patients with revised ISS 3 but not in patients with high-risk cytogenetics | ||
| N = 444 | PFS from R2: 60% | |||||||
| Einsele et al12 | 59 | VCD, VD, VAD, AD, idarubicin/dexamethasone | Four 35-d cycles of bortezomib | Greater than or equal to VGPR: 62% | PFS: 33.6 mo | Similar in both groups | Greater benefit in patients with greater than or equal to VGPR, similar effect in standard- and high-risk patients; 15% of patients discontinued TX because of AE | |
| N = 371 | Single ASCT | VGPR: 48% (P = .003) | PFS: 27.8 mo (P = .0058) | (P = .75) | ||||
| Sezer et al13 | 58.0; N = 51 | Bortezomib based in majority of patients | Four 35-d cycles of bortezomib | sCR/CR: 22%, greater than or equal to VGPR: 80% | 44.9 mo | 40.1 mo | No statistically significant differences | |
| Control: 57.0; N = 53 | Single or double ASCT | None | sCR/CR: 11%; greater than or equal to VGPR: 68% | 21.8 mo | 36.6 mo (P = .098) | |||
AD, doxorubicin-dexamethasone; AE, adverse event; CRD, cyclophosphamide-Revlimid-dexamethasone; CRDa, same as CRD, but 6 cycles; CTD, cyclophosphamide-thalidomide-dexamethasone; CTDa, same as CTD, but 6 cycles; EFS, event-free survival; HR, hazard ratio; ISS, International Staging System; MM, multiple myeloma; MR, minor response; n.a., not available; nCR, near complete remission; R2, second relapse; RVD, Revlimid-Velcade-dexamethasone; TX, treatment; VAD, vincristine-doxorubicin-dexamethasone; VCD, Velcade-cyclophosphamide-dexamethasone; VD, Velcade-dexamethasone; VGPR, very good partial response.
Median unless otherwise stated.
Taken together, bortezomib consolidation improves PFS but not OS; patients with less than VGPR benefit most, whereas conflicting results have been reported for those with high-risk cytogenetics.
Continuous therapy after ASCT (maintenance)
Interferon.
Thalidomide.
The first newcomer in the series of novel drugs was thalidomide and therefore, the next logical candidate to be studied as maintenance therapy after ASCT. Six prospective randomized trials have been reported; all of them showed significant improvement in PFS, with 2 studies also revealing a survival benefit.19 A meta-analysis including all 6 trials confirmed the PFS benefit, but a trend for better outcome was reported for survival only.20 Thalidomide was found to be active in patients with standard-risk cytogenetics, whereas in those with high-risk features, it was associated with shortened survival.21,22 Also, after relapse from thalidomide maintenance therapy, survival was shorter compared with that in untreated controls, indicating an untoward effect on the bone marrow stroma, clonal evolution, or both.21-23 Another limitation is the poor tolerance in some patients, particularly its neurotoxic side effect profile. Therefore, because of the introduction of better-tolerated drugs, thalidomide maintenance therapy generally is no longer used.
Lenalidomide.
The IFM group was the first to evaluate the impact of low-dose lenalidomide maintenance after ASCT, and they reported a significant increase in PFS but no improvement of OS.24 The study had been amended to limit the time of exposure to lenalidomide maintenance therapy to 2 years after it became clear that treatment induced a higher rate of secondary primary malignancies, thus limiting the median time on lenalidomide maintenance to 33 months in the entire group. The significant PFS benefit was subsequently confirmed by the Cancer and Leukemia Group B (CALGB)25 and the Groupo Italiano Malattie Ematologiche dell' Adulto (GIMEMA) group,26 but a significant improvement in OS was noted in the CALGB Trial only. A meta-analysis utilizing individual patient data showed a 26% reduction in the risk of mortality and a prolongation of the median overall survival by 2.5 years, establishing it as an important treatment standard.27 There are, however, a few limitations, because the 3 studies varied significantly in the induction therapy, and in the IFM Study, patients received 2 cycles of standard-dose Revlimid-dexamethasone (Rd) as consolidation therapy.
The benefit of lenalidomide maintenance therapy has now been confirmed by the Myeloma XI Study that included 828 patients.28 Lenalidomide maintenance therapy resulted in a significant increase in PFS (50 vs 28 months). Patients with high-risk cytogenetics did better with than without maintenance but worse than those with standard risk factors. Lenalidomide maintenance therapy is associated with side effects, most notably a moderately increased incidence in secondary primary malignancies, hematologic toxicity, infections, and thromboembolic complications. Still, the marked improvement of OS outweighs these complications, most of which can be managed by dose reduction and or symptomatic therapy.
