Abstract
Activation of the hemostatic system occurs in patients with sickle cell disease. The extent to which this activation contributes to sickle cell pathophysiology is uncertain. Clinical trials of anticoagulants or platelet inhibitors have demonstrated the ability to decrease biomarkers of hemostatic activation, but this has generally not resulted in improvement in clinically relevant outcomes. Venous thromboembolism (VTE: deep venous thrombosis and pulmonary embolism) has been until recently an underappreciated complication of sickle cell disease, with incident event and recurrence rates consistent with a strong thrombophilia. There is no strong evidence that management should differ than for other patients with VTE, with the possible exception that secondary prophylaxis be extended regardless of provocation, given the persistent strong thrombophilic state.
Learning Objectives
Review the potential contribution of hemostatic pathway activation to the pathophysiology of sickle cell disease
Present more recent information on incidence and outcomes of venous thromboembolism in patients with sickle cell disease
Introduction
Sickle cell disease (SCD) is the result of homozygous or compound heterozygous inheritance of mutation in the β-globin gene. The resulting substitution of the hydrophilic amino acid glutamic acid at the sixth position by the hydrophobic amino acid valine leads to the production of hemoglobin S (HbS). HbS polymerizes when deoxygenated, and this polymerization is associated with cell dehydration and increased red cell density. Many investigators have reported alteration in the hemostatic system in SCD both under steady state and during acute events, as well as increased thromboembolic events.1,2 Changes that have been described include increased expression of tissue factor on blood monocytes3,4 and endothelial cells,5 abnormal exposure of phosphatidylserine on the red cell surface,6 and increased microparticles, which all promote activation of coagulation cascade.7,8 SCD meets the requirements of Virchow’s triad (slow flow, activated procoagulant proteins, and vascular injury); therefore, it should not be surprising that sickle disease is accompanied by thrombosis.
In this section, we highlight the existing evidence for contribution of the clotting system to SCD pathophysiology. More recent studies of platelet inhibition and anticoagulation are discussed. We also review the data showing increased risk for venous thromboembolic events in patients with SCD. Stroke is not discussed, and the reader is referred to several recent more comprehensive reviews.1,2
Alterations of coagulation proteins and platelets
Many investigators have shown biomarker evidence for ongoing activation of the coagulation cascade both during steady state (clinically well) and during vaso-occlusive crisis (VOC) (Table 1). These markers denote an ongoing hypercoagulable state in SCD. Platelet- and red cell–derived microparticles are increased in patients with hemoglobin SS (HbSS).7,9-11 Activated and tissue factor–positive monocytes are also increased in those with HbSS4,12 and hemoglobin SC (HbSC).13 The predominance of data support the notion that platelet activation is enhanced during VOC, whereas the evidence for further coagulation activation is more mixed.1
Hemostatic alterations in patients with SCD
| Increased levels . | Decreased levels . |
|---|---|
| Platelet activation | Factor V |
| Platelet aggregation | Factor XII |
| Phosphatidylserine-rich platelets and red blood cells | Factor IX |
| Thrombin-antithrombin complexes | Protein C |
| Prothrombin fragment F 1.2 | Protein S |
| Plasmin-antiplasmin complexes | |
| Fibrinogen and fibrin-fibrinogen complex | |
| Fibrinopeptide A | |
| D-dimer | |
| Plasminogen activator inhibitor |
| Increased levels . | Decreased levels . |
|---|---|
| Platelet activation | Factor V |
| Platelet aggregation | Factor XII |
| Phosphatidylserine-rich platelets and red blood cells | Factor IX |
| Thrombin-antithrombin complexes | Protein C |
| Prothrombin fragment F 1.2 | Protein S |
| Plasmin-antiplasmin complexes | |
| Fibrinogen and fibrin-fibrinogen complex | |
| Fibrinopeptide A | |
| D-dimer | |
| Plasminogen activator inhibitor |
Adapted from De Franceschi et al.47
Proximal intrinsic pathway proteins alterations have been reported in patients with SCD. In a small study of homozygous SS disease, plasma prekallikrein levels were decreased during steady state, with a further reduction during VOC.14 There was an additional 50% decrease in kininogen during VOC compared with low levels of kininogen at baseline.15 The levels of factor XII, high molecular weight kininogen, and prekallikrein are slightly decreased in children with homozygous SS disease in steady state.16 Because components of the contact system are mediators of inflammation, including pain and local vasodilatation, activation of this system might play a role in inflammatory pathway perturbations, as well as coagulation pathway abnormalities that contribute to SCD pathophysiology.
