Abstract
Cardiovascular (CV) health has emerged as an important consideration in patients with chronic myeloid leukemia (CML) because of improved prognosis. Indeed, the success of BCR-ABL1 tyrosine kinase inhibitors (TKIs) has increased the focus on survivorship and late toxicity in oncological care. Survivorship issues in this population include CV disease prevention, given its prevalence in the general population. The introduction of BCR-ABL1 TKIs represented a unique concept of indefinite cancer therapy, only recently evolving to include “treatment-free remission.” Importantly, later-generation BCR-ABL1 TKIs have been associated with CV complications. Dasatinib has been associated with pleural/pericardial effusions and pulmonary hypertension, whereas nilotinib and ponatinib have been linked to the development of vascular occlusive events. There is currently a dearth of data with respect to the mechanisms of drug toxicities, the subsets of patients at risk, and prevention and treatment strategies to mitigate CV complications in patients with CML. Nevertheless, optimal patient CV risk assessment needs to become a more central tenet of patient care in CML. We propose several practical considerations for the practicing oncologist relative to the CV health of patients with CML, especially those on chronic TKI therapy.
Learning Objectives
Highlight and describe the current data on cardiovascular toxicities associated with BCR-ABL1 tyrosine kinase inhibitors (TKIs) for chronic myeloid leukemia (CML)
Suggest practical clinical recommendations and treatment algorithm for preventing and managing cardiovascular toxicities in the CML population treated with TKIs
Case presentation 1
A 64-year-old woman with a diagnosis of imatinib-resistant chronic-phase CML is started on treatment with nilotinib 400 mg twice daily. She has a history of hypertension and hyperlipidemia and was told previously that she had “high pressures” in her lungs. What cardiovascular tests should be done at baseline and in follow-up?
Case presentation 2
A 69-year-old woman with a diagnosis of resistant CML with an identified T315I mutation is started on treatment with ponatinib 45 mg once daily. She has a history of diabetes, hyperlipidemia, and coronary artery disease, necessitating coronary artery stenting 5 years ago. What baseline cardiovascular tests should be done? What cardiovascular parameters should be followed?
Overview
The advent of BCR-ABL1 tyrosine kinase inhibitors (TKIs) has dramatically altered the treatment of chronic myeloid leukemia (CML), turning a potentially fatal disease into a manageable condition in most patients.1 Imatinib was the first approved TKI; other drugs (dasatinib, nilotinib, bosutinib, ponatinib) have been approved for patients who are resistant or intolerant to prior TKI therapy.2-7 In addition, by virtue of accelerated and superior molecular response, dasatinib and nilotinib have been approved for frontline treatment of CML.5,6
Cardiovascular (CV) care has become an important consideration in patients with CML for several reasons. The success of TKIs, the chronicity of treatment, and TKIs’ impact on overall patient survival (>90% at 5 years) has introduced survivorship as a new theme in patient care.8 Owing to the high prevalence of CV disease in the general population, attention to CV health is an important aspect of care for all cancer survivors, including patients with CML. In contrast to imatinib, a TKI lacking novel serious nonhematological adverse events and generally deemed safe, subsequently developed TKIs have been associated with a number of CV complications (Table 1).9-12 These issues have heightened the need for introduction of CV risk assessment when patients are initiated on TKI therapy or when their TKI therapy is switched. In high-risk patients, incorporation of CV disease prevention and management is crucial; in some cases, it may also require the involvement of CV specialists. Indeed, at many tertiary care centers, direct referrals are made to cardio-oncologists (cardiologists who specialize in the care of patients with cancer) when a patient with CML is started on a TKI and CV risk is high.