Bortezomib.
The joint HOVON-65/GMMG-HD4 Trial randomized 827 patients to vincristine-doxorubicin-dexamethasone (VAD) or PAD induction followed by ASCT and maintenance with thalidomide in the former and bortezomib in the latter group29 ; 27% of patients on thalidomide and 47% on bortezomib completed the planned duration of 2 years of maintenance. Therapy led to an upgrade of response in 24% and 23% of patients on thalidomide or bortezomib maintenance, respectively. PFS was significantly longer in patients treated with PAD followed by bortezomib maintenance (35 vs 28 months), but OS rates at 5 years were similar (61% and 55%, respectively). The bortezomib group showed a significantly better PFS and OS in patients with creatinine levels of >2 mg/dL and/or deletion 17p13. Still, because of the differing induction regimens, results of maintenance are difficult to interpret. Peripheral neuropathy grade 3 to 4 developed in 8% of patients receiving thalidomide compared with 5% in the bortezomib maintenance group.
Bortezomib and thalidomide vs thalidomide vs interferon
The Programa para el Tratamiento de Hemopatías Malignas (PETHEMA) group compared VTD with TD and VBMCP/VBAD/B (vincristine, carmustine, melphalan, cyclophosphamide, prednisone/vincristine, carmustine, doxorubicin, dexamethasone/bortezomib) followed by ASCT.30 Thereafter, patients were randomized to maintenance for 3 years with interferon α-2b, thalidomide, or thalidomide plus bortezomib. The CR rate improved with maintenance by 21% with thalidomide plus bortezomib, 11% with thalidomide, and 17% with interferon α-2b (difference not significant). Maintenance therapy with thalidomide plus bortezomib yielded significantly longer PFS compared with thalidomide and interferon α-2b (50.6 vs 40.3 vs 32.5 months, respectively), but OS was similar between the 3 arms. Grade 2 to 3 peripheral neuropathy was observed in 48.8%, 34.4%, and 1% of patients treated with thalidomide plus bortezomib, thalidomide, and interferon α-2b, respectively.
Bortezomib-lenalidomide-dexamethasone
Nooka et al31 evaluated, in a phase 2 study, the efficacy of a combination of consolidation and maintenance with Revlimid-Velcade-dexamethasone (RVD) after ASCT in 46 high-risk patients defined as either cytogenetic (fluorescence in situ hybridization) or clinical high risk (primary plasma cell leukemia in 24%). RVD maintenance was given for up to 3 years, and single-agent lenalidomide was given thereafter. An stringent CR (sCR) was noted in 51% of patients, and 96% achieved greater than or equal to VGPR. Median PFS for all patients was 32 months, with a 3-year OS of 93%. The regimen was well tolerated, with no grade 3/4 neuropathy.
Carfilzomib, lenalidomide, and dexamethasone
A truly continuous therapy was investigated by the Chicago group.32 Treatment consisted of four 28-day cycles of carfilzomib, lenalidomide, and dexamethasone (KRd) induction followed by ASCT and 2 cycles of full-dose KRd; thereafter, it was given at reduced doses until cycle 18, after which single-agent lenalidomide was given. With continued therapy, deepening of response was noted, with an sCR rate of 20% post-ASCT to 69% after 4 cycles of KRd consolidation and 82% after 10 additional cycles of KRd maintenance. Similarly, MRD rates (next-generation sequencing [NGS]) increased from 66% by cycle 8 to 71% at the end of cycle 18. PFS and OS rates at 2 years were 97% and 99%, respectively. Of note, no differences in outcome were noted between high- and standard-risk patients. KRd was well tolerated; adverse events were mostly of grade 1/2. Most common grade 3/4 adverse events were lymphopenia (28%), neutropenia (18%), and infections (8%); 2 of 71 patients evaluated pretransplant had an asymptomatic decrease of ejection fraction.
Which patients should receive which type of continuous (maintenance) therapy after transplantation?