Decreases in anticoagulant proteins of hemostasis system would further promote a hypercoagulable state and have been reported in SCD.1,2 Increased plasma levels of plasminogen activator inhibitor-1 in both steady state and during acute events, and elevations in plasma plasmin-antiplasmin complexes, were also observed in patients with SCD in the noncrisis, steady state. The frequency of pain episodes in patients with SCD correlated with the extent of fibrinolytic activity (assessed by D-dimer levels) in the noncrisis steady state, suggesting D-dimer levels may predict the frequency of pain crises.17
Increased platelet activation markers such as P-selectin, CD63, activated glycoprotein IIb/IIIa, plasma soluble factor-3, plasma soluble factor-4, β-thromboglobulin, and platelet-derived soluble CD40 ligand were reported in patients with SCD, using a cytofluorimetric approach.1,2 In addition, platelet adherence to fibrinogen was increased through modulation of intracellular signaling pathways associated with increased αIIβ3-integrin activation.18 Increased phosphatidylserine-rich platelets have also been described in patients with SCD, which might accelerate the activation of the coagulation system.17 There is evidence hydroxyurea treatment lowers many markers of hemostatic activation and suggests another mechanism for therapeutic benefit.1
The pathophysiology of these changes is multifactorial and more extensively reviewed elsewhere.2 Abnormal phosphatidylserine exposure of the sickle red blood cell membrane alters the adhesive properties of sickle red blood cells,19 leading to an increase in capillary transit time and stasis, enhancing the potential for the activation of coagulation factors and cellular elements in the microvasculature and postcapillary venules. In addition, the exposed phosphatidylserine functions as a docking site for procoagulant proteins.20
In hemolytic states, cell-free hemoglobin is accumulated in plasma and interacts with nitric oxide, generating reactive oxygen species, and leading to endothelial damage. Free heme can also induce endothelial tissue factor expression. In addition, arginase I, which is released from the red blood cell during hemolysis, metabolizes arginine, which is the substrate for nitric oxide synthesis.21 Depletion of nitric oxide predisposes to thrombosis, as it has potent antithrombotic effects. A role for iron in hypercoagulability has also been recently suggested by demonstrating decreased parameters of clotting by thromboelastography in the plasma of patients with SCD after ex vivo iron chelation.22
Microparticles are small membrane vesicles released from cells when activated or during apoptosis. In healthy individuals, circulating MPs are mainly derived from platelets and, to a lesser extent, leukocyte and endothelial cells.23 Tissue factor–positive microparticles derived from red blood cells, platelets, endothelial cells, and monocytes are elevated in patients with sickle cell, both in steady state and during acute events compared with normal controls.24
Therapeutic implications of hemostatic system activation in SCD
Although hemostatic activation is somewhat downstream in the SCD pathophysiological cascade, it is plausible that a therapy targeted at decreasing coagulation activation might ameliorate or prevent sickle cell–related complications. Anticoagulants are widely used and studied, and their safety profiles are well described. All of this makes study of these agents attractive in SCD. A recent study in 2 sickle cell mouse models suggests targeting prothrombin may have a beneficial effect.25
Recent trials of platelet inhibitors in SCD
Desai and colleagues evaluated the safety and efficacy of eptifibatide, a synthetic peptide inhibitor of the αIIβ3 receptor, in patients with SCD during VOC.26 In a single-site, double-blind, placebo-controlled pilot trial, patients with SCD admitted for acute VOC were randomly assigned to receive eptifibatide or placebo at a ratio of 2:1. Thirteen patients (SS, 10; S/β0, 2; SC, 1) were randomly assigned to receive either eptifibatide (N = 9; 6 females; median age, 25 years) or placebo (N = 4; 3 females; median age, 31 years). There were no major bleeding episodes in either the eptifibatide or placebo groups (point estimate of difference: 0.00; 95% confidence interval [CI], −0.604 to 0.372) or differences in thrombocytopenia between the treatment groups (point estimate of difference: 0.11; 95% CI, −0.587 to 0.495). There were no differences in the median times to discharge, crisis resolution, or median total opioid use, although this pilot study was not adequately powered to determine differences in these end points. Eptifibatide appeared to be safe, but did not improve the times to crisis resolution or hospital discharge in this underpowered study.
