Abstract
Data on specific studies in cancer patients using direct oral anticoagulants (DOACs) for the prevention and treatment of venous thromboembolism (VTE) are still scarce. For preventing VTE with DOACs, current experience is still very limited, so definite conclusions cannot yet be drawn. However, DOACs have so far been compared with vitamin K antagonists (VKAs) in patients with acute VTE in 5 studies, and several hundreds of patients included in these studies had either active cancer, a history of cancer, or a new occurrence of cancer during the course of disease. Meta-analyses have revealed an at least similar efficacy and safety profile of DOACs compared with VKAs. A number of studies of cancer patients investigating primary prevention and treatment are underway, and some will be finalized soon. Nevertheless, we might need further trials, specifically on the prevention of VTE in patients who are at particularly high risk. This article also includes a personal opinion on the use of DOACs in cancer patients. In conclusion, the currently available data show that DOACs might be safe and efficacious in the treatment of VTE, however, this has yet to be proven in specifically designed trials in patients with cancer. With regard to prevention, thus far, even less data exist, and the outcomes of the ongoing studies have to be evaluated before DOACs may be used for primary prevention.
Learning Objectives
Learn about DOACs in general and the presently available data on treatment of venous thrombosis and/or pulmonary embolism in patients with cancer
Gain knowledge on DOACs in the prevention of venous thromboembolism in cancer patients
Learn about the recent guidelines in prevention and treatment of venous thromboembolism and the individualized approach in a patient with cancer
Introduction
In the framework of autopsy studies, pathologists recognized several decades ago that many patients with cancer had thrombosis in the vascular system of the lungs and that this led or contributed to death in >40% of patients.1 Later, a noninvasive method (light reflection rheography) revealed a 52% (135 in 285 patients) rate of thrombosis in the deep leg veins in hospice patients with advanced cancer.2 Since that time, research and knowledge on cancer-associated thrombosis have considerably advanced and increased, and it has been identified as one of the leading causes of mortality and morbidity in cancer patients.3 The association between cancer and thrombosis is two-sided: on the one hand, cancer patients develop venous thrombosis or pulmonary embolism much more frequently during the course of disease, and on the other hand, cancer can more frequently be found in patients with deep vein thrombosis (DVT) or pulmonary embolism (PE). Venous thromboembolism (VTE) is a term used to commonly summarize DVT and PE. The risk of developing VTE is ∼4 to 6.5 times higher in patients with malignancy than in those without it.4,5 Patients with cancer do not only have increased risk of deep leg or arm vein thrombosis or pulmonary embolism, but they also more frequently experience thrombosis at unusual sites, such as in the mesenteric or portal veins.6
It is common knowledge that cancer induces a hypercoagulable state, and this state is regarded as the main reason for the increased risk of VTE.7 It is not only the deep veins that may thrombose, but also the superficial veins, as was first noted by Trousseau,8 who described the association between thrombophlebitis and malignancy.
Superficial vein thrombosis, DVT, and PE cause a number of symptoms, such as pain and swelling, and thus have a major impact on the quality of life of cancer patients. Pulmonary embolism constitutes an immediate fatal risk. In the long term, patients may develop postthrombotic syndrome after DVE, and those with PE may develop pulmonary hypertension. The primary medical objective is therefore, in the first place, to prevent VTE in cancer patients altogether and, if it occurs, to treat it safely, efficaciously, and easily without additionally burdening the patients. Anticoagulants used for patients with VTE in general, but cancer patients specifically, should fulfill all of these criteria.
Anticoagulants for the prevention and treatment of cancer-associated thrombosis
For many decades, we have used 2 groups of anticoagulants, LMWH and VKAs, not only for patients without cancer, but also for those with malignancy. The most important anticoagulant shown to be effective for the prevention and treatment of cancer-associated VTE is LMWH. As will be discussed later, LMWH is still the most frequently recommended and most important anticoagulant for the prevention and treatment of VTE in cancer patients. VKAs have also been useful and used for many decades in cancer patients. However, patients with cancer have a higher risk of recurrent VTE despite sufficient anticoagulation with VKAs.9 Moreover, treatment of cancer patients with VKAs has turned out to be more unstable10 than for noncancer patients, which is probably due to several reasons: for example, the ability to take VKAs on a regular basis might be impaired because of nausea, or other medications might influence the effectiveness of VKAs. Thus, VKA treatment is cumbersome for cancer patients, because they need monitoring of the international normalized ratio more frequently than noncancer patients.10
Heparin, on the other hand, is only available as a parenteral drug, so it has to be used either IV (mostly unfractionated heparin) or subcutaneously (unfractionated heparin or LMWH). The daily needle prick for injection of LMWH causes pain and often a burning sensation at the site of injection, which many patients experience to be unpleasant. Furthermore, at the site of injection, a hematoma or allergic reaction may occur, which again constitutes a deterioration in a cancer patient’s quality of life.