Continuous therapy should be offered to all patients after transplant. A recent meta-analysis confirmed that lenalidomide maintenance therapy confers a significant OS gain for almost all subgroups of patients, with the exception of patients with large tumor mass (stage 3) and/or high-risk cytogenetics.27 The Myeloma XI Study also noted a PFS benefit of lenalidomide maintenance therapy in patients with high-risk cytogenetics, albeit smaller than that in standard-risk patients.28 Addition of prednisone to lenalidomide did not improve outcome compared with single-agent lenalidomide.33
Bortezomib-based therapy as induction and as single agent for maintenance therapy has been shown to overcome the negative impact of del17p and partly that of t(4;14) in the HOVON Trial, and therefore, seems suitable for patients with high-risk cytogenetics.
A combination of both lenalidomide and bortezomib should result in synergistic effects and likely improve the outcome, particularly in cytogenetic high-risk patients, but proof from randomized trials is lacking. There are, however, data from the Total Therapy 3 Study that used VRD maintenance for 2 years with excellent outcome,34 and these results are supported by a small phase 2 trial in patients with cytogenetic or clinical high-risk disease,31 which showed a high sCR and a high survival rate. Results might even be further improved by the novel 3-drug combination KRd (Table 3).
Randomized trials evaluating the role of continued (maintenance) therapy with lenalidomide, bortezomib, and bortezomib-based regimen in TE patients
| Study group or source . | Age (median), y; no. of patients . | Induction consolidation therapy . | Maintenance dose, duration of TX . | Improvement in quality of response . | TTP or PFS* . | OS* . | Survival after relapse . | Tolerance . |
|---|---|---|---|---|---|---|---|---|
| Attal et al24 | 55; N = 614 | VAD, VD, and other induction therapy, single or double ASCT; Consolidation: lenalidomide 25 mg/d, days 1-21, every 28 d, 2 cycles | Len 10-15 mg/d continuously† | CR: 25%† (P = .49); VGPR: 76%‡ (P = .13) | 42 mo | OS at 5 y: 79% | 12 mo | Discontinuation because of AEs: 21%; SPM: 26 |
| Placebo | CR: 23%† (P = .49); VGPR: 71%‡ (P = .13) | 24 mo; P < 108 | OS at 5 y: 73% | 12 mo | 15%; SPM: 6 | |||
| Sonneveld et al16 | 57; N = 827 | PAD | Bortezomib 1.3 mg/m2, biweekly for 2 y | CR/nCR: 49%; greater than or equal to VGPR: 76% | 35 mo | OS at 5 y 61% | n.a. | Grade 3 and 4 PNP 5% |
| VAD | Thal 50 mg/d for 2 y | CR/nCR: 38%; greater than or equal to VGPR: 61% | 28 mo; P = .02 | 55%; P = .007 | 8% | |||
| McCarthy et al25 | 59; N = 460 | Any, followed by ASCT | Len 10 mg/d with dose adaptations (5-15 mg/d) continuously† | n.a. | TTP, median 46 mo | No. of deaths 23 | n.a. | Discontinuation because of AEs: 12% or other reasons: 13%; SPM: 15 |
| Placebo | 27 mo; P < .0001 | 39; P = .018 | 78% of Eligible patients of the control group had been crossed over to lenalidomide TX | Because of AEs: 2% or other reasons: 6%; SPM: 6 | ||||
| Palumbo et al26 | 57; N = 273 | Rd-MPR or Rd-ASCT | Len 10 mg/d, days 1-21, continuously† | CR: 43%; | 41.9 mo | OS at 3 y 88.0% | Similar survival after relapse | All patient subgroups benefitted from maintenance therapy with the exception of stage 3 patients |
| None | CR: 28.7%, P < .001 | 21.6 mo | OS at 3 y 79.2%; P = .14 | |||||
| Gay et al33 | 56-57; N = 389 | Rd-CycloRd or ASCT × 1-2 | Len 10 mg/d, days 1-21 plus Pred (50) qod, d 11-28 | 37.5 mo 28.5 mo, | OS at 3 y 83% OS at 3 v 88% | PFS2 at 50 mo | ||
| Len 10 mg/d, days 1-21 | P = .3 | P = .21 | ASCT-LenPred: 66%; RCD-LenPred: 47%; ASCT-Len: 57%; RCD-Len: 51% | |||||