Therapeutic effect of thienopyridines have also been studied in SCD.1,2 Wun et al27 studied the third-generation thienopyridine, prasugrel, in a randomized, double-blind adaptive phase 2 study in adults with all genotypes of SCD. Patients were randomly assigned to prasugrel 5 mg daily (n = 41) or placebo (n = 21) for 30 days. Platelet function was significantly inhibited in prasugrel compared with placebo-treated patients with SCD. Biomarkers of in vivo platelet activation, including platelet surface P-selectin and plasma-soluble P-selectin, were significantly reduced in patients with SCD treated with prasugrel compared with placebo (all P < .05). Mean pain rate (percentage of days with pain) and intensity decreased in the prasugrel group but did not reach statistical significance (P = .30 and .24, respectively). Prasugrel was well tolerated and not associated with serious hemorrhagic events. Despite the small size and short duration of this study, there was a decrease in platelet activation biomarkers and a trend toward decreased pain.
Styles and colleagues performed a phase 2 study of prasugrel to characterize platelet inhibition and safety in children with SCD.28 It was an open-label, multicenter, adaptive design, dose-ranging study. Patients were assigned daily doses (0.06, 0.08, and 0.12 mg/kg) on the basis of pharmacodynamic measurements at the start of 2 dosing periods, each 14±4 days. Platelet inhibition was significantly higher at 0.12 mg/kg (56.3%±7.4%; least squares mean±SE) compared with 0.06 mg/kg (33.8%±7.4%) or 0.08 mg/kg (37.9%±5.6%). There were no hemorrhagic events. The researchers concluded that most children with SCD achieved clinically relevant platelet inhibition with titration of daily dose prasugrel.
Based on the study by Styles, the Determining Effects of Platelet Inhibition on Vaso-Occlusive Events (DOVE) trial was conducted. Children and adolescents aged 2 through 17 years with sickle cell anemia were randomly assigned to receive oral prasugrel or placebo for 9 to 24 months (N = 341).29 The primary end point was the rate of VOC, a composite of painful crisis or acute chest syndrome. The secondary end points were the rate of sickle cell–related pain and the intensity of pain, which were assessed daily with the use of pain diaries. The rate of VOC events per person-year was 2.30 in the prasugrel group and 2.77 in the placebo group (rate ratio, 0.83; 95% CI, 0.66-1.05; P = .12). There was a trend toward reduced rates of VOC in the 12- to 17-year-old age group and for those not taking hydroxyurea. The frequency of bleeding of hemorrhagic and nonhemorrhagic adverse events did not differ significantly between the groups. The researchers concluded that among children and adolescents with sickle cell anemia, the rate of VOC was not significantly lower among those who received prasugrel than among those who received placebo. There were no significant between-group differences in the safety findings.
