Abstract
Neutrophils are the most common type of leukocyte in human circulating blood and constitute one of the chief mediators for innate immunity. Defined as a reduction from a normal distribution of values, neutropenia results from a number of congenital and acquired conditions. Neutropenia may be insignificant, temporary, or associated with a chronic condition with or without a vulnerability to life-threatening infections. As an inherited bone marrow failure syndrome, neutropenia may be associated with transformation to myeloid malignancy. Recognition of an inherited bone marrow failure syndrome may be delayed into adulthood. The list of monogenic neutropenia disorders is growing, heterogeneous, and bewildering. Furthermore, greater knowledge of immune-mediated and drug-related causes makes the diagnosis and management of neutropenia challenging. Recognition of syndromic presentations and especially the introduction of next-generation sequencing are improving the accuracy and expediency of diagnosis as well as their clinical management. Furthermore, identification of monogenic neutropenia disorders is shedding light on the molecular mechanisms of granulopoiesis and myeloid malignancies.
Learning Objectives
To describe the heterogeneity and diagnostic approaches of disorders associated with inherited neutropenia
To identify the functional consequences and life-threatening complications of inherited neutropenias
Neutropenia: definition, epidemiology, and complications
Neutropenia is defined as a reduction from a normal range in the number of circulating neutrophils (also known as polymorphonuclear leukocytes or granulocytes). The lower limit of normal neutrophil counts varies based on age and ethnicity. Lower normal values may be found among neonates and individuals of African or Middle Eastern ancestry. Genome-wide association studies are confirming the genetic basis for this. For instance, individuals of African ancestry may harbor polymorphisms in the Duffy antigen receptor complex gene associated with mild neutropenia.1 Neutropenia may be mild, moderate, or severe.2 Severe neutropenia is characterized as an absolute neutrophil count (ANC) <0.5 × 109/L. The ANC consists of neutrophils and bands. Moderate neutropenia is defined as an ANC that ranges from 0.5 × 109/L to 1 × 109/L. Mild neutropenia is found when the ANC is between 1 × 109/L and 1.5 × 109/L. Neutropenia may be acute or chronic, with the latter defined as >3 months and documented on 3 separate occasions. Severe neutropenia implies the risk of life-threatening infections. However, there is chronic benign neutropenia, which may be characterized by an ANC <0.5 × 109/L with an absence of bacterial infections. Emerging evidence suggests that there is functional and phenotypic heterogeneity in neutrophils, which may depend on antecedent inflammation and determine subsequent host defense activities.3
When and how extensively to evaluate an individual with neutropenia remains a challenging question for the general practitioner, if not the hematologist. The initial consultation requires consideration of the degree and duration of neutropenia, the presence/absence of other cytopenias, the age of the patient, the presence or recent history of viral/viral-like infections, the presence of fever (38°C/oral) and/or organ dysfunction, recent or current drug consumption, and family history of blood disorders. A physical examination that focuses on the oral cavity, skin, lungs, and perirectal area for infection is important. Lymphadenopathy and hepatosplenomegaly must be determined. As discussed below, stigmata of specific syndromes should be ascertained. The initial laboratory screen consists of a complete blood count (CBC) with differential and reticulocyte count and a visual inspection of the blood smear. Anti–neutrophil and anti–nuclear antibodies and serial blood count determinations several times a week for 4 to 6 weeks are generally not as helpful as a thorough history, focused physical examination, and review of past CBCs. A history of fevers, mouth sores, pharyngitis, or fatigue that reoccurs every 21 ± 7 to 14 days strongly suggests cyclic neutropenia (CyN). CyN should be confirmed with CBC and differential performed every 3 to 4 days for 4 to 6 weeks and genetic testing. While this condition is almost always due to a germline ELANE mutation, its symptomatic presentation may be delayed until adolescence or adulthood. Occasionally, there will also be cyclic thrombocytopenia. If more than one blood lineage is affected with or without red blood cell macrocytosis, chronicity and severity of neutropenia, and/or stigmata of an inherited bone marrow failure syndrome (IBMFS), then a bone marrow aspirate and biopsy with cytogenetics, flow cytometry, and culture are indicated. Flow cytometry is performed to identify possible acute leukemia, paroxysmal nocturnal hemoglobinuria, or large granular leukemia. Metabolic (B12, folate, or copper) or autoimmune (lupus, connective tissue disorders, or common variable immune deficiency) causes should be evaluated. Next-generation sequencing (NGS) on peripheral blood should be performed later, if diagnosis remains elusive, as a bewildering array of monogenic disorders are being recognized. Unusual infections, such as warts or Mycobacterium, may suggest a specific monogenic disorder, such as GATA2 deficiency or WHIM (warts, hypogammaglobulinemia, infections, and myelokathexis) syndrome.