| Jackson et al18 | N = 428; Len: 53 | CTD/CTDa RCT/RCTa ASCT | 10 mg days 1-21 continuously† | Greater number of patients with deeper response (combined analysis of TE and TNE patients) | 50 mo | n.a. | n.a. | Longer duration of maintenance TX reduced risk of relapse; benefit seen in cytogenetic high-risk patients, albeit to lesser degree |
| Control: 54 | None | 28 mo; P < .0001 |
| Study group or source . | Age (median), y; no. of patients . | Induction consolidation therapy . | Maintenance dose, duration of TX . | Improvement in quality of response . | TTP or PFS* . | OS* . | Survival after relapse . | Tolerance . |
|---|---|---|---|---|---|---|---|---|
| Attal et al24 | 55; N = 614 | VAD, VD, and other induction therapy, single or double ASCT; Consolidation: lenalidomide 25 mg/d, days 1-21, every 28 d, 2 cycles | Len 10-15 mg/d continuously† | CR: 25%† (P = .49); VGPR: 76%‡ (P = .13) | 42 mo | OS at 5 y: 79% | 12 mo | Discontinuation because of AEs: 21%; SPM: 26 |
| Placebo | CR: 23%† (P = .49); VGPR: 71%‡ (P = .13) | 24 mo; P < 108 | OS at 5 y: 73% | 12 mo | 15%; SPM: 6 | |||
| Sonneveld et al16 | 57; N = 827 | PAD | Bortezomib 1.3 mg/m2, biweekly for 2 y | CR/nCR: 49%; greater than or equal to VGPR: 76% | 35 mo | OS at 5 y 61% | n.a. | Grade 3 and 4 PNP 5% |
| VAD | Thal 50 mg/d for 2 y | CR/nCR: 38%; greater than or equal to VGPR: 61% | 28 mo; P = .02 | 55%; P = .007 | 8% | |||
| McCarthy et al25 | 59; N = 460 | Any, followed by ASCT | Len 10 mg/d with dose adaptations (5-15 mg/d) continuously† | n.a. | TTP, median 46 mo | No. of deaths 23 | n.a. | Discontinuation because of AEs: 12% or other reasons: 13%; SPM: 15 |
| Placebo | 27 mo; P < .0001 | 39; P = .018 | 78% of Eligible patients of the control group had been crossed over to lenalidomide TX | Because of AEs: 2% or other reasons: 6%; SPM: 6 | ||||
| Palumbo et al26 | 57; N = 273 | Rd-MPR or Rd-ASCT | Len 10 mg/d, days 1-21, continuously† | CR: 43%; | 41.9 mo | OS at 3 y 88.0% | Similar survival after relapse | All patient subgroups benefitted from maintenance therapy with the exception of stage 3 patients |
| None | CR: 28.7%, P < .001 | 21.6 mo | OS at 3 y 79.2%; P = .14 | |||||
| Gay et al33 | 56-57; N = 389 | Rd-CycloRd or ASCT × 1-2 | Len 10 mg/d, days 1-21 plus Pred (50) qod, d 11-28 | 37.5 mo 28.5 mo, | OS at 3 y 83% OS at 3 v 88% | PFS2 at 50 mo | ||
| Len 10 mg/d, days 1-21 | P = .3 | P = .21 | ASCT-LenPred: 66%; RCD-LenPred: 47%; ASCT-Len: 57%; RCD-Len: 51% | |||||
| Jackson et al18 | N = 428; Len: 53 | CTD/CTDa RCT/RCTa ASCT | 10 mg days 1-21 continuously† | Greater number of patients with deeper response (combined analysis of TE and TNE patients) | 50 mo | n.a. | n.a. | Longer duration of maintenance TX reduced risk of relapse; benefit seen in cytogenetic high-risk patients, albeit to lesser degree |
| Control: 54 | None | 28 mo; P < .0001 |
AE, adverse event; CALGB, Cancer and Leukemia Group B; CI, confidence interval; CTD, cyclophosphamide-thalidomide-dexamethasone; CTDa, same as CTD, but dose attenuated; HOVON, Stichting Hemato-Oncologie voor Volwassenen Nederland; HR, hazard ratio; GMMG, German-speaking Myeloma-Multicenter Group; Len, lenalidomide; LenPred, lenalidomide-prednisone; n.a., not available; nCR, near complete remission; PAD, bortezomib-doxorubicin-dexamethasone; PNP, polyneuropathy; qod, every other day; RCD, Revlimid-cyclophosphamide-dexamethasone; RCT, Revlimid-cyclophosphamide-thalidomide; RCTa, same as RCT, but dose attenuated; SPM, secondary primary malignancy; TTP, time to progression; TX, treatment; VAD, vincristine-doxorubicin-dexamethasone; VD, Velcade-dexamethasone.