Despite the somewhat disappointing results of the DOVE trial, ticagrelor for SCD is being assessed in 2 ongoing studies. The first is a pharmacokinetic and pharmacodynamics phase 2 dose-ranging study enrolling patients aged 2 to 18 years (NCT02214121). The primary goal of the second study (NCT02482298) is to assess the effect of 2 doses of ticagrelor on the number of days with pain, pain intensity, and analgesic use in patients aged 18 to 30 years.
Anticoagulant therapy for patients with SCD without VTE
There have been a handful of studies examining chronic anticoagulation in SCD; most have been small and uncontrolled (Table 2). Schnog and colleagues30 performed a randomized, double-blind, placebo-controlled, crossover pilot study to assess the efficacy and safety of low adjusted-dose acenocoumarol. Treatment was either acenocoumarol or placebo for 14 weeks, after which treatment was discontinued for a period of 5 weeks. Patients were then crossed over. Efficacy was assessed by comparing the frequency of VOC, incident bleeding, and biomarkers of coagulation activation between acenocoumarol and placebo treatment of each patient. Twenty-two patients (14 homozygous [HbSS] and 8 compound heterozygous sickle-C [HbSC] aged 20-59 years) completed the entire study. Acenocoumarol treatment did not result in a significant reduction of VOC events. There was a marked reduction of the hypercoagulable state, as measured by biomarkers (decreased plasma levels of prothrombin F1.2 fragments [P = .002], thrombin-antithrombin complexes [P = .003], and D-dimer fragments [P = .001]) without the major bleeding. Even though no clinical benefit (pertaining to the frequency of painful crises) was detected in this pilot study, the authors concluded the value of low adjusted-dose acenocoumarol for preventing specific events (such as strokes) and as a long-term treatment of sickle cell patients should be subject of further study.
Recent studies of platelet inhibition and anticoagulation in SCD
| Author . | Genotypes . | Study type (N) . | Therapy . | Biomarker end point . | Clinical end point . |
|---|---|---|---|---|---|
| Desai et al26 | HbSS/Sβ thalassemia/SC | Randomized pilot13 | Eptifibatide vs placebo 2:1 | None reported | Estimated difference in major bleeding = 0.00 (95% CI, −0.604 to 0.372) |
| Wun et al27 | HbSS/Sβ thalassemia/SC | Randomized phase 2 (62) | Prasugrel vs placebo | Decrease in platelet P-selectin, soluble P-selectin, thromboxane B2, and CD40 ligand for those receiving prasugrel | 21% reduction in number of days with pain (P = .30) and 25% reduction in intensity (P = .24) |
| Styles et al28 | HbSS/Sβ0 thalassemia | Phase 2, open-label, adaptive-design, dose ranging study (33) | Prasugrel | Platelet inhibition higher at 0.12 mg/kg compared with 0.06 mg/kg or 0.08 mg/kg | Minor bleeding in 3 or 18 patients on escalating daily doses |
| Heeney et al29 | HbSS/Sβ0 thalassemia (aged 2-17) | Randomized phase 3 (341) | Prasugrel vs placebo | Decreased platelet reactivity units | VOC events 2.30 per person-year in prasugrel group and 2.77 in placebo group (rate ratio, 0.83; 95% CI, 0.66-1.05; P = .12). |
| Schnog et al30 | HbSS/SC | Randomized double-blind crossover pilot (phase 2) (22) | Acenocoumarol vs placebo | Decreased prothrombin 1.2, thrombin-antithrombin, and D-dimer on active drug | 3 VOC on acenocoumarol, 5 on placebo, not significantly different |
| Ahmed et al31 | HbSS/Sβ thalassemia/SC | Prospective observational (37) | Low-dose warfarin | Median (range) D-dimer 0.81 μg FEU/mL (0.34-1.8) on warfarin vs 3.1 μg FEU/mL (0.94-4) not receiving warfarin during VOC | None reported |