At present, we have a number of direct oral anticoagulants (DOACs) available that, as the term “oral” already illustrates, can be used per os. DOACs have become the treatment of choice for stroke prevention in patients with arterial fibrillation11 and in noncancer patients with DVT or PE.12
Table 1 lists the 4 DOACs that are currently licensed in many countries for the treatment of VTE. In Table 1, which is derived from Farge et al,13 the most important, oncology-relevant qualities of these drugs are summarized. Apixaban, edoxaban, and rivaroxaban are anti–factor Xa antagonists, and dabigatran is a factor IIa antagonist.
Characteristics of available DOACs for VTE treatment
| . | Dabigatran . | Rivaroxaban . | Apixaban . | Edoxaban . |
|---|---|---|---|---|
| Target | Flla | FXa | FXa | FXa |
| Therapeutic dose | 150 mg twice a day; 110 mg twice a day for patients aged >80 y; 110 mg twice a day following at least 5 d of parenteral anticoagulants for patients with a high risk of bleeding | 15 mg twice a day for 3 wk followed by 20 mg once a day | 10 mg twice a day for 7 d, followed by 5 mg twice a day | 60 mg once a day following ≥5 d of parenteral anticoagulants |
| Prodrug | Yes | No | No | No |
| Bioavailability | 3-7% | 10 mg dose: 100%; 20 mg dose: 100% when taken with food; 66% under fasting conditions; interindividual variability: 30-40% | ∼50%; interindividual variability: 30% | ∼62% |
| Activity onset | 1-3 h | 2-4 h | 3-4 h | 1-2 h |
| Half-life | 12-18 h | 5-13 h | 12 h | 10-14 h |
| Excretion (% of administered dose) | 80% renal (unchanged), 20% liver | 66% renal (half active drug unchanged and half inactive metabolites), 33% feces (inactive metabolites) | 25% renal, 75% feces | 50% renal (unchanged, 50% biliary or intestinal) |
| Considerations for renal insufficiency | Mild or moderate: dose adjustment recommended; severe: contraindicated if GFR <30 mL/min | Moderate (GFR 30-49 mL/min): dose adjustment recommended; severe: not recommended if GFR <30 mL/min | Mild or moderate, or if GFR >25-30 mL/min: no dose adjustment required; severe: not recommended if GFR <15 mL/min; no data available in patients with end-stage renal disease | Moderate (GFR, 30-50 mL/min): dose adjustment recommended; severe: not recommended if GFR <15 mL/min; no data available in patients with end-stage renal disease or on dialysis |
| Considerations for hepatic insufficiency | Liver enzymes twice normal limit or if acute liver diseases: not recommended | Moderate hepatic impairment: caution; hepatic disease with coagulopathy and clinically relevant bleeding risk: contraindicated | Mild or moderate hepatic impairment: caution, but no dose adjustment required; severe hepatic impairment: not recommended; hepatic disease with coagulopathy and clinical relevant bleeding risk: contraindicated | Mild hepatic impairment: no dose reduction; moderate or severe hepatic impairment: not recommended |
| Interaction | P-glycoprotein inducers or inhibitors | P-glycoprotein inducers or inhibitors; CYP3A4 and CYP2J2 | P-glycoprotein inducers or inhibitors; CYP3A4 | P-glycoprotein inducers or inhibitors; CYP3A4 |
| Antidote | Idarucizumab | None | None | None |
| . | Dabigatran . | Rivaroxaban . | Apixaban . | Edoxaban . |
|---|---|---|---|---|
| Target | Flla | FXa | FXa | FXa |
| Therapeutic dose | 150 mg twice a day; 110 mg twice a day for patients aged >80 y; 110 mg twice a day following at least 5 d of parenteral anticoagulants for patients with a high risk of bleeding | 15 mg twice a day for 3 wk followed by 20 mg once a day | 10 mg twice a day for 7 d, followed by 5 mg twice a day | 60 mg once a day following ≥5 d of parenteral anticoagulants |
| Prodrug | Yes | No | No | No |
| Bioavailability | 3-7% | 10 mg dose: 100%; 20 mg dose: 100% when taken with food; 66% under fasting conditions; interindividual variability: 30-40% | ∼50%; interindividual variability: 30% | ∼62% |
| Activity onset | 1-3 h | 2-4 h | 3-4 h | 1-2 h |
| Half-life | 12-18 h | 5-13 h | 12 h | 10-14 h |
