Abstract
Patients with cancer have a high risk of venous thromboembolism (VTE) and about one-half of these events are incidentally detected. The prognosis of incidental VTE appears to be similar to symptomatic events, with comparably high rates of recurrent VTE in this patient population. In the absence of major contraindications, anticoagulant treatment with low-molecular-weight heparin for 3 to 6 months is generally recommended for incidental proximal deep vein thrombosis as well as for incidental pulmonary embolism that involves multiple subsegmental or more proximal pulmonary arteries. The decision of whether to extend treatment beyond 3 to 6 months should be evaluated on a case-by-case basis after periodic reassessment of the risks factors for bleeding and recurrent VTE while also taking into account patient preferences. The clinical relevance of a single incidental subsegmental pulmonary embolism without concomitant deep vein thrombosis is uncertain and either a watchful approach or a shorter course of anticoagulation to minimize the bleeding risk may also be considered. Preliminary evidence suggests that anticoagulation treatment may be beneficial for cancer patients with incidental distal deep vein thrombosis or incidental splanchnic vein thrombosis.
Learning Objectives
Understand the incidence and prognostic relevance of incidental venous thromboembolism
Learn the management options for incidental deep vein thrombosis and pulmonary embolism
Introduction
Patients with cancer have a high risk of developing venous thromboembolic events (VTEs), including deep venous thrombosis (DVT) and pulmonary embolism (PE), because of a state of chronic hypercoagulability caused by the cancer itself and by cancer therapies such as chemotherapy or radiotherapy.1 About one-half of all VTEs in cancer patients are incidentally detected without any clinical suspicion of VTE at the time of diagnosis.2,3 Although incidental VTEs may be truly asymptomatic, as many as two-thirds of affected patients report symptoms such as cough and fatigue or signs indicative of potential DVT.4-7 These clinical findings, however, may be often regarded as poorly specific for the presence of VTE and considered a side effect of the underlying cancer or its associated treatment.
In this review, we discuss the clinical relevance of incidental VTE in patients with cancer and critically evaluate the evidence on the efficacy and safety of anticoagulation in these patients.
Prevalence of incidental VTE
The prevalence of incidental VTE seems to vary broadly from less than 1% to 15% or higher, with most studies reporting incidental PE (Tables 1-3).2,6,8-15 This large variation probably stems from differences in study populations (eg, tumor type and stage), frequency and type of imaging tests used, and single vs double reading of the diagnostic scans.2 Incidental VTEs are mostly diagnosed by multidetector computed tomography (MDCT) scans requested for baseline staging, treatment response evaluation, or routine surveillance while off anticancer treatment. Although these scans do not represent the standard imaging tests for the diagnosis of VTE (ie, compression ultrasonography for DVT, CT pulmonary angiography or ventilation/perfusion lung scan for PE), the high spatial and temporal resolution of modern MDCT in conjunction with high-concentration contrast media enables the detection of VTEs even in routine CT scans.
Incidental PE in cancer patients
| Reference . | Study design . | Cancer patients (N) . | Cancer type . | CT reassessment . | CT scanner . | Slice thickness . | iPE, n (%) . |
|---|---|---|---|---|---|---|---|
| 3 | Retrospective | 3270 | Mixed | Yes | 64-row MDCT | 5 mm | 129 (3.9) |