Neutropenia can occur at any age, and the age of presentation may be an important clue to its diagnosis. Epidemiology of severe neutropenia reveals considerable variability. Self-limiting infections are likely to be the most common cause of acute neutropenia in pediatrics. Fetal/neonatal autoimmune neutropenia presents within the first 6 months of life, with or without sepsis. While it is infrequent, with an incidence of <1 in 1000,4 autoimmune neonatal neutropenia is the most common cause of neutropenia in pediatrics. Beyond the first year of life, the most common cause of chronic neutropenia is idiopathic. Of 45 children with several neutropenia identified at a single US institution, 2 were autoimmune, 3 congenital, 3 familial, 6 cyclic, and 31 idiopathic.5 Not all were symptomatic. In a study from Taiwan, 29 pediatric patients were found to have chronic neutropenia (7 congenital, 7 autoimmune, and 15 idiopathic).6 According to the French National Registry of Primary Immunodeficiency Diseases, congenital neutropenias occur in ∼6 out of 1 000 000.7 This is likely to be an underestimate, as genetic disorders such as Shwachman-Diamond syndrome (SDS), GATA2 deficiency, and dyskeratosis congenita are being better recognized and genetically confirmed.
Severe chronic neutropenia is rare in adults.8 In a multi-institutional French study, 108 individuals were observed. They were mostly female (78%) and young adults (median age, 28 years)9 ; 23 out of 66 patients had neutrophil autoantibodies and 27 patients had severe bacterial infections, but there were no deaths or hematologic malignancies. Approximately half received filgrastim. As in children, the differential diagnosis for all forms of neutropenia is extensive.10
The importance of making an accurate diagnosis lies in providing optimal management and surveillance for transformation to either myelodysplastic syndrome (MDS) or acute myeloid leukemia (AML). Sometime in the near future, genetic engineering of hematopoietic stem cells will permit correction of monogenic neutropenia disorders. As we shall now review, many of these conditions are syndromic with nonhematopoietic deficits.
Monogenic disorders and syndromes
Severe congenital neutropenia
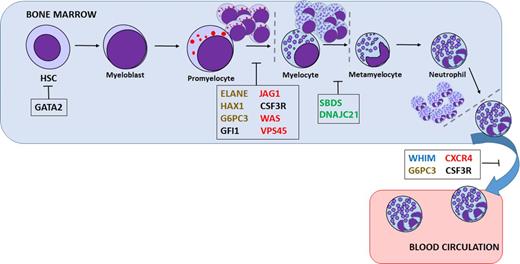
Severe congenital neutropenia (SCN) comprises a heterogeneous group of rare genetic disorders characterized by an impaired development of neutrophilic granulocytes and a marked propensity to develop AML and myelodysplasia (Figure 1). In the last decade, due to genetic sequencing, a number of new genetic defects in neutrophil differentiation and viability have been identified (Table 1). Still, the genetic cause in as many as 30% of SCN patients remains unidentified.
Presumed sites of differentiation block or cell death in various neutropenias. Mutations in a variety of genes are thought to be responsible for inherited neutropenia: granulocytic lineage factors (black), ribosomal proteins (green), membrane proteins (blue), cytoskeletal proteins (red), or metabolism/intracellular stress factors (brown). HSC, hematopoietic stem cell.
Presumed sites of differentiation block or cell death in various neutropenias. Mutations in a variety of genes are thought to be responsible for inherited neutropenia: granulocytic lineage factors (black), ribosomal proteins (green), membrane proteins (blue), cytoskeletal proteins (red), or metabolism/intracellular stress factors (brown). HSC, hematopoietic stem cell.
Monogenic disorders associated with neutropenia
| Disease . | Heritability . | Gene (year reported) . | Protein . | Function . | Pathology . | Intracellular location . | Pathophysiology . | Chromosome . | Predisposition to MDS/AML . | Mice knockout . |
|---|---|---|---|---|---|---|---|---|---|---|
| ELANE deficiency, SCN, and CyN | Autosomal dominant | ELANE (1999) | Neutrophil elastase | Involved in the inflammatory response | Neutropenia, monocytosis, eosinophilia, and osteopenia | Lysosome | UPR activation (misfolding proteins) and mislocalization (observed in neutrophil nucleus) | 19 | Yes | No neutropenia and high susceptibility to infections |
| GFI1 deficiency | Autosomal dominant | GFI1 (2003) | Growth factor independent-1 | Transcription factor involved in the maintenance of HSCs (proliferation and differentiation) | Neutropenia and reduction in HSC self-renewal capacity | Nucleolus | Reduction of myeloid differentiation due to transcriptional deregulation | 1 | Yes | Neutropenia and lymphopenia |
| HAX1 deficiency (Kostmann syndrome) | Autosomal recessive | HAX1 (2007) | HCLS1 associated protein X-1 | Maintains mitochondrial membrane potential and myeloid homeostasis messenger RNA processing | Neutropenia and strong susceptibility to infections and neuronal defects | Mitochondria, nucleus, cytoskeleton, and ER | Destabilization of mitochondrial membrane potential and increased apoptosis of myeloid and neuronal cells | 1 | Yes | Normal granulopoiesis and neurological defects |