| Excretion (% of administered dose) | 80% renal (unchanged), 20% liver | 66% renal (half active drug unchanged and half inactive metabolites), 33% feces (inactive metabolites) | 25% renal, 75% feces | 50% renal (unchanged, 50% biliary or intestinal) |
| Considerations for renal insufficiency | Mild or moderate: dose adjustment recommended; severe: contraindicated if GFR <30 mL/min | Moderate (GFR 30-49 mL/min): dose adjustment recommended; severe: not recommended if GFR <30 mL/min | Mild or moderate, or if GFR >25-30 mL/min: no dose adjustment required; severe: not recommended if GFR <15 mL/min; no data available in patients with end-stage renal disease | Moderate (GFR, 30-50 mL/min): dose adjustment recommended; severe: not recommended if GFR <15 mL/min; no data available in patients with end-stage renal disease or on dialysis |
| Considerations for hepatic insufficiency | Liver enzymes twice normal limit or if acute liver diseases: not recommended | Moderate hepatic impairment: caution; hepatic disease with coagulopathy and clinically relevant bleeding risk: contraindicated | Mild or moderate hepatic impairment: caution, but no dose adjustment required; severe hepatic impairment: not recommended; hepatic disease with coagulopathy and clinical relevant bleeding risk: contraindicated | Mild hepatic impairment: no dose reduction; moderate or severe hepatic impairment: not recommended |
| Interaction | P-glycoprotein inducers or inhibitors | P-glycoprotein inducers or inhibitors; CYP3A4 and CYP2J2 | P-glycoprotein inducers or inhibitors; CYP3A4 | P-glycoprotein inducers or inhibitors; CYP3A4 |
| Antidote | Idarucizumab | None | None | None |
Reprinted with permission from Elsevier/The Lancet Oncology.13
Flla. thrombin; FXa, factor Xa; GFR, glomerular filtration rate.
Each of the phase 3 studies in patients with VTE that investigated DOACs in patients with DVT or PE and compared these novel treatments with warfarin also included a minority of patients with a history of cancer or active cancer. In the chapter on treatment of cancer-associated VTE, these studies14-22 are discussed in more detail and with specific focus on cancer patients.
In contrast to the general population with VTE, up to now, specific studies on patients with cancer-associated thrombosis and the use of DOACs are very limited. Furthermore, in cancer patients, the ideal comparator with DOACs is not warfarin, but LMWH, because LMWH is still regarded as the gold standard for treating cancer-associated thrombosis.12,13 Even fewer specific studies are available regarding prophylaxis of VTE by using DOACs. There is one published phase 2 study that specifically aimed at investigating the safety of a DOAC (apixaban) in comparison with LMWH (see below),23 and a limited number of patients with cancer (∼7%) were included in a thrombosis prophylaxis study of rivaroxaban in acutely ill patients.24
Prevention of cancer-associated VTE
When patients are admitted to the hospital with active cancer and additional risk factors for thrombosis, such as immobilization, infection, or heart failure, they receive primary thrombosis prophylaxis with LMWH,25 a practice that is well established in many countries. Usually, thrombosis prophylaxis is terminated when patients are discharged from the hospital. Thrombosis prophylaxis in ambulatory cancer patients has been studied and deemed effective26,27 ; however, in an unselected group of ambulatory cancer patients, even for those with advanced disease, the rate of cancer-associated thrombosis is low (<4% in the placebo groups),26,27 so that even a 50% reduction in the VTE rate is hardly regarded as a sufficient reason for initiating long-term usage (3-6 months) of a parenteral medication in a cancer patient. For patients with a tumor entity that is associated with a particularly high risk of thrombosis (eg, in pancreatic cancer), the rate of VTE during the course of disease is much higher (15.1%), which could be reduced to 6.4% with enoxaparin.28 Thus, for such a high-risk group of patients, primary thrombosis prophylaxis seems more reasonable.