| 44 | Prospective | 1090 | Mixed | Yes | NR | 2.5 mm | 14 (1.28) |
| 6 | Retrospective | 207 | Lung | No | NR | NR | 5 (2.4) |
| 45 | Prospective | 588 | Mixed | Yes | 4-row MDCT | 5-8 mm | 10 (1.7) |
| 46 | Prospective | 2085 | Mixed | NR | NR | 2 mm | 44 (2.1) |
| 47 | Retrospective | 410 | Mixed | Yes | 4-row MDCT | 5 mm | 14 (3.4) |
| 48 | Prospective | 385 | Mixed | No | 4-row MDCT | 5-8 mm | 10 (2.6) |
| 49 | Retrospective | 403 | Mixed | Yes | 4-row MDCT | 3.75 mm | 16 (4.0) |
| 50 | Retrospective | 397 | Mixed | Yes | NR | 8 mm | 13 (3.3) |
| 51 | Retrospective | 787 | Mixed | Yes | 16-row MDCT | 2.5 mm | 15 (1.9) |
| 52 | Prospective | 343 | Mixed | Yes | 4- or 16-row MDCT | 1-3 mm | 18 (5.2) |
| 53 | Retrospective | 765 | Mixed | Yes | 16-row MDCT | 2.5 mm | 17 (2.2) |
| 54 | Retrospective | 8014 | Lung | No | NR | NR | 180 (2.2) |
| 8 | Retrospective | 342 | Mixed | Yes | 4- or 16-row MDCT | 1-3 mm | 6 (1.8) |
| 55 | Retrospective | 1921 | Mixed | Yes | NR | NR | 24 (1.2) |
| 9 | Prospective | 407 | Mixed | Yes | 64-row MDCT | 1 and 5 mm | 18 (4.4) |
| 10 | Retrospective | 135 | Pancreas | No | NR | NR | 4 (3.0) |
| 11 | Retrospective | 13 783 | Mixed | No | 4- or 64-row MDCT | 5-7 mm | 202 (1.47) |
| 12 | Retrospective | 1331 | Mixed | No | PET-CT | 3 mm | 9 (0.7) |
| 56 | Retrospective | 453 | Prostate | No | NR | NR | 3 (0.66) |
| 57 | Retrospective | 838 | Mixed | No | NR | NR | 3 (0.36) |
| 13 | Retrospective | 220 | Gastrointestinal | No | NR | NR | 13 (5.9) |
| 14 | Retrospective | 141 | Lung | Yes | 6- or 64-row MDCT | 2.5 mm | 21 (14.9) |
| 15 | Prospective | 999 | Mixed | Yes | 64-row MDCT | 1.25 and 5 mm | 51 (5.1) |
| Reference . | Study design . | Cancer patients (N) . | Cancer type . | CT reassessment . | CT scanner . | Slice thickness . | iPE, n (%) . |
|---|---|---|---|---|---|---|---|
| 3 | Retrospective | 3270 | Mixed | Yes | 64-row MDCT | 5 mm | 129 (3.9) |
| 44 | Prospective | 1090 | Mixed | Yes | NR | 2.5 mm | 14 (1.28) |
| 6 | Retrospective | 207 | Lung | No | NR | NR | 5 (2.4) |
| 45 | Prospective | 588 | Mixed | Yes | 4-row MDCT | 5-8 mm | 10 (1.7) |
| 46 | Prospective | 2085 | Mixed | NR | NR | 2 mm | 44 (2.1) |
| 47 | Retrospective | 410 | Mixed | Yes | 4-row MDCT | 5 mm | 14 (3.4) |
| 48 | Prospective | 385 | Mixed | No | 4-row MDCT | 5-8 mm | 10 (2.6) |
| 49 | Retrospective | 403 | Mixed | Yes | 4-row MDCT | 3.75 mm | 16 (4.0) |
| 50 | Retrospective | 397 | Mixed | Yes | NR | 8 mm | 13 (3.3) |
| 51 | Retrospective | 787 | Mixed | Yes | 16-row MDCT | 2.5 mm | 15 (1.9) |
| 52 | Prospective | 343 | Mixed | Yes | 4- or 16-row MDCT | 1-3 mm | 18 (5.2) |
| 53 | Retrospective | 765 | Mixed | Yes | 16-row MDCT | 2.5 mm | 17 (2.2) |
| 54 | Retrospective | 8014 | Lung | No | NR | NR | 180 (2.2) |
| 8 | Retrospective | 342 | Mixed | Yes | 4- or 16-row MDCT | 1-3 mm | 6 (1.8) |
| 55 | Retrospective | 1921 | Mixed | Yes | NR | NR | 24 (1.2) |
| 9 | Prospective | 407 | Mixed | Yes | 64-row MDCT | 1 and 5 mm | 18 (4.4) |
| 10 | Retrospective | 135 | Pancreas | No | NR | NR | 4 (3.0) |
| 11 | Retrospective | 13 783 | Mixed | No | 4- or 64-row MDCT | 5-7 mm | 202 (1.47) |
| 12 | Retrospective | 1331 | Mixed | No | PET-CT | 3 mm | 9 (0.7) |
| 56 | Retrospective | 453 | Prostate | No | NR | NR | 3 (0.66) |
| 57 | Retrospective | 838 | Mixed | No | NR | NR | 3 (0.36) |
| 13 | Retrospective | 220 | Gastrointestinal | No | NR | NR | 13 (5.9) |
| 14 | Retrospective | 141 | Lung | Yes | 6- or 64-row MDCT | 2.5 mm | 21 (14.9) |
| 15 | Prospective | 999 | Mixed | Yes | 64-row MDCT | 1.25 and 5 mm | 51 (5.1) |
iPE, incidental pulmonary embolism; NR, not reported; PET, positron emission tomography.