| Qari et al32 | HbSS | Randomized double-blind phase 3 (253) | Tinzaparin vs placebo | None reported | Reduction in days with worse pain (mean ± SD) (1.28 ± 0.2 vs 1.74 ± 0.15), VOC duration (2.57 ± 0.45 vs 4.35 ± 0.78), and length of stay (7.08 ± 1.8 vs 12.06 ± 2.2), all P < .05 |
| Author . | Genotypes . | Study type (N) . | Therapy . | Biomarker end point . | Clinical end point . |
|---|---|---|---|---|---|
| Desai et al26 | HbSS/Sβ thalassemia/SC | Randomized pilot13 | Eptifibatide vs placebo 2:1 | None reported | Estimated difference in major bleeding = 0.00 (95% CI, −0.604 to 0.372) |
| Wun et al27 | HbSS/Sβ thalassemia/SC | Randomized phase 2 (62) | Prasugrel vs placebo | Decrease in platelet P-selectin, soluble P-selectin, thromboxane B2, and CD40 ligand for those receiving prasugrel | 21% reduction in number of days with pain (P = .30) and 25% reduction in intensity (P = .24) |
| Styles et al28 | HbSS/Sβ0 thalassemia | Phase 2, open-label, adaptive-design, dose ranging study (33) | Prasugrel | Platelet inhibition higher at 0.12 mg/kg compared with 0.06 mg/kg or 0.08 mg/kg | Minor bleeding in 3 or 18 patients on escalating daily doses |
| Heeney et al29 | HbSS/Sβ0 thalassemia (aged 2-17) | Randomized phase 3 (341) | Prasugrel vs placebo | Decreased platelet reactivity units | VOC events 2.30 per person-year in prasugrel group and 2.77 in placebo group (rate ratio, 0.83; 95% CI, 0.66-1.05; P = .12). |
| Schnog et al30 | HbSS/SC | Randomized double-blind crossover pilot (phase 2) (22) | Acenocoumarol vs placebo | Decreased prothrombin 1.2, thrombin-antithrombin, and D-dimer on active drug | 3 VOC on acenocoumarol, 5 on placebo, not significantly different |
| Ahmed et al31 | HbSS/Sβ thalassemia/SC | Prospective observational (37) | Low-dose warfarin | Median (range) D-dimer 0.81 μg FEU/mL (0.34-1.8) on warfarin vs 3.1 μg FEU/mL (0.94-4) not receiving warfarin during VOC | None reported |
| Qari et al32 | HbSS | Randomized double-blind phase 3 (253) | Tinzaparin vs placebo | None reported | Reduction in days with worse pain (mean ± SD) (1.28 ± 0.2 vs 1.74 ± 0.15), VOC duration (2.57 ± 0.45 vs 4.35 ± 0.78), and length of stay (7.08 ± 1.8 vs 12.06 ± 2.2), all P < .05 |
Adapted from Ataga and Key.48
Anticoagulation has also been studied in the acute VOC setting. Ahmed and colleagues31 measured plasma D-dimer in 37 adult patients with SCD who were hospitalized for VOC. D-dimer level in patients who were receiving low-dose warfarin was compared with the level in patients who were not receiving any anticoagulation treatment. Patients were receiving warfarin either for venous thromboembolism (VTE) or catheter prophylaxis; this was not a randomized study. Overall median D-dimer level in 65 samples was 2.7 μg fibrinogen equivalent units (FEU)/mL (range 0.34-4). Patients who were receiving low-dose warfarin had a median D-dimer level of 0.81 μg FEU/mL (0.34-1.8) compared with 3.1 μg FEU/mL (0.94-4) in those patients who were not receiving anticoagulation treatment. Using analysis of variance to model D-dimer levels, only warfarin was significantly correlated with low D-dimer levels after controlling for other variables. The researchers concluded that patients with SCD during VOC have an elevated D-dimer level and that low-dose anticoagulation treatment is associated with a significant reduction in the D-dimer levels. There was no assessment of clinically important outcomes.