| G6PC3 deficiency | Autosomal recessive | G6PC3 (2009) | Glucose-6-phosphatase catalytic subunit 3 | Hydrolyzes glucose-6- phosphate to glucose and phosphate in the ER | Neutropenia, cardiac and urogenital abnormalities, thrombocytopenia, and bone defects | ER | Impaired intracellular glucose homeostasis, UPR activation, and elevated apoptosis of myeloid cells | 17 | Yes | Neutropenia but no cardiac or urogenital defects |
| VPS45 deficiency | Autosomal recessive | VPS45 (2013) | Vacuolar protein sorting 45 | Protein trafficking of intracellular molecules into distinct organelles | Neutropenia, neutrophil disfunction, myelofibrosis, hepatomegaly, and nephromegaly | Golgi apparatus and endosome | Abnormal neutrophil maturation and function, with a reduction in motility and increased apoptosis | 1 | ? | N/A |
| JAG1 deficiency | Autosomal recessive | JAG1 (2014) | Protein jagunal homolog 1 | Membrane/ protein transport from ER to Golgi | Problem in glycosylation of proteins, including CSF3R, which leads to aberrant surface expression of the receptor, and thus G-CSF treatment is not effective | ER | ER defects in granulocytes and absence of granules, abnormal N-glycosylation of multiple proteins, and increased apoptosis | 20 | Yes | Knockout lethal |
| CSF3R deficiency | Autosomal dominant or recessive | CSF3R (1994) | Colony-stimulating factor 3 receptor | Receptor for G-CSF | Reduced number of neutrophils in peripheral blood, full maturation of neutrophil granulocytes in the bone marrow, andrefractory to G-CSF treatment | Membrane | Abnormal neutrophil differentiation | 1 | Yes | Reduced numbers of neutrophils in peripheral blood |
| Wiskott-Aldrich syndrome | X-linked recessive | WAS (1994) | Wiskott-Aldrich syndrome protein | Regulator of the cytoskeleton and the control of cell division | Neutropenia, lymphopenia, and reduced lymphocyte proliferation; also affects phagocyte activity | Cytoskeleton | WASP structure is affected, leading to conformational changes that induce increased actin polymerization | X | Yes | Normal |
| WHIM syndrome | Autosomal dominant | CXCR4 (2003) | C-X-C motif chemokine receptor 4 | Membrane receptor for CXCL12; activation involved in cell growth and differentiation | Warts, hypogammaglobulinemia, infections, severe neutropenia, no maturation arrest, and myelokathexis | Membrane | Bone marrow hyperplasia, lymphopenia | 2 | Yes | Haematopoietic and cardiac defects |
| GATA2 deficiency | Autosomal dominant | GATA2 (2013) | GATA binding protein 2 | Transcription factor essential for hematopoietic differentiation and lymphatic formation | Neutropenia, monocytopenia, and thrombocytopenia | Nucleus | Abnormal neutrophil maturation | 3 | Yes | Knockout lethal |
| SDS, classical | Autosomal recessive | SBDS (2003) | Shwachman-Diamond-Bodian-syndrome | Ribosome biogenesis, mitotic spindle stabilization | Mild neutropenia, exocrine pancreatic insufficiency, skeletal dysplasia, hepatic and cardiac disorders | Nucleus, cytosol, and cytoskeleton | Defects in maturation of 60S ribosomal subunit and thereby decrease of 80S actively translating ribosomes | 7 | Yes | Knockout lethal |
| SDS, nonclassical | Autosomal recessive | DNAJC21 (2016) | DnaJ heat shock protein family (Hsp40) member C21 | Ribosome biogenesis | Neutropenia, exocrine pancreatic insufficiency, skeletal dysplasia, and hepatic and cardiac disorders | Nucleus and cytosol | Defects in maturation of 60S ribosomal subunit and thereby decrease of 80S actively translating ribosomes | 5 | Yes? | N/A |
| Disease . | Heritability . | Gene (year reported) . | Protein . | Function . | Pathology . | Intracellular location . | Pathophysiology . | Chromosome . | Predisposition to MDS/AML . | Mice knockout . |
|---|---|---|---|---|---|---|---|---|---|---|
| ELANE deficiency, SCN, and CyN | Autosomal dominant | ELANE (1999) | Neutrophil elastase | Involved in the inflammatory response | Neutropenia, monocytosis, eosinophilia, and osteopenia | Lysosome | UPR activation (misfolding proteins) and mislocalization (observed in neutrophil nucleus) | 19 | Yes | No neutropenia and high susceptibility to infections |
| GFI1 deficiency | Autosomal dominant | GFI1 (2003) | Growth factor independent-1 | Transcription factor involved in the maintenance of HSCs (proliferation and differentiation) | Neutropenia and reduction in HSC self-renewal capacity | Nucleolus | Reduction of myeloid differentiation due to transcriptional deregulation | 1 | Yes | Neutropenia and lymphopenia |
| HAX1 deficiency (Kostmann syndrome) | Autosomal recessive | HAX1 (2007) | HCLS1 associated protein X-1 | Maintains mitochondrial membrane potential and myeloid homeostasis messenger RNA processing | Neutropenia and strong susceptibility to infections and neuronal defects | Mitochondria, nucleus, cytoskeleton, and ER | Destabilization of mitochondrial membrane potential and increased apoptosis of myeloid and neuronal cells | 1 | Yes | Normal granulopoiesis and neurological defects |
| G6PC3 deficiency | Autosomal recessive | G6PC3 (2009) | Glucose-6-phosphatase catalytic subunit 3 | Hydrolyzes glucose-6- phosphate to glucose and phosphate in the ER | Neutropenia, cardiac and urogenital abnormalities, thrombocytopenia, and bone defects | ER | Impaired intracellular glucose homeostasis, UPR activation, and elevated apoptosis of myeloid cells | 17 | Yes | Neutropenia but no cardiac or urogenital defects |