Incidental DVT of the extremities in cancer patients
| Reference . | Study design . | Cancer patients (N) . | Cancer type . | Scanner . | Incidental DVT, n (%) . | Most proximal vein . | UEDVT . | ||
|---|---|---|---|---|---|---|---|---|---|
| . | . | . | . | . | . | Inferior vena cava . | Ileofemoral . | popliteal . | . |
| 44 | Prospective | 1090 | Mixed | NR | 3 (0.3) | 0 | 3 | NR | NR |
| 6 | Retrospective | 207 | Lung | NR | 3 (1.45) | NR | NR | NR | 3 |
| 50 | Retrospective | 339 | Mixed | NR | 23 (6.8) | 1 | 23 | 0 | 0 |
| 10 | Retrospective | 135 | Pancreas | NR | 6 (4.44)* | 0 | 5 | NR | 1 |
| 12 | Retrospective | 1331 | Mixed | PET-CT | 8 (0.6) | 0 | 4 | 0 | 3 |
| 56 | Retrospective | 453 | Prostate | NR | 13 (2.9) | NR | NR | NR | NR |
| 57 | Retrospective | 838 | Mixed | NR | 8 (0.95) | 4 | 4 | NR | 1 |
| Reference . | Study design . | Cancer patients (N) . | Cancer type . | Scanner . | Incidental DVT, n (%) . | Most proximal vein . | UEDVT . | ||
|---|---|---|---|---|---|---|---|---|---|
| . | . | . | . | . | . | Inferior vena cava . | Ileofemoral . | popliteal . | . |
| 44 | Prospective | 1090 | Mixed | NR | 3 (0.3) | 0 | 3 | NR | NR |
| 6 | Retrospective | 207 | Lung | NR | 3 (1.45) | NR | NR | NR | 3 |
| 50 | Retrospective | 339 | Mixed | NR | 23 (6.8) | 1 | 23 | 0 | 0 |
| 10 | Retrospective | 135 | Pancreas | NR | 6 (4.44)* | 0 | 5 | NR | 1 |
| 12 | Retrospective | 1331 | Mixed | PET-CT | 8 (0.6) | 0 | 4 | 0 | 3 |
| 56 | Retrospective | 453 | Prostate | NR | 13 (2.9) | NR | NR | NR | NR |
| 57 | Retrospective | 838 | Mixed | NR | 8 (0.95) | 4 | 4 | NR | 1 |
UEDVT, upper extremity DVT.
Events.
Incidental SVT in cancer patients
| Reference . | Study design . | Cancer patients (N) . | Cancer type . | Scanner . | Patients with incidental SVT, n (%) . | Site of SVT, n . |
|---|---|---|---|---|---|---|
| 44 | Prospective | 1090 | Mixed | NR | 3 (0.27) | Superior mesenteric vein = 1 |
| Pelvic = 1 | ||||||
| Gonadal = 1 | ||||||
| 6 | Retrospective | 207 | Lung | NR | 3 (1.45) | Portal vein = 2 |
| Hepatic vein = 1 | ||||||
| 10 | Retrospective | 135 | Pancreas | NR | 31 (22.96) | 47 DVTs* |
| Portal vein = 18 | ||||||
| Splenic = 14 | ||||||
| Superior mesenteric vein = 11 | ||||||
| Inferior mesenteric vein = 4 | ||||||
| Hepatic = 1 | ||||||
| Gonadal = 1 | ||||||
| 12 | Retrospective | 1331 | Mixed | PET-CT | 2 (0.15) | Portal vein = 1 |
| Superior mesenteric vein = 1 | ||||||
| 57 | Retrospective | 838 | Mixed | NR | 8 (0.95) | Portal vein = 5 |
| Superior mesenteric vein = 3 | ||||||
| Renal vein = 1 | ||||||
| 58 | Retrospective | 1442 | Mixed | 64-row MDCT | 36 (2.5) | NR |
| Reference . | Study design . | Cancer patients (N) . | Cancer type . | Scanner . | Patients with incidental SVT, n (%) . | Site of SVT, n . |
|---|---|---|---|---|---|---|
| 44 | Prospective | 1090 | Mixed | NR | 3 (0.27) | Superior mesenteric vein = 1 |
| Pelvic = 1 | ||||||
| Gonadal = 1 | ||||||
| 6 | Retrospective | 207 | Lung | NR | 3 (1.45) | Portal vein = 2 |
| Hepatic vein = 1 | ||||||
| 10 | Retrospective | 135 | Pancreas | NR | 31 (22.96) | 47 DVTs* |
| Portal vein = 18 | ||||||
| Splenic = 14 | ||||||
| Superior mesenteric vein = 11 | ||||||
| Inferior mesenteric vein = 4 | ||||||
| Hepatic = 1 | ||||||
| Gonadal = 1 | ||||||
| 12 | Retrospective | 1331 | Mixed | PET-CT | 2 (0.15) | Portal vein = 1 |
| Superior mesenteric vein = 1 | ||||||
| 57 | Retrospective | 838 | Mixed | NR | 8 (0.95) | Portal vein = 5 |
| Superior mesenteric vein = 3 | ||||||
| Renal vein = 1 | ||||||
| 58 | Retrospective | 1442 | Mixed | 64-row MDCT | 36 (2.5) | NR |
*Events.