Median unless otherwise stated.
Until progression of disease or intolerance.
Patients on placebo were not allowed to cross over to lenalidomide after PD.
Taken together, patients who achieved at least stable disease after ASCT should receive continuous therapy. Patients with high-risk cytogenetics benefit from bortezomib or bortezomib plus lenalidomide therapy. Presently, it is unclear whether continued therapy provides additional benefit in patients who have already achieved MRDneg.
Newly diagnosed NTE patients
Velcade-melphalan-prednisone-thalidomide–Velcade-thalidomide vs VMP
The comparison of standard VMP with Velcade-melphalan-prednisone-thalidomide (VMPT) for 9 cycles followed by Velcade-thalidomide (VT) maintenance for 2 years (the GIMEMA-MM-03-05 Trial) provided clear evidence for the benefit of continuous therapy in elderly patients. The trial become positive only after long (median 54 months) follow-up.35 VMPT-VT resulted in a higher CR rate (38% vs 24%), PFS (35.3 vs 24.8 months), 5-year OS rate (61% vs 51%), and time to next therapy (46.6 vs 27.8 months). Survival from relapse was identical in both groups. The most frequent grade 3 to 4 adverse events included neutropenia (38%), thrombocytopenia (22%), peripheral neuropathy (11%), and cardiologic events (11%). All of these, except for thrombocytopenia, were significantly more frequent in the VMPT-VT patients and particularly more frequent in patients ages 75 years old or older.
VT vs Velcade-prednisone
The Spanish GEM05MAS65 Trial compared VT with Velcade-prednisone (VP) maintenance for up to 3 years in 178 patients after induction therapy with 6 cycles of either VMP or VTP.36 Maintenance therapy increased the CR rate from 24% after induction to 46% with VT and 39% with VP. Median PFS was longer with VT compared with VP maintenance (39 vs 32 months). Furthermore, a tendency for an increased 5-year OS rate was noted with VT (69% and 50%, respectively). In both groups, CR rates were similar between high- and standard-risk patients, but PFS and OS rates at 4 years were significantly inferior in high-risk patients treated with either VT (54% vs 79%, respectively) or VP (53% vs 69%, respectively). The incidence rates of grade 3 to 4 peripheral neuropathy were 9% for VT and 3% for VP.
Melphalan-prednisone-Revlimid vs Melphalan-prednisone-Revlimid, followed by Revlimid maintenance
The MM-015 Study randomized patients to 9 cycles of Melphalan-prednisone-Revlimid, followed by Revlimid maintenance (MPR-R) vs Melphalan-prednisone-Revlimid (MPR) without maintenance therapy vs Melphalan-prednisone (MP).37 Response rates were superior with MPR-R and MPR (77% and 68%, respectively, vs 50% with MP). PFS was significantly longer with MPR-R than with MPR or MP (31 vs 14 vs 13 months, respectively). The PFS benefit associated with MPR-R was noted in patients 65 to 75 years of age but not in those older than 75 years of age. A landmark analysis after the end of induction therapy showed a 66% reduction in the rate of progression with MPR-R compared MPR, which was age-independent. However, OS was similar in the 3 groups. Grade 4 neutropenia was reported in 35%, 32%, and 8% in the MPR-R, MPR, and MP groups, respectively, and second primary tumors were reported in 7%, 7%, and 3% of the patients in the MPR-R, MPR, and MP groups, respectively.
Rd continuous vs Rd for 18 cycles vs Melphalan-prednisone-thalidomide for 18 cycles
In the Frontline Investigation of Revlimid and Dexamethasone versus Standard Thalidomide (FIRST) Trial, 1623 patients were randomized to continuous Rd vs Rd for 18 cycles vs Melphalan-prednisone-thalidomide (MPT) for 18 cycles.38 Median PFS rates were 25.5, 20.7, and 21.2 months in the continuous Rd, Rd for 18 cycles, and MPT arms, respectively. At an interim analysis, a significantly higher 4-year OS rate was noted for both Rd (59%) and Rd for 18 cycles (56%) compared with MPT (51%). In elderly patients >75 years of age, the benefit of continuous treatment was significant regarding response rates (71% vs 55%) but less pronounced regarding PFS (21.2 vs 19.4 months), whereas in younger patients, a more profound PFS benefit, with a median PFS of 27.4 in the Rd vs 21.8 months in the MPT group, was noted. Patients with standard-risk cytogenetics, deeper response (greater than or equal to VGPR), and good tolerance of prolonged treatment had the greatest benefit from continuous therapy. Median durations of therapy were 18.4 and 16.6 months in patients assigned to continuous or Rd for 18 cycles therapy, respectively; in fact, only a subgroup of 39% of patients was able to receive Rd for ≥2 years. OS was not significantly different between the patients in the Rd and the Rd for 18 cycles groups. Table 4 lists more details of the trials discussed above.