Until now, studies on primary thrombosis prophylaxis in cancer patients with DOACs have been very limited. There is one phase 2 trial with apixaban for the prevention of thromboembolism in patients with metastatic cancer.23 The aim of this study was to evaluate the safety and efficacy of apixaban as primary thrombosis prophylaxis in patients with metastatic cancer who received chemotherapy. Eligible patients had either first-line or second-line chemotherapy for advanced or metastatic lung, breast, gastrointestinal, or bladder cancer, cancer of unknown origin, ovarian or prostate cancer, or myeloma or selected lymphoma. During the study, it was decided that, the patients were also allowed to receive bevacizumab in addition to standard chemotherapy. Patients were randomized in a 1:1:1:1 ratio to receive apixaban at a dosage of 5, 10, or 20 mg per day or placebo. The duration of this study was 12 weeks, and visits occurred at weeks 3, 6, 9, and 12. The primary outcome for the assessment of tolerability was the occurrence of either major bleeding or clinically relevant nonmajor bleeding (CRNM). As a secondary outcome, the authors defined symptomatic DVT or PE of grade ≥3 as an adverse event considered to be related to the study drug in addition to death. There was no screening for VTE, but objective tests had to be used to diagnose VTE. In addition to the clinical outcomes, blood samples were taken at baseline, twice during the study, and at the end of the study at week 12 for the investigation of prothrombin fragment 1+2 and D-dimer. In total, 125 patients were randomized to 1 of 4 treatment regimens. Approximately 30 patients each were allocated to the various apixaban dosages or placebo. Between 63% and 80% of patients of the various groups completed the 12 weeks of the study. Between 18% and 40% of the various groups were patients with breast cancer, the second largest group was patients with colorectal cancer. With regard to study outcomes, there were 3 major bleeding events in total, none in the 5- and 10-mg of apixaban dosage groups, but 2 in the 20-mg apixaban arm, and 1 in the placebo arm. CRNM was also rare, with 1 occurrence in the 5-mg and 10-mg apixaban arm each, 2 in the 20-mg arm, and none in the placebo group. There were 3 VTE events, all in the 29 evaluated patients with placebo. The grade 3 adverse events were found in the 5- and 20-mg apixaban group, 1 in the 20-mg group, and 2 in the 5-mg group. The event in the 20-mg arm was adjudicated as a major bleed.
In addition, D-dimer and prothrombin fragment 1+2 levels were measured in these patients during the course of disease. The d-dimer levels were rather constant over time and did not differ between patients with placebo and those on apixaban. In contrast, there was a trend for the prothrombin fragment 1+2 levels, which were comparable between the groups at baseline, but tended to decrease during treatment with apixaban, and there was also some dose-response relationship, with the lowest values in patients on the higher doses of apixaban. However, the differences were statistically not significant, most probably due to the low number of patients in the study.
It can be concluded that apixaban is well tolerated in patients with active cancer who are undergoing chemotherapy. There was a trend toward a higher risk of bleeding in patients receiving 20 mg of apixaban per day. The VTE events certainly have to be interpreted with caution, because the numbers of patients in the various groups were low. However, it should be kept in mind that all patients who had a VTE event (n = 3) were in the placebo group, which at the end had a VTE event rate of 10% and thus resembles the findings from other studies of patients with advanced tumors.7
Rivaroxaban was used for thrombosis prophylaxis in 8101 acutely ill medical patients in the MAGELLAN trial.24 Patients ≥40 years of age and hospitalized for various acute medical illnesses with risk factors for VTE randomly received either 40 mg enoxaparin subcutaneously once daily for 10 ± 4 days or oral rivaroxaban 10 mg once daily for 35 ± 4 days. The primary efficacy outcome was the composite of asymptomatic proximal DVT, symptomatic DVT, symptomatic nonfatal PE, and VTE-related death up to day 10 + 4 and up to day 35 ± 4. The primary safety outcome was the composite of treatment-emergent major bleeding and CRNM.29 In both the rivaroxaban and the enoxaparin/placebo group, 7.3% of patients had active cancer. Furthermore, 17.3% and 16.7% of patients had a history of cancer, respectively. The composite primary efficacy outcome on day 10 was similar in patients on rivaroxaban and enoxaparin. On day 35, the primary efficacy outcome was found in 5.7% of patients on placebo and 4.4% of patients on rivaroxaban. With regard to major bleeding, patients on rivaroxaban had a clinically relevant bleeding rate of 2.8% compared with 1.2% in the enoxaparin/placebo group. Other safety outcomes did not differ between the groups. The subgroup analysis of cancer patients revealed a primary efficacy outcome on day 35 in a higher number of patients on rivaroxaban (9.9%; 20 of 202 patients) compared with those on enoxaparin/placebo (7.4%; 15 of 203 patients). This difference was not statistically significant. Looking at the safety outcome (clinically relevant bleeding), cancer patients performed similarly to the other patients, with a significantly higher bleeding risk in those on rivaroxaban of 5.4% (16 of 294 patients) compared with 1.7% in those on enoxaparin/placebo (5 of 290 patients).