The collimation of MDCT influences test accuracy with smaller slice thickness carrying a higher sensitivity, reduced artifacts, and increased detection of peripheral thrombosis such as subsegmental PEs.9,15 In a large prospective evaluation of 999 cancer patients undergoing contrast-enhanced chest CT examination for cancer follow-up, 30% of the 5-mm scans erroneously interpreted as negative were correctly reclassified when the collimation was decreased to 1.25 mm.15 Confident diagnosis of a filling defect at thick slices can be difficult with the potential of false positives resulting from partial voluming, respiratory or cardiogenic movement artifacts, and the presence of adjacent peribronchial lymph nodes. Tresoldi and colleagues found that thinner reconstructions allowed a confident diagnosis of 3% to 10% of cases that remained uncertain at the first 5-mm evaluation.15 False-positive results may still be a concern when peripheral thrombosis such as subsegmental PE is incidentally detected. Confirmation by CT pulmonary angiography is often not feasible in these cases because of additional radiation and contrast exposure for the patient as well as increased health care costs. Interobserver variability may also complicate the diagnosis of incidental VTE. Two studies showed a high level of agreement between radiologists for proximal incidental PEs,16,17 but a disappointingly lower agreement for filling defects that involved segmental and subsegmental branches.17 Therefore, it is important to review the images of reported incidental peripheral filling defects with an experienced radiologist to avoid false-positive diagnosis of incidental subsegmental PE in this patient population and an unnecessary exposure to potentially harmful anticoagulation treatment.
Data on the prevalence of incidental DVT of the extremities are sparse and show a large variation from less than 1% to about 7% (Table 2). These estimates may significantly underestimate the actual prevalence of incidental DVT because systematic assessment of the veins down to the trifurcation area or in the upper limb is not routinely performed.
SVT
Although generally regarded as a relatively rare type of VTE, splanchnic vein thrombosis (SVT) is increasingly diagnosed in patients with cancer, which may be partly explained by the frequent detection of incidental SVT on repeated imaging tests performed for cancer staging and response assessment.18-20 As for VTE at other sites, the prevalence of incidental SVT ranges broadly from less than 2%8,12 to more than 20%10 (Table 3). The type of cancer evaluated as well as type and frequency of imaging tests performed may affect these estimates, as suggested by a recent retrospective cohort of 135 pancreatic cancer patients in whom a diagnosis of incidental SVT was confirmed in as many as 23% of patients.10 In a small retrospective series of 18 cancer patients with incidental SVT, thrombosis more frequently involved the portal (61%) and superior mesenteric (50%) veins, with multiple veins affected in 44% of the patients.21
Prognosis of incidental VTE
About 60% of all incidental PEs involve the main or lobar arteries; bilateral lung involvement occurs in approximately one-third of cases (Table 4). Few small studies have suggested that incidental PE may present a lower embolic burden when compared with symptomatic PE.3,22 However, these studies may have underestimated the actual thrombotic load.
Radiologic characteristics of incidental pulmonary embolism in cancer patients
| Reference . | iPE (N) . | Most proximal arterial thrombus location, n (%) . | Bilateral (%) . | |||||
|---|---|---|---|---|---|---|---|---|
| Main . | Lobar . | Segmental . | Subsegmental . | Central . | Peripheral . | |||
| 5 | 66 | 4 (6.1) | 14 (21.2) | 38 (57.6) | 10 (15.2) | 18 (27.3) | 48 (72.7) | NR |