Randomized trials evaluating the role of continued (maintenance) therapy with lenalidomide and bortezomib-based regimen in TNE patients
| Study group . | Age (median), y; no. of patients . | Induction therapy . | Maintenance dose, duration of TX . | Improvement in quality of response . | EFS or PFS* . | OS† . | Tolerance . |
|---|---|---|---|---|---|---|---|
| Mateos et al36 | 73; N = 260 | VMP or VTP | VT: bortezomib 1.3 mg/m2 days 1, 4, 8, 11 every 12 wk for 3 y; thalidomide 50 mg/d for 3 y | CR IFneg 23% ⇒ 44% | PFS 32 mo | OS at 2 y 86% | Grade 3 and 4 PNP |
| VT: 7% | |||||||
| VP: 2% | |||||||
| VP: bortezomib as above; prednisone 50 mg every 2 d for 3 y | 23% ⇒ 39% | 24 mo | 81% P = .7 | Discontinuation because of AEs | |||
| HR, 1.4; 95% CI, 0.8-2.1 | VT: 8% | ||||||
| P = .1 | VP: 5% | ||||||
| Palumbo et al35 | 71; N = 511 | VMPT-VT | Bortezomib 1.3 mg/m2 days 1 and 15 every 4 wk | CR | PFS at 3 y | OS at 3 y | Grade 3 and 4 neutropenia 38%, 28.1% |
| 38% | 60% | 88.8% | |||||
| VMP | Thalidomide 50 mg/d until PD or intolerance | 24% | 42% | 89.2% | Cardiologic 10.4%, 5.5% | ||
| P = .0008 | P < .07 | P = .9 | |||||
| Palumbo et al37 | 71; N = 355 | MPR-R | MP plus revlimid 10 mg, days 1-21, 9 cycles followed by revlimid 10 mg maintenance until PD or intolerance | CR: 9.9% | PFS: 31 mo | OS at 3 y 70% 62%, P = .81 | Grade 3 neutropenia 67%, 5% during maintenance |
| PR: 61.1% | Grade 3 neutropenia 64% | ||||||
| MPR | MP plus revlimid 10 mg, days 1-21, 9 cycles | CR: 3.3% | PFS: 14 mo P < .001 | ||||
| PR: 64.7% | |||||||
| Benboubker et al38 | 73; N = 535 | Ld continuous | Lenalidomide 25 mg, days 1-21; dexamethasone 40 mg once weekly | ORR: 75% | 25.5 mo | OS at 4 y 59% | Grade 3 and 4 AEs: 85% Grade 3 and 4 infections: 29% |
| N = 541 | Ld18 | Ld for 18 cycles | ORR:73% | 20.7 mo | 56% P = .31 | Grade 3 and 4 AEs: 80%; Grade 3 and 4 infections: 22% |
| Study group . | Age (median), y; no. of patients . | Induction therapy . | Maintenance dose, duration of TX . | Improvement in quality of response . | EFS or PFS* . | OS† . | Tolerance . |
|---|---|---|---|---|---|---|---|
| Mateos et al36 | 73; N = 260 | VMP or VTP | VT: bortezomib 1.3 mg/m2 days 1, 4, 8, 11 every 12 wk for 3 y; thalidomide 50 mg/d for 3 y | CR IFneg 23% ⇒ 44% | PFS 32 mo | OS at 2 y 86% | Grade 3 and 4 PNP |
| VT: 7% | |||||||
| VP: 2% | |||||||
| VP: bortezomib as above; prednisone 50 mg every 2 d for 3 y | 23% ⇒ 39% | 24 mo | 81% P = .7 | Discontinuation because of AEs | |||
| HR, 1.4; 95% CI, 0.8-2.1 | VT: 8% | ||||||
| P = .1 | VP: 5% | ||||||
| Palumbo et al35 | 71; N = 511 | VMPT-VT | Bortezomib 1.3 mg/m2 days 1 and 15 every 4 wk | CR | PFS at 3 y | OS at 3 y | Grade 3 and 4 neutropenia 38%, 28.1% |
| 38% | 60% | 88.8% | |||||
| VMP | Thalidomide 50 mg/d until PD or intolerance | 24% | 42% | 89.2% | Cardiologic 10.4%, 5.5% | ||
| P = .0008 | P < .07 | P = .9 | |||||
| Palumbo et al37 | 71; N = 355 | MPR-R | MP plus revlimid 10 mg, days 1-21, 9 cycles followed by revlimid 10 mg maintenance until PD or intolerance | CR: 9.9% | PFS: 31 mo | OS at 3 y 70% 62%, P = .81 | Grade 3 neutropenia 67%, 5% during maintenance |
| PR: 61.1% | Grade 3 neutropenia 64% | ||||||
| MPR | MP plus revlimid 10 mg, days 1-21, 9 cycles | CR: 3.3% | PFS: 14 mo P < .001 | ||||
| PR: 64.7% | |||||||
| Benboubker et al38 | 73; N = 535 | Ld continuous | Lenalidomide 25 mg, days 1-21; dexamethasone 40 mg once weekly | ORR: 75% | 25.5 mo | OS at 4 y 59% | Grade 3 and 4 AEs: 85% Grade 3 and 4 infections: 29% |
| N = 541 | Ld18 | Ld for 18 cycles | ORR:73% | 20.7 mo | 56% P = .31 | Grade 3 and 4 AEs: 80%; Grade 3 and 4 infections: 22% |