The interpretation of this data for cancer patients must be made with caution. The study was not designed to specifically investigate cancer patients. The trend with regard to bleeding was similar when compared with the whole group of patients. However, there was no decrease in the number of VTE events for patients on prolonged rivaroxaban compared with the short-term enoxaparin patients, and then placebo phase, which was rather unexpected. When looking at the subgroup analysis given in the supplement (Figure S1 in Cohen et al24 ), the cancer patients were the only patients with a relative risk (RR) >1, favoring enoxaparin/placebo; however, the 95% confidence interval (CI) was wide (precise data not given) and included 1.
Another oral anti–factor Xa antagonist, which was only recently approved in the United States, is betrixaban. Betrixaban was investigated in patients who were hospitalized for acute medical illnesses. Betrixaban given for 35 to 42 days was compared with subcutaneous enoxaparin given for 10 ± 4 days. Twelve percent of patients had a history of cancer. Overall, the primary efficacy outcome (composite of asymptomatic proximal DVT and symptomatic VTE) occurred in 6.9% of patients receiving betrixaban and in 8.5% of patients receiving enoxaparin (RR, 0.81; 95% CI, 0.65-1.0), whereas major bleeding complications occurred with a comparable frequency of ≤0.7% in both treatment groups. Betrixaban is an extremely interesting substance for primary thrombosis prophylaxis in cancer patients, and follow-up studies in ambulatory cancer patients are an important next step.30,31
It can be concluded that, at present, the data on primary thrombosis prophylaxis with DOACs in patients with active cancer are too few to draw any reliable conclusions. Additional studies (eg, the Apixaban for the Prevention of Venous Thromboembolism in Cancer Patients [clinicaltrials.gov identifier NCT02048865] and CASSINI studies [clinicaltrials.gov identifier NCT02555878] are needed that investigate whether apixaban or rivaroxaban are able to decrease thromboembolic events in ambulatory cancer patients who are preselected on the basis of a high anticipated VTE risk using the validated Khorana score (cut off risk score ≥2).32
Treatment of cancer-associated VTE
The efficacy and safety of DOACs was assessed in the various pivotal studies that led to the approval of the DOACs apixaban, dabigatran, edoxaban, and rivaroxaban for the treatment and secondary prophylaxis of DVT and PE in the United States, Canada, the European Union, and many other countries. In each of these studies, patients with active cancer were included, however, their numbers were relatively small, and the comparator was not LMWH, but warfarin, which is known to be inferior to LMWH in the treatment of cancer-associated thrombosis.33 Data on the outcomes and safety of cancer patients in the DOAC pivotal trials were nicely summarized in a recent review.34
In the Apixaban for the Initial Management of Pulmonary Embolism and Deep-Vein Thrombosis as First-Line Therapy trial (5400 patients),14 apixaban was started immediately after VTE diagnosis and was compared with enoxaparin/warfarin, with a follow-up time of 6 months. There were 81 patients with active cancer in the apixaban group and 78 in the enoxaparin/warfarin group. In addition, 365 patients (179 patients on apixaban and 175 patients on warfarin) had a history of cancer. In the subgroup analysis of patients with cancer, VTE or VTE-related death occurred in 3.7% of patients on apixaban and 6.4% of patients on warfarin. For those with a history of cancer, the related numbers were 1.1% and 6.3%, respectively. There were also fewer major bleeding events in the patients on apixaban (in patients with active cancer, the rate 2.3% vs 5.0%; in those with a history of cancer, the rate was 0.5% vs 2.8%).
The RE-COVER I and II studies investigated the use of dabigatran vs warfarin. Both treatment groups received enoxaparin in the initial phase for 5 days.15,16 The specific data on the subgroup of patients with cancer were published in 2015.17 There were 114 patients with active cancer receiving dabigatran, and 107 receiving warfarin. The treatment duration was 6 months. The number of patients with a history of cancer were not given, however, 59 patients in the dabigatran group and 55 patients in the warfarin group developed cancer during the course of the study. The rate of VTE or VTE-related death was comparable between patients with active cancer on dabigatran and those on warfarin (3.5% and 4.7%), and the rate of major bleeding was also quite similar between the groups (3.8% and 3.0%). For those patients who developed cancer during the study, both the recurrence rate (8.5% vs 13.0%) and the bleeding rate (3.7% vs 7.7%) were lower in patients on dabigatran than those on warfarin.