| 4 | 70 | 7 (10.0) | 26 (37.1) | 20 (28.6) | 17 (24.3) | 33 (47.1) | 37 (52.9) | NR |
| 6 | 5 | 3 (60) | NR | 1 (20) | 1 (20) | 3 (60) | 2 (40) | 2 (40) |
| 45 | 13 | 4 (30.8) | 4 (30.8) | 5 (38.5) | 0 (0.0) | 8 (61.5) | 5 (38.5) | 6 (46.2) |
| 49 | 16 | 0 (0.0) | 8 (50.0) | 7 (43.8) | 1 (6.3) | 8 (50.0) | 8 (50.0) | 6 (37.5) |
| 54 | 113 | NR | NR | 56 (49.6) | 0 (0.0) | 57 (50.4) | 56 (49.6) | 32 (28.3) |
| 9 | 18 | 4 (22.2) | 5 (27.8) | 6 (33.3) | 3 (16.7) | 9 (50.0) | 9 (50.0) | NR |
| 11 | 202 | 62 (30.7) | 62 (30.7) | 65 (32.2) | 13 (6.4) | 124 (61.4) | 78 (38.6) | NR |
| 12 | 9 | 4 (44.4) | 2 (22.2) | 3 (33.3) | 0 | 6 (66.7) | 3 (33.3) | NR |
| 57 | 3 | NR | NR | 2 (66.7) | 0 (0.0) | 1 (33.3) | 2 (66.7) | NR |
| 14 | 21 | 2 (9.5) | 6 (28.6) | 13 (61.9) | 0 (0.0) | 8 (38.1) | 13 (61.9) | 5 (23.8) |
| 15 | 51 | 3 (5.9) | 11 (21.6) | 27 (52.9) | 10 (19.6) | 14 (27.4) | 37 (72.6) | 14 (27.5) |
| 23 | 45 | NR | NR | 30 (66.7) | 4 (8.9) | 11 (24.4) | 34 (75.6) | NR |
| 24 | 56 | NR | NR | NR | NR | 36 (64.3) | 20 (35.7) | 23 (41.1) |
| Total | 674 | 93 (13.8) | 130 (19.3) | 260 (38.6) | 59 (8.7) | 325 (48.2) | 349 (51.8) | 67 (30.2) |
| Reference . | iPE (N) . | Most proximal arterial thrombus location, n (%) . | Bilateral (%) . | |||||
|---|---|---|---|---|---|---|---|---|
| Main . | Lobar . | Segmental . | Subsegmental . | Central . | Peripheral . | |||
| 5 | 66 | 4 (6.1) | 14 (21.2) | 38 (57.6) | 10 (15.2) | 18 (27.3) | 48 (72.7) | NR |
| 4 | 70 | 7 (10.0) | 26 (37.1) | 20 (28.6) | 17 (24.3) | 33 (47.1) | 37 (52.9) | NR |
| 6 | 5 | 3 (60) | NR | 1 (20) | 1 (20) | 3 (60) | 2 (40) | 2 (40) |
| 45 | 13 | 4 (30.8) | 4 (30.8) | 5 (38.5) | 0 (0.0) | 8 (61.5) | 5 (38.5) | 6 (46.2) |
| 49 | 16 | 0 (0.0) | 8 (50.0) | 7 (43.8) | 1 (6.3) | 8 (50.0) | 8 (50.0) | 6 (37.5) |
| 54 | 113 | NR | NR | 56 (49.6) | 0 (0.0) | 57 (50.4) | 56 (49.6) | 32 (28.3) |
| 9 | 18 | 4 (22.2) | 5 (27.8) | 6 (33.3) | 3 (16.7) | 9 (50.0) | 9 (50.0) | NR |
| 11 | 202 | 62 (30.7) | 62 (30.7) | 65 (32.2) | 13 (6.4) | 124 (61.4) | 78 (38.6) | NR |
| 12 | 9 | 4 (44.4) | 2 (22.2) | 3 (33.3) | 0 | 6 (66.7) | 3 (33.3) | NR |
| 57 | 3 | NR | NR | 2 (66.7) | 0 (0.0) | 1 (33.3) | 2 (66.7) | NR |
| 14 | 21 | 2 (9.5) | 6 (28.6) | 13 (61.9) | 0 (0.0) | 8 (38.1) | 13 (61.9) | 5 (23.8) |
| 15 | 51 | 3 (5.9) | 11 (21.6) | 27 (52.9) | 10 (19.6) | 14 (27.4) | 37 (72.6) | 14 (27.5) |
| 23 | 45 | NR | NR | 30 (66.7) | 4 (8.9) | 11 (24.4) | 34 (75.6) | NR |
| 24 | 56 | NR | NR | NR | NR | 36 (64.3) | 20 (35.7) | 23 (41.1) |
| Total | 674 | 93 (13.8) | 130 (19.3) | 260 (38.6) | 59 (8.7) | 325 (48.2) | 349 (51.8) | 67 (30.2) |
Several studies reported a similar prognosis for incidental and symptomatic VTE in patients with cancer.5,23-26 In a prospective study of cancer patients newly diagnosed with VTE, the incidence of recurrent VTE (11% vs 18%), major bleeding (7% vs 10%), and overall survival (71% vs 71%) were comparable in patients with incidental and symptomatic VTE over a mean follow-up of 477 days.24 Similar findings have consistently been reported in other studies,6,17 whereas some investigators found better survival rates in cancer patients with incidental VTE.6,14 Differences in study populations, type of incidental VTE, and thrombotic load evaluated could explain these discrepant findings. In a case-control study that compared mortality rates of 70 cancer patients with incidental PE with 137 control patients without PE, the former group had a significantly lower median survival (8 vs 12 months; hazard ratio [HR], 1.51; 95% confidence interval [CI], 1.01-2.27), with a difference that was apparently driven by the poor prognosis of proximal PEs.4 Survival of patients with incidental subsegmental PEs (n = 17) was comparable to that of controls without PE and significantly better than survival of patients with incidental proximal PE (7 months vs 12 months; HR, 1.70; 95% CI, 1.06-2.74).