AE, adverse event; CI, confidence interval; EFS, event-free survival; FIRST, Frontline Investigation of Revlimid and Dexamethasone versus Standard Thalidomide; GIMEMA, Gruppo Italiano Malatti E Matologiche dell'Adulto; HR, hazard ratio; IFneg, negative immunofixation; Ld18, lenalidomide given over 18 cycles; ORR, objective response rate; PD, progression of disease; PETHEMA, Programa para el Tratamiento de Hemopatías Malignas; PNP, polyneuropathy; PR, partial response; TX, treatment; VTP, Velcade-thalidomide-prednisone.
Median unless otherwise stated.
Continuous therapy (maintenance) after induction
Thalidomide maintenance therapy after induction therapy revealed an increase in PFS in elderly patients with standard-risk cytogenetics, but it resulted in significantly inferior survival (35 vs 47 months) in those with high-risk cytogenetics when TE and TNE patients were analyzed together.27 Preliminary results with lenalidomide maintenance after induction therapy in elderly patients showed a significant increase in PFS.20,28
Relapsed disease
Prospective comparisons between fixed duration and continuous therapy in patients with relapsed disease are not available as yet. Several reports on retrospective comparisons claiming a benefit for continuous therapy have been published, but all are affected by an inherent statistical bias, because patients were retrospectively allocated to different risk groups based on outcome measures. Nevertheless, none showed any advantage for short fixed duration therapy. Furthermore, experts recommend (until proven otherwise) continuous therapy, particularly in patients with high-risk disease, and short disease-free interval. In standard-risk patients with asymptomatic relapse after a long disease-free interval, a shorter (fixed duration) approach may be more appropriate.
Taken together, continuous Rd therapy extends PFS over fixed duration Rd in standard-risk patients. VMPT-VT showed improved OS after long follow-up but has not been taken up as a frequently used regimen because of toxicity concerns. MPR-R extends PFS, but usually, the chemo-free combination lenalidomide-dexamethasone is preferred. Thalidomide maintenance after induction therapy is an option in standard-risk patients, but lenalidomide maintenance should be preferred, because it shows significant activity in standard-risk patients and also, albeit to a lesser degree in high-risk patients, and is better tolerated.
Which TNE patients should receive which type of continuous therapy?
The majority of elderly TNE patients benefit in terms of prolonged PFS from continuous therapy. Thus, long-term treatment should be offered to all who accept that an increase in overall survival was noted only in one but not the other 2 of 3 randomized trials.35,37,38 It is difficult to make general recommendations for a specific regimen, because treatment selection depends on patient-specific factors. For fit elderly patients, a 3-drug regimen, including a proteasome inhibitor, an immunomodulatory agent, and dexamethasone, is the obvious choice. For patients who are either cytogenetic standard or high risk, VRD or KRd followed by Rd or R maintenance treatment seems to be a valuable option, whereas in patients 75 years of age or older or frail patients, Rd seems to be the most logical choice. The latter is true for patients with standard-risk cytogenetics, but it is not optimal for those with high-risk disease. In the high-risk group, a bortezomib-based regimen seems preferable, although there is little evidence from randomized trials. The optimal duration of continuous treatment is poorly defined. Patients with excellent response seem to benefit most from continuous therapy (Figure 2).