The Hokusai-VTE study investigated the use of edoxaban vs warfarin in 8240 patients with VTE.18 Data on the subgroup of patients with cancer were published separately in 2016.19 A total of 109 patients were randomized to the edoxaban group and 99 to the warfarin group. In addition, quite a high number had a history of cancer. In total, 378 on edoxaban and 393 on warfarin had either active cancer or a history of cancer. The rate of VTE or VTE-related death was 4% for those receiving edoxaban vs 7% in those receiving warfarin. Major bleeding occurred at a rate of 3% in each of the 2 patient groups. A total of 78 patients in the edoxaban group and 97 patients in the VKA group developed cancer during the study. For these patients, the VTE recurrence rate was higher (17% for patients receiving edoxaban and 20% for patients receiving a VKA).
In the EINSTEIN-DVT20 and -PE trials,21 which were conducted separately, a separate evaluation of cancer patients was performed.22 Rivaroxaban was compared with enoxaparin/warfarin or acenocoumarol (hereafter, VKA) in an open-label study. The definition of the subgroup of patients with cancer in this trial was either active cancer at inclusion or cancer diagnosed during anticoagulant treatment and was thus different than the other studies. According to their definition, 354 patients with active cancer were included in the rivaroxaban arm, and 301 were randomized to receive a VKA. Recurrent VTE occurred in 5% of patients allocated to the rivaroxaban group and in 7% of patients allocated to the VKA group. With regard to safety, the rate of major bleeding was significantly lower in patients receiving rivaroxaban (2% vs 5%; hazard ratio, 0.42; 95% CI, 0.18-0.99). The authors point out that the risk of developing a recurrent VTE was highest for those patients who had a new diagnosis of cancer during the study in both groups (10% in patients on rivaroxaban and 12% in those on a VKA).
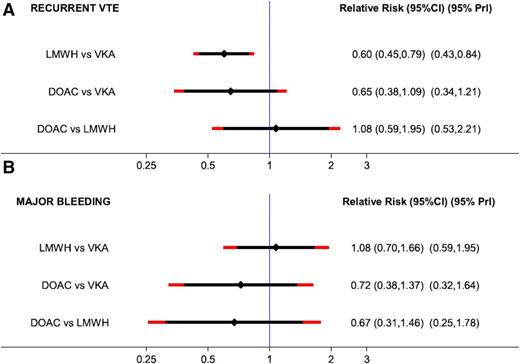
There are several meta-analyses in the literature that include these specific patients and evaluate the efficacy and safety of DOACs compared with warfarin in patients with cancer.35-39 We would like to specifically present data on a network meta-analysis published in 2015 that focused on the treatment of VTE in patients with cancer and evaluated the efficacy and safety of anticoagulants by comparing 6 trials that investigated LMWH in comparison with VKAs and 4 studies (described above) that compared DOACs with VKAs. This network meta-analysis provides a very detailed overview of the study characteristics, the patients, and the recurrence and bleeding rates.37 In the pairwise meta-analysis of the 6 trials (LMWH vs VKA), the risk for recurrent VTE was highly in favor of LMWH (RR, 0.60; 95% CI, 0.45-0.79), and the risk of major bleeding did not differ (RR, 1.07; 95% CI, 0.66-1.73). Comparing DOACS with VKA, the risk for recurrent VTE nonsignificantly favored DOACs (RR, 0.65; 95% CI, 0.38-01.09) as did the risk of major bleeding (RR, 0.79; 95% CI, 0.39-1.09). In the network meta-analysis, an indirect network comparison was used that compared LMWH with DOACs via their common comparator VKA in the star-shaped network (Figure 1). The comparison between DOACs and LMWH indicated comparable efficacy (risk ratio, 1.08; 95% CI, 0.59-1.95), and a nonsignificant RR toward improved safety with DOACs (RR, 0.67; 95% CI, 0.31-1.46). The results prevailed after adjusting for different risks for recurrent VTE and major bleeding between LMWH vs VKA and DOAC vs VKA studies. The results are shown as a forest plot in Figure 2. In addition, estimates from the network meta-analysis were adjusted to a 10% 6-month risk of recurrent VTE (reflecting efficacy) and a 5% 6-month risk of major bleeding (reflecting safety) in the VKA arm. The risk ratios and 95% CIs of the unadjusted and adjusted network meta-analysis are shown in Table 2.