Isolated subsegmental PE
The clinical relevance of PE confined to 1 or more subsegmental branches (ie, isolated symptomatic or incidental subsegmental PE) is unclear. Symptomatic subsegmental PE might have limited clinical relevance, with a number of retrospective studies showing no recurrent VTE during 3 months of follow-up in patients with isolated symptomatic subsegmental PE in whom anticoagulant therapy was withheld.27 By contrast, in a combined post hoc analysis of 2 large prospective cohort studies, den Exter and colleagues suggested that subsegmental PE is associated with similar rates of recurrent VTE, bleeding, and mortality as PEs that involve more proximal arteries.28 In a recent cross-sectional chart review of 2213 patients who underwent CT pulmonary angiography, 28% of 82 patients diagnosed with symptomatic subsegmental PE had active malignancy and only 22% of them received anticoagulant treatment.29 Major bleeding complications occurred in 2 of the 43 patients who received anticoagulation for subsegmental PE, whereas none of the patients with subsegmental PE developed recurrent VTE.
In most studies, incidental subsegmental PE accounted for less than 10% of all incidental PEs, although a prevalence as high as 20% to 24% has been reported.2,30 In a recent individual patient data meta-analysis, van der Hulle and colleagues pooled data of 926 cancer patients with incidental PE from 11 cohorts, including 9 with a retrospective design.30 Recurrent VTE seemed to occur at similar rates in patients with subsegmental PE and those with a more proximal PE (7.8% and 5.5%) with a high risk of recurrence in subsegmental PEs among cancer patients that were left untreated (4 of 42). None of these studies analyzed the prognosis of multiple vs single subsegmental PEs separately.
SVT
The prognosis of incidental SVT is unclear. Whereas some studies reported a prognosis similar to patients without incidental SVT, others have not. A small case series of cancer patients showed that the overall median survival was similar in patients with incidental SVT compared with age and stage-matched controls without SVT (11 vs 12 months).21 Another study reported comparable 3- and 6-month survival rates in patients with incidental and symptomatic SVT.10 Similarly, a retrospective cohort study including 626 patients with pancreatic cancer undergoing chemotherapy has reported that the overall survival was comparable in patients diagnosed with incidental SVT and those who did not develop any VTE (P = .17).31 Finally, a large international prospective cohort of 604 patients with SVT (22% solid cancer, 9% hematological malignancy), with approximately one-third of incidental events, reported that 9% (12/136) of patients with cancer developed a recurrent VTE for an incidence of 7.6 per 100 patient-years and an overall rate of major bleeding complications of 4.4 per 100 patient-years over a follow-up period of 2 years.19 In a post hoc analysis conducted on the subgroup with incidental SVT, the long-term prognosis was comparable to symptomatic SVT with a non-negligible incidence of recurrent VTE; 11% (7/62) of patients with cancer and incidental SVT developed a recurrent event for an incidence of 8.1 per 100 patient-years.20
Screening for incidental VTE
The high prevalence of incidental VTE raises the question about the usefulness of early detection of these VTEs by means of screening tests with potential benefits to patients from timely institution of treatment. Candidate patients could be those with pancreatic, hepatobiliary, upper gastrointestinal tract, brain, or ovarian cancer3,11 and patients with advanced or metastatic disease2,3,5,6 in whom a higher prevalence of incidental VTE has been reported. The utility of screening for incidental DVT was evaluated in 35 cancer (mostly pancreatic and gastroesophageal) patients starting a new chemotherapy regimen and regarded to be at high risk of VTE.32 Serial ultrasonography was performed before initiation of chemotherapy and repeated at 4-week intervals for up to 16 weeks. Ultrasonography detected incidental DVT in 3 (9.3%) cases at baseline, none at weeks 4 and 8, and 1 DVT (5.6%) at week 12. These findings suggest the potential usefulness of ultrasonography to screen for incidental DVT in high-risk populations before chemotherapy with low yield of serial testing. In another prospective study that adopted an even more extensive screening strategy with CT and whole-leg compression ultrasonography, incidental VTE was diagnosed in 27 of 117 patients (23%). More than one-half of the VTEs were distal DVTs of the leg (n = 18), 6 were proximal DVT, 2 were catheter-related thrombosis, and 2 were incidental PEs.33 The clinical significance of these incidental VTEs detected on screening ultrasonography is unknown and needs further assessment before it can be integrated in clinical practice. Furthermore, the cost-effectiveness of these screening approaches requires further evaluation.
Management
International clinical guidelines recommend 3 to 6 months of low-molecular-weight heparin (LMWH) treatment of cancer-associated symptomatic VTE.34 The decision to continue or withdraw treatment after this initial period as well as the type and dose of anticoagulant should be evaluated case-by-case considering the benefit-to-risk ratio, drug availability, and patient preference.