CV changes associated with TKIs in patients with CML and provisional recommendations for CV treatment
| TKI . | CV changes . |
|---|---|
| Dasatinib | Pleural/pericardial effusion |
| Pulmonary hypertension | |
| Vascular events+/− | |
| Nilotinib | QT prolongation |
| Hyperglycemia | |
| Hypertension+/− | |
| Hyperlipidemia+/− | |
| Vascular events | |
| Ponatinib | Hypertension |
| Vascular events |
| TKI . | CV changes . |
|---|---|
| Dasatinib | Pleural/pericardial effusion |
| Pulmonary hypertension | |
| Vascular events+/− | |
| Nilotinib | QT prolongation |
| Hyperglycemia | |
| Hypertension+/− | |
| Hyperlipidemia+/− | |
| Vascular events | |
| Ponatinib | Hypertension |
| Vascular events |
Vascular events include coronary, cerebral, and peripheral events. CML, chronic myeloid leukemia; CV, cardiovascular; TKI, tyrosine kinase inhibitor. +/−Conclusive data are lacking.
The TKIs approved for CML therapy have very diverse effects on the CV system, including potential toxicities associated with the specific TKI. There is a dearth of data regarding the mechanisms of these effects, the precise phenotype of toxicity, and why some patients are particularly at risk, hindering precise algorithms for patient care.1,12 Herein we provide a broad review of the toxicities associated with TKIs and practical recommendations for CV risk assessment and management in this population. The reader is referred to several excellent reviews for a deeper discussion of mechanisms of CV toxicities associated with TKIs.1,12-14
Imatinib
Imatinib was the first TKI tested and approved for CML.2 Early data regarding imatinib indicated an acceptable CV profile.2,15 Although 1 case series suggested a signal for congestive heart failure (cardiomyopathy), subsequent long-term follow-up of patients on imatinib revealed a low incidence of cardiomyopathy.8,16 Indeed, some preclinical data suggested that imatinib may have favorable metabolic and vascular effects.17,18 In addition, in animal models, imatinib reversed experimentally induced pulmonary hypertension, which motivated several trials to test the efficacy of imatinib for the treatment of patients with pulmonary hypertension.19-21
Despite the absence of an obvious CV toxicity signal with imatinib, we recommend appropriate CV risk assessment as an integral and important aspect of care for all patients. The “ABCDE” approach to CML care comprises a simple checklist that allows easy identification of CV risks that should be monitored in all patients (Table 2).
Practical ABCDE steps to reduce CV disease in patients with CML receiving TKI treatment
| ABCDE prevention steps . | Actions/measures . |
|---|---|
| A | Awareness of CV disease signs and symptoms |
| Aspirin in select patients | |
| Ankle–brachial index measurement to monitor for peripheral artery disease | |
| B | Blood pressure control |
| C | Cigarette and tobacco cessation |
| Cholesterol level, including regular monitoring and treatment, if needed | |
| D | Diabetes mellitus regular monitoring and treatment, if needed |
| Diet and weight management | |
| E | Exercise |
| ABCDE prevention steps . | Actions/measures . |
|---|---|
| A | Awareness of CV disease signs and symptoms |
| Aspirin in select patients | |
| Ankle–brachial index measurement to monitor for peripheral artery disease | |
| B | Blood pressure control |
| C | Cigarette and tobacco cessation |
| Cholesterol level, including regular monitoring and treatment, if needed | |
| D | Diabetes mellitus regular monitoring and treatment, if needed |
| Diet and weight management | |
| E | Exercise |
CML, chronic myeloid leukemia; CV, cardiovascular; TKI, tyrosine kinase inhibitor.