| VPS45 deficiency | Autosomal recessive | VPS45 (2013) | Vacuolar protein sorting 45 | Protein trafficking of intracellular molecules into distinct organelles | Neutropenia, neutrophil disfunction, myelofibrosis, hepatomegaly, and nephromegaly | Golgi apparatus and endosome | Abnormal neutrophil maturation and function, with a reduction in motility and increased apoptosis | 1 | ? | N/A |
| JAG1 deficiency | Autosomal recessive | JAG1 (2014) | Protein jagunal homolog 1 | Membrane/ protein transport from ER to Golgi | Problem in glycosylation of proteins, including CSF3R, which leads to aberrant surface expression of the receptor, and thus G-CSF treatment is not effective | ER | ER defects in granulocytes and absence of granules, abnormal N-glycosylation of multiple proteins, and increased apoptosis | 20 | Yes | Knockout lethal |
| CSF3R deficiency | Autosomal dominant or recessive | CSF3R (1994) | Colony-stimulating factor 3 receptor | Receptor for G-CSF | Reduced number of neutrophils in peripheral blood, full maturation of neutrophil granulocytes in the bone marrow, andrefractory to G-CSF treatment | Membrane | Abnormal neutrophil differentiation | 1 | Yes | Reduced numbers of neutrophils in peripheral blood |
| Wiskott-Aldrich syndrome | X-linked recessive | WAS (1994) | Wiskott-Aldrich syndrome protein | Regulator of the cytoskeleton and the control of cell division | Neutropenia, lymphopenia, and reduced lymphocyte proliferation; also affects phagocyte activity | Cytoskeleton | WASP structure is affected, leading to conformational changes that induce increased actin polymerization | X | Yes | Normal |
| WHIM syndrome | Autosomal dominant | CXCR4 (2003) | C-X-C motif chemokine receptor 4 | Membrane receptor for CXCL12; activation involved in cell growth and differentiation | Warts, hypogammaglobulinemia, infections, severe neutropenia, no maturation arrest, and myelokathexis | Membrane | Bone marrow hyperplasia, lymphopenia | 2 | Yes | Haematopoietic and cardiac defects |
| GATA2 deficiency | Autosomal dominant | GATA2 (2013) | GATA binding protein 2 | Transcription factor essential for hematopoietic differentiation and lymphatic formation | Neutropenia, monocytopenia, and thrombocytopenia | Nucleus | Abnormal neutrophil maturation | 3 | Yes | Knockout lethal |
| SDS, classical | Autosomal recessive | SBDS (2003) | Shwachman-Diamond-Bodian-syndrome | Ribosome biogenesis, mitotic spindle stabilization | Mild neutropenia, exocrine pancreatic insufficiency, skeletal dysplasia, hepatic and cardiac disorders | Nucleus, cytosol, and cytoskeleton | Defects in maturation of 60S ribosomal subunit and thereby decrease of 80S actively translating ribosomes | 7 | Yes | Knockout lethal |
| SDS, nonclassical | Autosomal recessive | DNAJC21 (2016) | DnaJ heat shock protein family (Hsp40) member C21 | Ribosome biogenesis | Neutropenia, exocrine pancreatic insufficiency, skeletal dysplasia, and hepatic and cardiac disorders | Nucleus and cytosol | Defects in maturation of 60S ribosomal subunit and thereby decrease of 80S actively translating ribosomes | 5 | Yes? | N/A |
ER, endoplasmic reticulum; HSC, hematopoietic stem cell; G-CSF, granulocyte colony-stimulating factor; N/A, not applicable.
ELANE (SCN1).
Severe congenital neutropenia (online Mendelian inheritance in man [OMIM] #202700) and CyN (OMIM #162800) are inherited bone marrow failure syndromes caused by mutations in ELANE (formerly ELA2).11 Mutations in ELANE are the most common cause of SCN (40% to 60% of affected individuals). SCN is characterized by a maturation arrest of differentiation at the level of promyelocytes, peripheral blood absolute neutrophil counts <500/μL, and early onset of severe bacterial infections.12 In contrast, CyN is characterized by recurrent episodes of severe neutropenia at 21-day intervals. To date, >70 different mutations of the ELANE gene have been described in patients with CyN or SCN.13 Some of these mutations are present in both SCN and CyN patients, but some are restricted to SCN patients without a clear explanation of how a given genetic lesion may be associated with different phenotypes.14 Interestingly, mice carrying mutations in Elane did not show an effect in murine granulopoiesis, unless challenged by bortezomib.15-17
ELANE encodes for neutrophil Elastase, which has an important role in the inflammatory response.14 The molecular mechanisms by which ELANE mutations disrupt granulopoiesis is not well understood. A number of studies have demonstrated that ELANE mutations result in neutrophil elastase protein misfolding, induction of endoplasmic reticulum (ER) stress, activation of the unfolded protein response (UPR), and ultimately a block in granulocytic differentiation.18 However, not all ELANE mutations induce UPR, and moreover, different mutations differentially affect the 3 branches of the UPR.19 These differences might be explained by the abnormal accumulation of the protein in different intracellular compartments.