The indications for the management of incidental VTEs in patients with cancer are largely extrapolated from randomized studies in cancer-associated symptomatic VTE.35 In the only randomized study on the treatment of incidental VTE published to date, 64 patients with cancer and incidental PE were randomized to warfarin (dose-adjusted to achieve an international normalized ratio between 2.0 and 3.0) or fondaparinux (7.5 mg subcutaneously once daily) for 90 days.36 There were no differences in recurrent PE (7 of 32 warfarin patients and 6 of 32 fondaparinux patients, P = .32) or in thrombotic events in other locations (6 in both groups, P = .46). Major bleeding occurred less frequently in patients treated with fondaparinux (n = 3) than in those on warfarin (n = 6) (9% vs 19%, P < .01). However, the small size of the study, the poorly reported patient characteristics, outcomes, and quality of treatment, as well as the use of off-label fondaparinux as control, seriously limit the generalizability and validity of these findings.
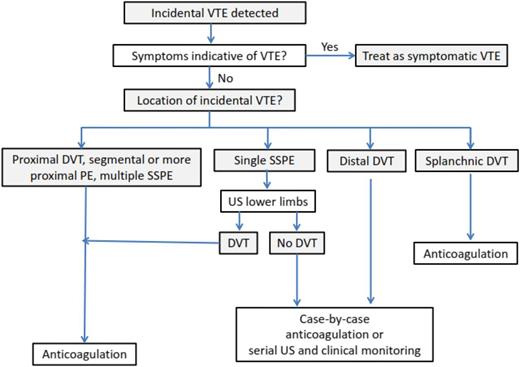
In the individual patient data meta-analysis by van der Hulle and colleagues, 79% of patients received prolonged therapeutic LMWH, 11% vitamin K antagonists, 4.4% another treatment including an inferior vena cava filter or unfractionated heparin, and 5.7% received no treatment.30 No information on the intensity of anticoagulation was provided, but the 6-month risks of recurrent VTE, major bleeding and mortality were 5.8%, 4.7%, and 37%, respectively. Recurrent VTE occurred at similar rates in patients treated with LMWH and vitamin K antagonists (6.2% vs 6.4%). However, the risk of major bleeding complications was fourfold higher in patients on vitamin K antagonists compared with those using LMWH (13% vs 3.9%; HR, 3.9; 95% CI, 1.6-10). In a subanalysis of the Registro Informatizado de Enfermedad TromboEmbólica registry, 20 recurrent VTEs and 45 major bleeding events were diagnosed in 715 cancer patients with incidental PE during a mean 235 days of anticoagulant therapy.37 The rate of major bleeding exceeded that of symptomatic PE (10.1/100 patient-years vs 3.17/100 patient-years, respectively), as did the rate of fatal bleeding compared with the rate of fatal PE (2.66 vs 0.66 deaths per 100 patient-years, respectively). After anticoagulant treatment discontinuation, there were 27 VTEs (14 symptomatic PEs and 13 DVTs) and 5 major bleeding events during a mean follow-up of 117 days. The rate of major bleeding was lower than the rate of symptomatic PE (3.00 vs 8.37 events per 100 patient-years, respectively), with no differences in fatal events. Interpretation of these studies should be done with caution given their limitations, including indication or treatment biases, and their relatively small sample size. Nonetheless, these data suggest that patients with incidental VTE are at high risk of recurrent VTE and major bleeding; the risk-benefit ratio seems to support the same management as symptomatic events using LMWH for 3 to 6 months35 (Figure 1). Similar considerations as for symptomatic VTE apply to the extension of LMWH treatment beyond this period.35 This is consistent with current clinical practice guidelines recommendations.34 Warfarin could be an alternative if LMWH is not available, affordable, or acceptable to patients. Data on the use of direct oral anticoagulants for VTE treatment in patients with cancer are scarce.34,35 Although not contraindicated, these agents cannot be recommended until further evidence from ongoing studies becomes available (NCT02073682, NCT02583191, ISRCTN86712308).