Dasatinib
Although initially approved for salvage treatment, dasatinib was approved for frontline CML therapy because of a superior molecular response when compared with imatinib in a head-to-head trial (DASISION trial).22,23 Early clinical trials with dasatinib identified 2 potential safety signals. A small percentage of patients (1% with QT >500 ms) had QT prolongation on screening electrocardiograms, and 14.3% of patients in the dasatinib arm in the trial had pleural effusion.22 In 2009, the first case of dasatinib-associated pulmonary arterial hypertension (PAH) was reported.24 Furthermore, a French pulmonary hypertension registry reported 9 patients with dasatinib-associated PAH.11 These patients had moderate to severe precapillary pulmonary hypertension. Improvements were seen in most patients after TKI withdrawal, although 2 patients died during follow-up as a result of cardiopulmonary causes.11 Importantly, no other TKI-associated PAH was noted in the registry. Data derived from 5-year follow-up of the DASISION trial showed that 5% of patients treated with dasatinib developed pulmonary hypertension compared with 0.4% in the imatinib arm.25 In 2011, the U.S. Food and Drug Administration (FDA) issued a warning regarding cardiopulmonary risks associated with dasatinib. From a practical standpoint, PAH symptoms may be nonspecific symptoms, such as dyspnea. A strong clinical suspicion is needed on the part of the treating physician for diagnosis. In patients with symptoms that may suggest pulmonary hypertension, we recommend a chest x-ray to rule out pleural effusion and an echocardiogram with Doppler flow studies as an adequate noninvasive assessment for PAH. Subsequent referral to a cardiologist may be indicated, depending on the results of these investigations. Other potential CV risks with dasatinib have been less clear, with data from the DASISION trial suggesting a higher risk of arterial ischemic events in the dasatinib arm than in the imatinib arm (5% vs 2% risk after 5 years, respectively).25
Nilotinib
Like dasatinib, nilotinib’s initial approval as a second-line agent was followed by frontline approval based on a head-to-head trial with imatinib (ENESTnd trial).26,27 Similarly to dasatinib, QT prolongation seen in screening electrocardiograms prompted a black box warning for nilotinib.26 In 2011, however, multicenter case series suggested that a subset of patients treated with nilotinib was developing severe peripheral arterial disease (PAD).28 In an initial study, 11 (6.2%) of 179 patients had severe PAD, with 8 patients requiring angioplasty, 8 patients requiring stent placement, and 4 patients requiring subsequent lower limb amputation.28 Following these initial data, retrospective studies done at multiple institutions have suggested a higher-than-expected incidence of peripheral (lower limb or cerebral) or cardiac ischemic events.6,9,28-30 Further data derived from the ENESTnd trial suggested a higher risk of hyperglycemia in the nilotinib arm than in the imatinib arm (36% vs 20% for all grades and 6% vs 0% for grade ≥3, respectively).27 Subsequent studies also suggested an increased body mass index as well as hyperlipidemia in nilotinib-treated patients.31,32 All three are CV risk factors and can accelerate the development of atherosclerosis, a systemic vascular disease that can manifest with both coronary and peripheral vascular events. The 5-year follow-up data derived from the ENESTnd trial confirm a higher risk of CV risk factors, including hypertension, hyperlipidemia, and hyperglycemia, in the nilotinib arms than in the imatinib arm.33 Importantly, a risk of CV events (defined as ischemic heart disease, ischemic cerebrovascular events, and PAD) was reported in 7.5% of patients randomized to nilotinib 300 mg twice daily and in 13.4% of patients randomized to nilotinib 400 mg twice daily, compared with 2.1% of patients randomized to imatinib 400 mg daily.33 Interestingly, CV events occurred more frequently in patients with a higher CV risk profile. The data derived from the ENESTnd trial strongly suggest a higher risk of CV risk factors and CV events in patients treated with nilotinib than in those treated with imatinib.
Ponatinib
Ponatinib was developed as a next-generation TKI with superior activity against resistant CML, including the highly drug-resistant T315I mutation.4 An initial phase 2 study (PACE) showed considerable response in patients who had failed other TKIs, leading to drug approval via the FDA accelerated approval program.4 However, at a median follow-up of 12 months, 6% of patients had coronary events, 3% had cerebrovascular events, and 4% had peripheral vascular events.4 At 28 months, cumulative events were 10%, 7%, and 7%, respectively.34 Interval analysis of CV toxicity (at 24 months) led to transient withdrawal of ponatinib from the market by the FDA, mandatory ponatinib dose reductions in patients continuing on the PACE trial, halting of the EPIC trial (a frontline study of ponatinib vs imatinib), and transition of EPIC patients to other therapies. Retrospective analysis of the PACE database suggests a higher risk in patients with CV risk factors or CV disease, as well as a signal for CV events occurring in a ponatinib dose–dependent manner. In addition, ≥26% of patients developed hypertension after initiating ponatinib, which may have contributed to CV events.34 These observations have prompted other studies to test lower doses of ponatinib in the hope of minimizing toxicity.35,36
Definition of vascular toxicity
A major shortcoming of the various studies assessing the risk of CV events in CML trials (and by extension associating potential toxicity with each TKI) is the lack of uniform definitions of vascular events. In CV practice, vascular events can arise from any number of pathologies, including atherosclerosis, thrombosis, vasospasm, or other potential etiologies (such as vasculitis). Moreover, in large cardiology clinical trials, an independent committee adjudicates CV events; no such CV event adjudication has occurred in CML trials. Additionally, ambiguity is perpetuated by the introduction of various terminologies by the oncology community (for example, peripheral arterial occlusive disease [PAOD]), which are defined differently depending on the study and give little insight into the pathophysiology of the vascular event. As a result, mere estimation of the frequency of CV events with each TKI has been difficult.