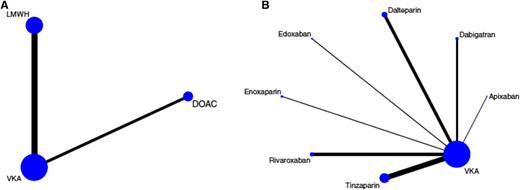
Network plots of included studies on the treatment of cancer-associated VTE. Nodes (blue dots) and edges (black connecting lines between nodes) are scaled according to the number of patients in the respective studies. Consequently, the larger the size of the respective trial(s), the larger the nodes and edges. (A) Pooled network as analyzed in the network meta-analysis. As indicated by the size of the nodes and edges, most evidence exists for VKAs, followed by LMWH and non-VCA oral anticoagulants (DOACs). (B) Full trial network showing individual LMWH and DOAC drugs. Again, the size of the nodes and edges is proportional to the size of the respective studies, and thus the amount of evidence for the drug within the trial network. The length of the edges does not convey information, and differences in edge lengths are simply for better graphical presentation. Reprinted with permission from Elsevier/Thrombosis Research.37
Network plots of included studies on the treatment of cancer-associated VTE. Nodes (blue dots) and edges (black connecting lines between nodes) are scaled according to the number of patients in the respective studies. Consequently, the larger the size of the respective trial(s), the larger the nodes and edges. (A) Pooled network as analyzed in the network meta-analysis. As indicated by the size of the nodes and edges, most evidence exists for VKAs, followed by LMWH and non-VCA oral anticoagulants (DOACs). (B) Full trial network showing individual LMWH and DOAC drugs. Again, the size of the nodes and edges is proportional to the size of the respective studies, and thus the amount of evidence for the drug within the trial network. The length of the edges does not convey information, and differences in edge lengths are simply for better graphical presentation. Reprinted with permission from Elsevier/Thrombosis Research.37
Forest plot of the RRs with 95% predictive intervals (95% PrI): network meta-analysis (NMA). 95% CIs are black, and 95% PrIs are red. (A) Estimates for recurrent VTE. (B) Estimates for major bleeding. Reprinted with permission from Elsevier/Thrombosis Research.37
Forest plot of the RRs with 95% predictive intervals (95% PrI): network meta-analysis (NMA). 95% CIs are black, and 95% PrIs are red. (A) Estimates for recurrent VTE. (B) Estimates for major bleeding. Reprinted with permission from Elsevier/Thrombosis Research.37
RRs of recurrent VTE and major bleeding with 3 anticoagulant strategies for the treatment of cancer-associated VTE: network meta-analysis
| Unadjusted network meta-analysis . | Adjusted network meta-analysis . | ||||
|---|---|---|---|---|---|
| VKA | 0.60 (0.45-0.79) | 0.65 (0.38-1.09) | VKA | 0.67 (0.46-0.97) | 0.93 (0.20-4.40) |
| 1.08 (0.70-1.66) | LMWH | 1.08 (0.59-1.95) | 1.30 (0.83-2.05) | LMWH | 0.71 (0.14-3.51) |
| 0.72 (0.39-1.37) | 0.67 (0.31-1.46) | DOAC | 0.52 (0.21-1.29) | 0.40 (0.15-1.19) | DOAC |
| Unadjusted network meta-analysis . | Adjusted network meta-analysis . | ||||
|---|---|---|---|---|---|
| VKA | 0.60 (0.45-0.79) | 0.65 (0.38-1.09) | VKA | 0.67 (0.46-0.97) | 0.93 (0.20-4.40) |
| 1.08 (0.70-1.66) | LMWH | 1.08 (0.59-1.95) | 1.30 (0.83-2.05) | LMWH | 0.71 (0.14-3.51) |
| 0.72 (0.39-1.37) | 0.67 (0.31-1.46) | DOAC | 0.52 (0.21-1.29) | 0.40 (0.15-1.19) | DOAC |
The 3 × 3 table on the left reports RRs from unadjusted network meta-analysis, and the 3 × 3 table on the right is the RRs from an adjusted network meta-analysis, in which the 6-month risks of recurrent VTE and major bleeding were calibrated to 10% and 5%, respectively. The RRs (95% CI) of recurrent VTE and major bleeding for each respective comparison can be found within the rectangle defined by the two anticoagulant strategies. The upper of the two results pertains to recurrent VTE, whereas the lower result pertains to major bleeding. The reference category (ie, denominator) for the relative risk is always higher to the left. For example, in the left 3 × 3 table, a rectangle defined by LMWH and DOAC includes 2 results, namely 1.08 (0.59-1.59) and 0.67 (0.31-1.46). The upper of these two (ie, 1.08) is the RR of recurrent VTE for DOAC vs LMWH (with LMWH as the denominator, ie, a 1.08-fold higher risk of recurrent VTE with DOAC compared with LMWH), whereas the lower of these 2 results (ie, 0.67) is the RR of major bleeding for DOAC vs LMWH (ie, 0.67-fold lower risk with DOAC compared with LMWH). Reprinted with permission from Elsevier/Thrombosis Research.37
*Estimates from the unadjusted network meta-analysis.