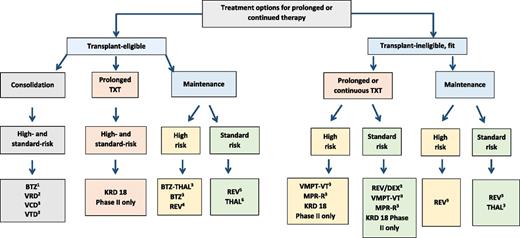
Treatment options for consolidation and prolonged or continuous therapy based on results of randomized trials. KRd has not been tested in randomized comparisons. Improved PFS was seen in (1) 3 of 3, (2) 1 of 2, (3) 1 of 1, (4) 1 of 4, (5) 4 of 4, and (6) 6 of 6 randomized trials. TXT, treatment; BTZ, bortezomib; VCD, Velcade-cyclophosphamide-dexamethasone; THAL, thalidomide; REV, Revlimid; DEX, dexamethasone.
Treatment options for consolidation and prolonged or continuous therapy based on results of randomized trials. KRd has not been tested in randomized comparisons. Improved PFS was seen in (1) 3 of 3, (2) 1 of 2, (3) 1 of 1, (4) 1 of 4, (5) 4 of 4, and (6) 6 of 6 randomized trials. TXT, treatment; BTZ, bortezomib; VCD, Velcade-cyclophosphamide-dexamethasone; THAL, thalidomide; REV, Revlimid; DEX, dexamethasone.
Open questions
Many questions remain unresolved, such as the optimal duration of continuous or maintenance therapy, which may vary depending on several factors, such as quality of response at the start of prolonged therapy, biological features of the disease, patient characteristics, and tolerance. One of the pertinent questions for the future with more patients achieving MRDneg is the role of maintenance in those patients. Recent data show that the impact of MRDneg is independent of the treatment used, but an MRDneg patient may still harbor around 7 × 105 to 10 × 105 myeloma cells. These residual cells usually show additional mutations compared with their baseline status, may remain quiescent for a long time, or more often, may evolve as relapsing disease.39 Whether additional therapy will constrain this tumor population is unclear and presumably dependent on multiple factors, including the genetic machinery of the residual clones, the treatment administered, and most likely, the composition of the stromal environment.29
Future treatment strategies
For the near future, continuing the exploration of the full potential of existing therapies is on the agenda. This includes the evaluation of the recently introduced monoclonal antibodies daratumumab and elotuzumab and proteasome inhibitors carfilzomib and ixazomib for continued therapy and attempts to tailor therapy to the needs of an individual patient to avoid over- and undertreatment, particularly in patients with specific risk features and response categories, including the growing number of patients with MRDneg . To achieve cure, myeloma cells should be eradicated completely or reduced to a small fraction that can be controlled by the immune system. The importance of the latter becomes increasingly clear, with immunotherapies curing cancers that were not curable by conventional chemotherapy. Presently, multiagent regimens using drugs with different modes of activity seem the logical consequence. Augmenting the immune system is an attractive strategy40 that has already highlighted some of its potential with the introduction of daratumumab, checkpoint inhibitors, and chimeric antigen receptor-T cells.
Correspondence
Heinz Ludwig, Wilhelminen Cancer Research Institute, Department of Medicine I, Center for Medical Oncology, Hematology and Outpatient Department and Palliative Care, Wilhelminenspital, Montleartstr 37, 1160 Vienna, Austria; e-mail: heinz.ludwig@wienkav.at.
References
Competing Interests
Conflict-of-interest disclosure: H.L. has received research funding from Takeda and AMGEN and has been affiliated with the Speakers Bureau for Takeda, AMGEN, Celgene, Cilag-Janssen, and BMS. N.Z. has received research funding, consulted, and received honoraria from Celgene, Cilag-Janssen, AMGEN, Takeda, and BMS.
Author notes
Off-label drug use: Bortezomib was used as consolidation and maintenance therapy. Carfilzomib-lenalidomide-dexamethasone was used for continued therapy. Thalidomide, lenalidomide, and bortezomib in various combinations were used for consolidation and/or maintenance therapy.