GFI1 (SCN2).
GFI1 is a transcription factor important in myeloid and lymphoid differentiation. In 2002, Karsunky et al created knockout (Gfi1−/−) mice and showed that they were neutropenic and accumulate immature monocytic cells in blood and bone marrow.20 One year later, Person et al screened for GFI1 mutations in SCN patients without ELANE mutations. In their study, they found 2 dominant-negative mutations that disable transcriptional repressor activity, and as a consequence, they show severe maturation arrest of myeloid cells and immunodeficient lymphocytes (OMIM #613107).21 In 2010, Khandanpour et al reported a new variant allele in GFI1 associated with AML. They showed different subcellular localization between 2 different variants of GFI1: GFI136S was found in the nucleus with a dotted pattern, and GFI136N (more abundant in AML patients) was found in the nuclear/cytoplasmic border. This different subnuclear localization could lead to partial or specific loss of GFI1 functions.22
HAX1 (SCN3).
Kostmann syndrome (OMIM #610738) was eponymously given to describe the original SCN patients, who were subsequently determined to harbor mutations in HAX1. The HAX1 gene that is transmitted in an autosomal-recessive fashion and is often associated with neurological symptoms. The main clinical features are severe neutropenia and strong susceptibility to bacterial and fungal infections. HAX1 mutations have been reported mainly in Sweden and Kurdistan.
HAX1 is critical for maintaining the inner mitochondrial membrane potential and is a major regulator of myeloid homeostasis that controls apoptosis in neutrophil development.23 Moreover, HAX1 has also been reported to be involved in calcium homeostasis, cell motility, and messenger RNA processing, stability, and localization.24 Hax1-deficient mice are not neutropenic, but they show lymphocytopenia and neuronal cell death.25
G6PC3 (SCN4).
This was first described in 2009 (OMIM# 612541) as an autosomal-recessive syndrome linked to a mutation in the glucose-6-phosphatase catalytic subunit 3 (G6PC3) gene. This gene encodes for glucose-6-phosphatase (G6Pase), which is involved in the final step of the gluconeogenic and glycogenolytic pathways.26 SCN4 is also associated with cardiac and urogenital abnormalities, thrombocytopenia, and bone deformities.27 In 2010, McDermont et al28 found that G6PC3 defects result in a neutrophil release defect rather than differentiation arrest; 4 years later, Desplantes et al proposed that defects in neutrophil release from the bone marrow might be due to ER stress and protein glycosylation.29
VPS45 (SCN5).
In 2013, 2 Israeli groups reported that biallelic mutations in VPS45 were the cause of a new syndromic form of SCN (OMIM #615285) among Bedouin Arabs characterized by severe neutropenia, myelodysplasia, myelofibrosis, nephromegaly, hepatomegaly, osteosclerosis, and susceptibility to life-threatening bacterial infections.30-32 The VPS45 gene encodes a protein associated with protein trafficking through the endosomal system and the release of inflammatory mediators. Defective VPS45 leads to abnormal neutrophil maturation and function with decrease in motility and increase apoptosis.30
JAGN (SCN6).
JAGN1 (protein jagunal homolog 1) deficiency has been recently identified as one of the causes of severe neutropenia (OMIM #616022). In 2014, Boztug et al reported 14 patients from 9 families with SCN with 9 different homozygous mutations in JAGN1.33 JAGN1 is a factor that is necessary in the differentiation and viability of neutrophils. Patients with biallelic mutations in JAG1 have granulocytes characterized by ultrastructural defects in the ER, absence of granules, abnormal N-glycosylation of multiple proteins, and increased apoptosis.33,34
Wirnsberger et al showed that Jag1−/− mice died at embryonic day 8.5. Consequently, they created a conditional knockout that deleted Jag1 only in hematopoietic cells. In their conditional model, they did not see any effect on the number of neutrophils but they found a reduction in mobilization and tissue recruitment of neutrophils after injection of Candida albicans.35 With these results, they concluded that Jag1 plays an important role in microbial pathogenesis. The failure to phenocopy SCN in the Jag1- or Elane-defective mice suggests that murine myelopoiesis has different control features than that in humans.
CSF3R (SCN 7).
Autosomal-dominant mutations in CSF3R, affecting the extracellular domain, were reported a decade ago in association with refractoriness to filgrastim.36,37 More recently (in 2014), Triot et al described a new SCN characterized by recessively inherited CSF3R mutations (OMIM #617014).38 CSF3R is a protein localized in the cell membrane that, in response to granulocyte colony-stimulating factor (G-CSF) stimulation, promotes proliferation and differentiation of neutrophils.39 In SCN7, patients show full myeloid cell maturation in the bone marrow but peripheral neutropenia and nonresponse to G-CSF treatment.38 Mutations found in these patients were missense mutations and truncations. Transfection studies in bone marrow cells with the missense mutation showed that the mutant protein was retained in the ER and not expressed on the cell membrane, causing a decrease in downsignaling compared to wild-type. The truncating mutations caused loss of function.38,40
WAS.