Interestingly, in the case of nilotinib, there have been a number of small studies that may provide a window onto specific CV toxicities where atherosclerosis is the potential complication. CV events associated with nilotinib, for example, have become more apparent with longer follow-up of trials such as ENESTnd. In addition, an intriguing prospective study involving 159 patients with imatinib or nilotinib showed a higher incidence of abnormal ankle–brachial index (ABI) in patients on nilotinib (relative risk, 10.3).37 Abnormal ABIs in patients treated with first- and second-line nilotinib were present in 26% and 35.7%, respectively, compared with 6.3% for first-line imatinib.37 The significance of abnormal ABI stems from its sensitivity and specificity for detecting PAD; these data suggest that the underlying pathophysiology explaining the TKI’s effect may be a buildup of systemic atherosclerosis in various arterial beds.
Cardio-oncological considerations
Data derived from a number of studies suggest that the vascular events associated with CML TKIs appear to be arterial in nature; all arterial beds (coronary, cerebral, and peripheral) appear to be affected. In addition, post hoc analyses suggest that older patients with a history of CV risk factors such as diabetes are at increased risk for vascular events. From a practical standpoint, these data suggest several clinical recommendations for all patients with CML, especially those who need to be treated with nilotinib or ponatinib. Table 3 outlines some clinical recommendations for patients with CML on TKIs. At baseline (prior to treatment start), all patients should be assessed for CV disease by history and physical examination. In addition, basic CV risk factors such as blood pressure, fasting glucose, and fasting lipids should be monitored. In follow-up (1 month and 3 months), CV symptoms should be further assessed (and treated if necessary). CV risk factors (such as hypertension, hyperlipidemia, and diabetes) should be further assessed at 3 months using appropriate testing. An ABI should be considered in patients treated with nilotinib or ponatinib at baseline and follow-up.
Clinical recommendations for assessing cardiotoxicity in patients with CML receiving TKI treatment
| Assessment . | Imatinib . | Bosutinib . | Dasatinib . | Nilotinib . | Ponatinib . |
|---|---|---|---|---|---|
| Baseline assessment | |||||
| Cardiovascular assessment | X | X | X | X | X |
| Blood pressure check | X | X | X | X | X |
| Fasting glucose | + | + | + | X | X |
| Fasting lipid panel | + | + | + | X | X |
| Echocardiogram | + | + | +* | + | + |
| Electrocardiogram | X | X | X | X | X |
| Ankle–brachial index | + | + | + | X | X |
| 1-mo follow-up | |||||
| Cardiovascular assessment | + | + | X | X | X |
| Blood pressure check | + | + | + | + | X |
| 3- to 6-mo follow-up | |||||
| Cardiovascular assessment | X | X | X | X | X |
| Blood pressure check | + | + | + | X | X |
| Fasting glucose | + | + | + | X | + |
| Fasting lipid panel | + | + | + | X | X |
| Echocardiogram | + | + | +* | + | + |
| Electrocardiogram | + | + | + | X | X |
| Ankle–brachial index | + | + | + | X | X |
| Assessment . | Imatinib . | Bosutinib . | Dasatinib . | Nilotinib . | Ponatinib . |
|---|---|---|---|---|---|
| Baseline assessment | |||||
| Cardiovascular assessment | X | X | X | X | X |
| Blood pressure check | X | X | X | X | X |