Few aspects of the management of incidental VTE remain controversial. Although full doses of LMWH are suggested during the initial treatment phase of incidental VTE,34,35 physicians need to consider that the age of incidentally detected clots may vary from days to months depending on the interval between imaging tests. A radiologic suspicion of an older clot, especially when VTE is not extensive, could prompt the use of a shorter course of full-intensity anticoagulation with earlier tapering of the dose.34,35
Isolated subsegmental PE
Single isolated subsegmental PE may have limited clinical relevance with uneventful follow-up in patients left untreated and survival rates that are similar to those of cancer patients without PE,4,38 although data have been conflicting.30 Routine anticoagulation could potentially unnecessarily expose these patients to the risk of anticoagulant-related bleeding without meaningful benefits. Therefore, it is important to initially review the images of reported incidental single isolated subsegmental PE to avoid false positives and confirm the diagnosis. In patients with a confirmed diagnosis, a conservative strategy using serial ultrasonography would potentially be reasonable, especially in cancer patients at high risk of bleeding.35 Some relatively small studies have suggested that concomitant incidental DVT is not an infrequent finding in patients with incidental PE.4,5 Therefore, the use of bilateral lower limb compression ultrasonography could be helpful to ascertaining the degree of thrombotic burden in a patient with an isolated, single subsegmental PE and enhance the confidence with which to recommend anticoagulation.35 If the decision has been made to treat cancer patients with single, isolated subsegmental PE without DVT, a shorter duration of anticoagulation seems reasonable to minimize bleeding risks. The decision to initiate anticoagulant therapy in these cases should carefully consider patient preferences, effect on patient quality of life, and costs.35 Physicians’ attitudes toward isolated subsegmental PE has been explored in several surveys over the past several years.39-41 The proportion of clinicians who are comfortable with withholding anticoagulation in patients with isolated subsegmental PE has ranged from 5% in the presence of concomitant cancer39 to 31% in patients without cancer if additional imaging tests could be performed to exclude a higher thrombotic burden.40 In a third survey, the majority of the physicians chose to anticoagulate cancer patients with incidental subsegmental PE despite the uncertain clinical significance of these PEs.41
Distal DVT
Whether cancer patients with incidental distal DVT require treatment is controversial. Two recent studies evaluated the clinical course of symptomatic distal DVT in patients with cancer.42,43 In a substudy of a multicenter, prospective, observational cohort of patients with VTE, the prognosis of 92 cancer patients with symptomatic isolated distal DVT was compared with 92 matched cancer patients with symptomatic proximal DVT and 184 patients without cancer with an isolated distal DVT.42 Relative to patients with cancer and proximal DVT, those with cancer-related isolated distal DVT had a similar risk of death (40.8% vs 38.3% patient-years), major bleeding (3.8% vs 3.6% patient-years), and a higher risk of VTE recurrence (5.4% vs 11.5% patient-years). Of 16 recurrent events that occurred in patients with cancer-related isolated distal DVT, 75% were major VTEs (6 PE and 6 proximal DVT). In another recent retrospective cohort of patients with cancer and symptomatic isolated distal DVT, the risk of death (44.8%) and VTE recurrence (13.2 events per 100 patient-years) were substantial during a mean follow-up of 13.9 months.43
Although incidental distal DVT was not evaluated in these studies, the findings suggest that distal DVT may have a poor prognosis in patients with cancer. Based on the observed high risk of recurrent VTE, a course of anticoagulation could be preferable over a more watchful approach with serial compression ultrasonography to detect thrombus extension into the proximal veins35 ; however, doses and duration of anticoagulant treatment remain to be established.
SVT
The prognostic relevance of SVT and the need to provide anticoagulant treatment of incidental SVT are a matter of debate. Small case series have suggested that SVT may be complicated by recurrent VTE if left untreated with rates as high as 29%.21 In the prospective cohort of 604 patients with SVT mentioned previously, anticoagulant therapy was associated with a reduction in thrombotic complications without an increase in the risk of major bleeding.19 Generalization of these findings to the whole group of patients with cancer and SVT is hampered by the relatively small number of cancer patients included (n = 136), confounding by indication, and the heterogeneity in duration and dosage of anticoagulant treatment provided. The broad variation in the management of SVT as observed in this and earlier studies could be the result of a lack of robust data from randomized controlled trials as well as the physicians' perception of the delicate balance between bleeding and thrombotic risk in these patients. Patients with SVT frequently present concomitant bleeding risk factors such as esophageal varices or thrombocytopenia secondary to hypersplenism, which should be weighed against risk factors for DVT extension or recurrence. In patients with incidental SVT who are neither actively bleeding nor have a very high risk of bleeding, anticoagulant therapy is suggested when thrombosis appears to be acute or shows progression or extension over time.35 Anticoagulant treatment should be continued for at least 3 months.18,34,35 Long-term or indefinite anticoagulation should be evaluated on a case-by-case basis with periodic assessment of the risk-to-benefit ratio, patient burden and preferences, and health care costs.
In conclusion, anticoagulant treatment is recommended for incidental proximal DVT and incidental PE that involves multiple subsegmental or more proximal pulmonary arteries. The clinical relevance of isolated subsegmental PE without concomitant DVT is uncertain and either a watchful approach or a shorter course anticoagulation may be considered. Preliminary evidence suggests that incidental distal DVT and SVT may benefit of anticoagulant treatment.
Correspondence
Marcello Di Nisio, Department of Medicine and Ageing Sciences, G. D'Annunzio University, Via dei Vestini 31, 66100 Chieti, Italy; e-mail: mdinisio@unich.it.
References
Competing Interests
Conflict-of-interest disclosure: M.D.N. is on the Board of Directors or an advisory committee for Daiichi Sankyo, BMS, and Pfizer and has received honoraria from Daiichi Sankyo and Bayer Health Care. M.C. has received research funding and honoraria from BMS, Leo Pharma, Bayer, Pfizer, and Sanofi.
Author notes
Off-label drug use: None disclosed.