Mutations of WAS, encoding the Wiskott-Aldrich syndrome protein (WASP), are associated with X-linked SCN (OMIM #301000). WASP is a hematopoietic-specific member of a conserved family of proteins that participates in the dynamic regulation of actin polymerization. Nearly 150 WAS missense, nonsense, and frameshift mutations have been identified. In most cases, they cause reduction or totally absence of WASP levels and/or activity and diminished actin polymerization in hematopoietic cells, reduce proliferation, and increase apoptosis.12,41,42 In contrast to those loss-of-function mutations that result in Wiskott-Aldrich syndrome (thrombocytopenia and immunodeficiency without neutropenia), gain-of-function mutations produce neutropenia and MDS. Bone marrow examination of patients shows a maturation arrest at the promyelocyte/myelocyte stage.43
WHIM.
WHIM syndrome was first described as an autosomal-dominant condition in 1977,44 and 13 years later, Wetzler et al suggested the name WHIM, an acronym for warts, hypogammaglobulinemia, infections, and myelokathexis (OMIM #193670).45 It is an autosomal-dominant disease caused by mutations in CXCR4.46 CXCR4 encodes C-X-C chemokine receptor type 4, a membrane receptor expressed in cells of both the immune and the central nervous systems.47,48 Mutations in this protein cause an impaired desensitization and internalization of CXCR4 in response to its ligand, CXCL12, a chemokine involved in hematopoietic cell trafficking and lymphoid tissue architecture, leading to increased responsiveness to chemokine ligand and retention of neutrophils in bone marrow.47,49,50 Patients with WHIM syndrome have a severe neutropenia with no maturation arrest but a defect in neutrophil release from the bone marrow. These individuals also have a severe reduction in peripheral B lymphocytes. Warts are typically due to human papillomavirus, but they do not typically respond to antiwart therapy. Despite antibiotics and filgrastim, chronic sinopulmonary infections often lead to complications.51 The CXCR4 antagonist plerixafor may be effective.52
Moderate congenital neutropenias
Shwachman-Diamond syndrome.
SDS was first described in 1964.53 SDS is an autosomal disorder characterized by exocrine pancreatic insufficiency, bone marrow dysfunction, and skeletal abnormalities (OMIM #260400). Hematologic abnormalities are a major cause of morbidity and mortality and include cytopenia, MDS, and AML. Neutropenia occurs in 88% to 98% of patients and has been identified as early as the neonatal period.54,55 Mutations in Shwachman-Bodian-Diamond syndrome (SBDS) has been detected in 80% to 90% of SDS patients.56,57 The most common mutation in SDS patients are either a frameshift (p.84Cfs3) or nonsense (p.K62X) mutation, but >30 other mutations have been reported.56,58,59 SBDS together with EFL1, a GTPase, triggers the GTP-dependent release of EIF6 from 60S preribosomes in the cytoplasm, thereby activating ribosomes for translation competence by allowing 80S ribosome assembly and facilitating EIF6 recycling to the nucleus, where it is needed for 60S rRNA processing and nuclear export.60
This past year, DNAJC21 mutations have been identified in patients with moderate neutropenia and pancreatic insufficiency but who did not harbor mutations in SBDS.61,62 DNAJC21 belongs to the highly conserved family of DnaJ (heat shock protein 40) involved in protein translation, folding, unfolding, translocation, and degradation. Like SBDS, DNAJC21 is involved in ribosome biogenesis in the maturation of 60S ribosomal subunit and ubiquitously expressed in human tissues.61,62 The identification of mutations in these 2 genes that are involved in 60S maturation and translational activation of ribosomes suggests a common pathway for SDS.63 The precise mechanism for the neutropenia (as well as other manifestations) remains elusive, however.
GATA2 deficiency.
Since 2011, GATA2 deficiency has been established as a new MDS/AML predisposition condition (OMIM #614172). One report described a mutation in GATA2 in a family with a history of chronic mild neutropenia evolving to AML and/or MDS.64 GATA2 is a transcription factor required for stem cell homeostasis, and its downregulation in needed for differentiation, and thus it is tightly regulated by different transcription factors downstream of multiple cytokines.64-67 Several excellent reviews of relatively large patient cohorts have reported the protean manifestations of GATA2-deficiency, its overlap with bone marrow failure syndromes, and predisposition to MDS/AML.68-71
SAMD9/SAMD9L.