| Fasting glucose | + | + | + | X | X |
| Fasting lipid panel | + | + | + | X | X |
| Echocardiogram | + | + | +* | + | + |
| Electrocardiogram | X | X | X | X | X |
| Ankle–brachial index | + | + | + | X | X |
| 1-mo follow-up | |||||
| Cardiovascular assessment | + | + | X | X | X |
| Blood pressure check | + | + | + | + | X |
| 3- to 6-mo follow-up | |||||
| Cardiovascular assessment | X | X | X | X | X |
| Blood pressure check | + | + | + | X | X |
| Fasting glucose | + | + | + | X | + |
| Fasting lipid panel | + | + | + | X | X |
| Echocardiogram | + | + | +* | + | + |
| Electrocardiogram | + | + | + | X | X |
| Ankle–brachial index | + | + | + | X | X |
Assessments are done at baseline, 1-mo follow-up, and 3- to 6-month follow-up. CV screening should be considered for periods beyond 6 mo in all patients, but particularly for high-risk patients. +, as clinically indicated; CML, chronic myeloid leukemia; CV, cardiovascular; X, recommended; TKI, tyrosine kinase inhibitor.
Patients treated with dasatinib should be considered for an echocardiogram if cardiopulmonary symptoms are present.
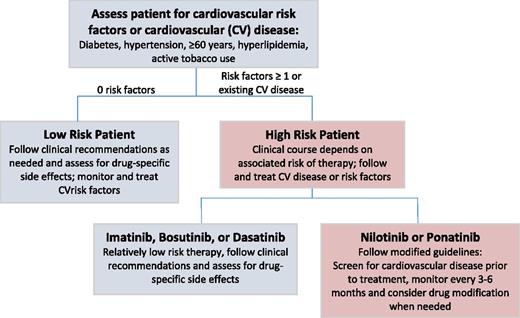
Given the number of TKI options already approved for CML treatment (with additional investigational TKIs and trials begetting broader indications for currently available TKIs), CV considerations may also play a role when selecting a specific TKI (Figure 1). Oncological considerations aside, a patient who has high CV risk (either CV disease at baseline or multiple cardiac risk factors) and who would benefit from a high-CV-risk TKI (eg, ponatinib) is someone who needs to be more thoroughly assessed for CV issues and, if necessary, referred to a cardiologist or cardio-oncologist.
Algorithm for determining the clinical management of low- and high-risk patients with CML treated with TKIs.
Algorithm for determining the clinical management of low- and high-risk patients with CML treated with TKIs.
Conclusions and future directions
The advent of TKIs for CML therapy has truly heralded a new era in hematological malignancies, where survivorship is a more relevant aspect of patient care. Increasingly, hematologists and oncologists must consider long-term effects of chronic TKI therapy. Given the high prevalence of CV disease in the general population, CV disease prevention and treatment strategies are relevant considerations for every patient with CML. The realization that subsequent-generation TKIs may increase CV disease risk adds further urgency to development of ways to mitigate CV risk in this population. This review outlines practical steps that patients and physicians can take in addressing these risks. However, we readily acknowledge the need for additional data in this area.
Correspondence
Javid Moslehi, Cardio-Oncology Program, Vanderbilt University Medical Center, 2220 Pierce Ave, Nashville, TN 37232; e-mail: javid.moslehi@vanderbilt.edu.
References
Competing Interests
Conflict-of-interest disclosure: M.C.B. declares no competing financial interests. M.J.M. has received research funding and has consulted for Bristol-Myers Squibb, Novartis Oncology, Pfizer, Takeda, and ARIAD Pharmaceuticals, Inc. J.M. has received research funding and has consulted for Novartis, Pfizer, Bristol-Myers Squibb, Takeda, ARIAD Pharmaceuticals, Inc., Acceleron Pharma, Pharmacyclics, Daiichi Sankyo, and Regeneron Pharmaceuticals.
Author notes
Off-label drug use: None disclosed.