†Estimates from a network meta-analysis adjusted to a 10% 6-month risk of recurrent VTE in the VKA arm (efficacy) and a 5% 6-month risk of major bleeding in the VKA arm (safety).
We still lack the head-to-head comparison between a DOAC and LMWH. However, several studies are currently ongoing that are comparing the use of edoxaban, rivaroxaban with dalteparin, and apixaban vs LMWH in patients with VTE and cancer. The first results will probably be available by the end of 2017.
Guidelines
The various guidelines on the prevention and treatment of cancer-associated VTE are summarized in the recent review by Ay et al.7 The guidelines (ACCP,12 ASCO,40 BCSH,41 EMN,42 ESMO,43 International Clinical Practice Guidelines,13 ISTH,44 NCCN45 ), all precisely illustrated in tables in Ay et al,7 recommend treating acute cancer-associated VTE with LMWH and also recommend long-term and extended treatment with LMWH. There are differences in the strength of the recommendations; for example, the International Clinical Practice Guidelines have a strong recommendation,13 whereas the ACCP guidelines12 have a less strong recommendation in favor of LMWH in these patients. It has already been noted that regular injections may considerably decrease the quality of life of a patient.
The International Clinical Practice Guidelines have a particular focus on DOAC use and specifically discuss the use of DOACs in patients with cancer.13 Their conclusion is that DOACs are not inferior to VKAs in the treatment of VTE, however, there is a clear need for a direct comparison between LMWH and DOACs.
It must be mentioned, however, that treatment with LMWH is a considerable cost burden for the insurance system and for patients in some countries.
Guidance on experience, patient preferences, and values
Trials that specifically deal with the prevention and treatment of VTE in cancer patients are still limited. Nevertheless, data can be extracted from large trials, and the meta-analyses performed on these patients show that DOACs can be regarded as efficacious and safe in patients with cancer.37 Although we know that patients might also accept long-term subcutaneous injection,46 they usually prefer an oral medication. There are also patients who either have local reactions at the site of the LMWH injection or who are afraid of needles or the associated pain during the injection. Furthermore, there are those who are not able to perform self-injections, either because they are too afraid of doing so or because they are too impaired in their constitution and performance. Although such problems may be overcome by nurses or other health professionals or by educating family members to perform the injection, an oral medication can of course be administered more easily and is thus preferable.
Based on the efficacy and safety data that are available from previous studies and that have been evaluated in meta-analyses, the International Clinical Practice Guidelines group considers DOACs to be a potential treatment of VTE for patients with stable cancer who do not receive systemic anticancer therapy and for cases in which a VKA is an acceptable, but not available treatment option.13
After 3 to 6 months of treatment with LMWH, we have no data comparing LMWH either with a VKA or with DOACs. In all current guidelines, individual assessment of the benefit-risk ratio, tolerability, and each patient’s individual preference are the basis for decisions on further anticoagulation therapy. It is our opinion, and that of many others, that, at the present stage, DOACs are a good option for patients with active and nonactive cancer. Of course, the interactions between DOACs and chemotherapeutic agents, antiangiogenic therapies, and novel treatments have yet to be considered. With regard to primary prevention, at present, we would not consider DOACs as a possible treatment option for either inpatients with an acute illness or for ambulatory patients with or without systematic anticancer therapy.
Acknowledgments
The authors thank Tanja Altreiter for proofreading the manuscript.
Correspondence
Ingrid Pabinger, Clinical Division of Haematology and Haemostaseology, Department of Medicine I, Medical University of Vienna, Währinger Gürtel 18-20, 1090 Vienna, Austria; e-mail: ingrid.pabinger@meduniwien.ac.at.
References
Author notes
Conflict-of-interest disclosure: I.P. has consulted for and received honoraria from Boehringer-Ingelheim, Pfizer, Sanofi, and Daiichi-Sachyo. The remaining author declares no competing financial interests.
Off-label drug use: The author will discuss (but not recommend) thrombosis prophylaxis in cancer patients with direct oral anticoagulants.