Ataxia-pancytopenia (AP) syndrome (OMIM #159550) is a rare autosomal-dominant disorder characterized by cerebellar ataxia, variable hematologic cytopenias, and predisposition to bone marrow failure and AML. Chen et al (2016) reported that missense mutations in SAMD9L (sterile α motif domain–containing 9-like) are the cause. SAMD9L plays a role in regulating cell proliferation and apoptosis.72 Buonocore et al (2017) recently defined a new form of syndromic adrenal hypoplasia, MIRAGE (myelodysplasia, infection, restriction of growth, adrenal hypoplasia, genital phenotypes, and enteropathy) syndrome (OMIM #617053), which is caused by mutations in SAMD9.73
According to the OMIM database, there are more than 175 genetic disorders with neutropenia. Other monogenic conditions that result in or are associated with moderate to severe neutropenias include glycogen storage disease type Ib (G6PT),74 X-linked Barth syndrome with cardiomyopathy (TAZ1),75 Cohen syndrome (developmental delay, facial dysmorphism, retinal dystrophy, truncal obesity, and joint laxity) due to mutated protein sorter VPS13B,76 endosomal adaptor p14 deficiency,77 and poikiloderma with neutropenia (C16orf67). Poikiloderma with neutropenia is characterized by poikiloderma (hypopigmentation, hyperpigmentation, telangiectasias, and atrophy), facial dysmorphism, pachyonychia, short stature, and neutropenia. This condition also predisposes to MDS. Although first described among US Navajo Indians, the condition has been reported in ∼37 individuals from across the world. Biallelic mutations are found in the C16orf57 gene, now referred to as USB1. USB1 is a phosphodiesterase that regulates the stability of spliceosomal U6-RNA.78 Thus, there is both phenotypic and genomic overlap with other conditions such as dyskeratosis congenita.79
Pathophysiology.
While a wide range of affected genes result in severe to moderate neutropenia, the mechanisms by which neutropenias arise are poorly understood. A major obstacle to greater understanding is the paucity of accurate experimental models. Most of the genes are expressed ubiquitously; a few are more selectively expressed in granulocytes and their precursors. This genetic diversity in congenital neutropenias suggests that various pathways are involved in their pathogenesis, with enhanced apoptosis as a final common pathway. New insights in the understanding of these disorders include induction of the UPR, defective ribosome assembly, and p53-dependent and p53-independent apoptosis.80
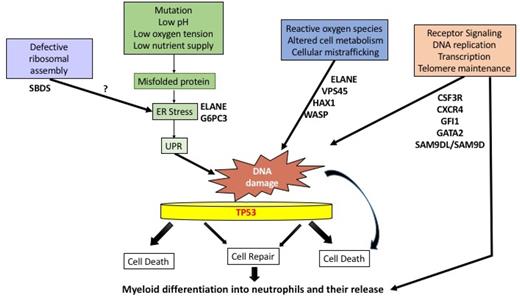
A critical component of the innate immune response,81 the neutrophil may have been adapted to a short and vulnerable but meaningful life. Rapid turnover of neutrophils occurs with recently calculated half-lives of 3 to 5 days.82 This requires prodigious production of up to 2 × 1011 cells per day.81 Stressful conditions such as low pH, low oxygen tension, low nutrient supply, accumulation of misfolded proteins, and ER stress can cause cell death, especially in a vulnerable cell like the neutrophil. Because of the tremendous amount of cell production and turnover, the UPR may play a general role in congenital neutropenias. Also, every time a cell divides, it invites mutagenesis through stochastic or deterministic processes.83 DNA damage can be caused by a number of endogenous and exogenous agents, including reactive oxygen species, altered cell metabolism, xenobiotics, radiation, and UPR/ER stress. DNA damage repair mechanisms may have important implications in the transformation process of normal cells into cancer cells due to inaccurate DNA repair (Figure 2).84 A role for TP53 has been proposed in congenital and acquired marrow failure syndromes.80,85
Proposed pathways for neutropenia. Excessive stress responses, such as the UPR, ER stress, or reactive oxygen species (ROS), may lead to DNA damage, with or without faulty repair by p53-dependent or p53-independent pathways. These events, in turn, may lead to a subsequent block in differentiation or cell death. Other stressful stimuli include radiation exposure, infections, low nutrient supply, or faulty protein synthesis due to defective ribosome assembly. In other cases, neutropenia may result from defective differentiation and release due to receptor/transcription factor defects in signal transduction.
Proposed pathways for neutropenia. Excessive stress responses, such as the UPR, ER stress, or reactive oxygen species (ROS), may lead to DNA damage, with or without faulty repair by p53-dependent or p53-independent pathways. These events, in turn, may lead to a subsequent block in differentiation or cell death. Other stressful stimuli include radiation exposure, infections, low nutrient supply, or faulty protein synthesis due to defective ribosome assembly. In other cases, neutropenia may result from defective differentiation and release due to receptor/transcription factor defects in signal transduction.
Congenital neutropenias as MDS/AML predisposition syndromes.
The IBMFS are also cancer/leukemia predisposition syndromes. In particular, SCN, SDS, and GATA2 deficiency carry a 1000 to 10 000-fold increase risk of MDS/AML. Several key patterns are emerging: the pharmacologic use of G-CSF (filgrastim) in SCN, existence of cooperating gene mutations, and recurrent cytogenetic abnormalities, particularly −7/del (7q). The contribution of filgrastim to the process is controversial. Epidemiological studies suggest that the greater the dose of filgrastim used the greater the risk of MDS/AML.86 Patients with SCN are treated with a higher dose of filgrastim and for a longer duration than patients with other IBMFS or acquired plastic anemia. Furthermore, ∼70% of MDS/AML secondary to SCN is associated with somatically acquired, truncating mutations of the G-CSF receptor (CSF3R).87 As the disease progresses from SCN to MDS/AML, a second (or subsequent) mutation occurs in RUNX1.88 Other genetic changes include monosomy 7 and mutations in ASXL1, SUZ2, or CSF3R.87 Another set of cooperating genes in leukemogenesis is found with GATA2 deficiency and ASXL1 mutation.84 A retrospective review of reported MDS/AML arising in the 8 most common IBMFS demonstrated the frequency and outcome of chromosome 7 abnormalities. Monosomy 7 or del(7q) occurred in ∼17%, with a frequency comparable between types of IBMFS.89 Because of the haploinsufficiency, it has been hypothesized that the loss of a tumor suppressor gene contributes to MDS/AML. Several genes have been proposed, including CUX1, EZH2, and MLL3.90
NGS as the next step in diagnosis
NGS, also known as massive parallel sequencing or deep sequencing, is transforming diagnostics by making the process more accurate and less expensive.
The Canadian Inherited Bone Marrow Failure Registry recently reported their use of a 72-gene panel on peripheral blood from a cohort of patients.91 Among patients with suspected IBMFS, 44 out of 75 (59%) harbored causal mutations. Interestingly, 9% of the patients had a diagnosis changed from one disorder to another (eg, Fanconi anemia to dyskeratosis congenita or Diamond-Blackfan anemia to SDS). Of 83 patients with unclassified IBMFS, NGS identified genetic causes in 15 (18%). Among patients with neutropenias, affected genes were WAS, G6PC3, GATA2, and CXCR4.
Using NGS, Keel and associates studied 208 children and adults with suspected aplastic anemia or MDS.92 A total of 5% of the aplastic anemia patients were found to have germline mutations in DKC, MPL, or TP53, and 13% of the MDS patients harbored germline mutations in FANCA, MPL, RTEL4, SBDS, GATA2, RUNX1, TERT, TINF, and TP53. Most of the affected patients were in the pediatric age range, suggesting that NGS is more cost-effective in this age group.
NGS permits evaluation for a wide range of mutations and quantification of variant alleles (useful in determining mosaicism). Several robust panels are offered by Clinical Laboratory Improvement Amendments laboratories, such as the University of Chicago and University of Washington. Turnaround time is from 2 to 8 weeks. With >50 genes interrogated on gene panels that are frequently being updated by new genomic discoveries, the costs are ∼$2000 to $4000. A 2015 cost–benefit analysis of NGS performed by the Canadian Inherited Bone Marrow Failure registry suggested the price per patient for NGS (without attendant costs of phlebotomy, shipping, and sample preparation) was <$500.91 It is likely that costs and turnaround time will decrease.
Additional benefits to greater use of NGS include earlier identification of cancer/leukemia predisposition syndromes, identification of genetic disorders that confer increased toxicity in chemotherapy and/or myeloablative transplantation regimens, better scrutiny of related stem cell donors, and more accurate family planning and genetic counseling. NGS must be considered a standard practice. Clinical judgment dictates when to use it during the evaluation of a person, pediatric or adult, suspected of single-lineage or multiple-lineage cytopenia.
Management.
Management depends on clinical judgment and supportive laboratory findings for the specific disorder and its severity. Treatment of neutropenia is not always indicated. Patients without an underlying syndrome or history of recurrent infections are unlikely to benefit from filgrastim. Filgrastim or its pegylated form is highly effective in SCN, and moderate neutropenia associated with conditions such as SDS. We recommend titering the dose of filgrastim to achieve an ANC of 0.5 to 0.75 × 109/L. There are no data to suggest therapeutic differences between filgrastim and the biosimilars. Those with moderate neutropenia and a benign course may be followed by observation and no prophylactic antibiotics. Whether to hospitalize for fever depends on other physical findings and whether recovery is expected within days. Compliance to filgrastim or peg-filgrastim can be an issue, especially in adolescents. Some patients will not respond to filgrastim (>10 μg/kg per day), and they need to be evaluated for CSF3R mutations in the external domain. Indications for an allogeneic stem cell transplant include filgrastim nonresponsive patients and those requiring high doses of filgrastim, the presence of a mutated CSF3R, or the appearance of frank myelodysplasia or increased bone marrow or peripheral blasts. The SCN International Registry recommends yearly surveillance marrows. A CBC with differential should be performed every 3 months. Prophylactic antibiotics and/or antifungal therapy should be used only for those with documented infections.
Acknowledgments
This work is dedicated to the memory of Laurence Boxer and Niels Borregaard, two hematologists who died in 2016 and taught us much about the neutrophil and neutropenia.
This work was supported by grants from the National Institutes of Health, the Department of Defense, and the Shwachman-Diamond Syndrome Foundation.
Correspondence
Seth J. Corey, Departments of Pediatrics, Micro/Immunology, and Human and Molecular Genetics, Box 980037, 401 College St, Richmond, VA 23298; e-mail: seth.corey@vcuhealth.org.
References
Competing Interests
Conflict-of-interest disclosure: S.J.C. has been affiliated with the Speakers Bureau for Jazz Pharmaceuticals. U.O. declares no competing financial interests.
Author notes
Off-label drug use: None disclosed.