A randomized double-blind clinical trial was performed to test the safety and efficacy of a low-molecular-weight heparin, tinzaparin, for the management of VOC.32 Two hundred fifty-three patients with acute painful crisis, but with no other complications of SCD, were randomly assigned to tinzaparin at 175 IU/kg (a therapeutic dose), subcutaneously once daily, along with supportive care, including morphine analgesia; in the comparator group, 126 patients received placebo and the same supportive care. The maximal treatment period was 7 days. Tinzaparin-treated patients had significantly fewer total hospital days (mean, 7.08 vs 12.06 days), crisis duration (mean, 2.57 vs 4.35 days), and days of severest pain score (mean, 1.28 vs 1.74) compared with placebo-treated patients. There were 2 minor bleeding events in the tinzaparin group. These provocative findings should be confirmed in future studies, especially as one could argue that at a minimum, low-molecular-weight heparin at prophylactic doses should be routine in hospitalized sickle cell patients. On the basis of this study and the effect of prothrombin depletion in mouse models,25 clinical trials of rivaroxaban and apixaban in patients with SCD are ongoing (registered at www.clinicaltrials.gov as NCT02072668 and NCT02179177).
Evidence for increased venous thromboembolic events in SCD
Using the National Discharge Survey, Stein and colleagues reported on the frequency of deep venous thrombosis (DVT) or pulmonary embolism (PE) as a discharge diagnosis for patients with SCD.33 In patients younger than 40 years, 7000 of 1 581 000 (0.44%) patients with SCD had a discharge diagnosis of PE compared with 59 000 of 48 611,000 (0.12%) African Americans without SCD. The prevalence of DVT was similar in patients younger than 40 years with SCD, at 7000 of 1 581 000 (0.44%), and in African Americans who did not have SCD, at 193 000 of 48 611 000 (0.40%). The authors speculated that high prevalence of apparent PE in patients with SCD compared with non-SCD African American patients of the same age and the comparable prevalence of DVT in both groups was compatible with an increased risk for in situ PE in patients with SCD. This report did not track individual patients over time.
Novelli et al used similar administrative discharge data from Pennsylvania to estimate the incidence of PE among hospitalized patients with SCD between 2001 and 2006.34 The incidence of inpatient PE was higher in the SCD population than in the non-SCD Pennsylvania population, although this incidence figure was based on a population estimate and not derived through the database itself. The PE prevalence among SCD discharges aged 50 years or younger (0.57%) was similar to that in non-SCD PA discharges (0.60%) and was unchanged after adjustment for race. Among SCD discharges, those developing PE were significantly older, with a longer length of stay, greater severity of illness, and higher mortality (χ-square, P < .001) than SCD without a PE. Among PE discharges, SCD had a similar severity of illness score but underwent fewer computed tomography (CT) scans than non-SCD with PE. The researchers also performed a case-control study using data from the University of Pittsburgh system and could not identify any clinical or laboratory feature, with the exception of length of stay, that was associated with development of PE for hospitalized patients with SCD. However, only 14 cases and 28 controls were included. They concluded that the incidence of PE is higher and chest CT use is lower in inpatients with SCD than non-SCD inpatients, and suggested that PE may be underdiagnosed in patients with SCD.
More recently, Naik and colleagues examined the single-institution experience cross-sectional study of 404 patients with SCD cared for at the Sickle Cell Center for Adults at Johns Hopkins in Baltimore, Maryland.35 They found 101 patients (25%) had a history of VTE, with a median age at diagnosis of 29.9 years. Non-catheter-related VTE was found in 18.8% of patients, and catheter-related in 7.7%. Sickle variant genotypes conferred a higher risk for non-catheter-related VTE compared with sickle cell anemia genotypes (SS/Sβ0; relative risk [RR], 1.77; 95% CI, 1.18-2.66). Tricuspid regurgitant jet velocity ≥2.5 m/s also was associated with non-catheter-related VTE (RR 1.65; 95% CI, 1.12-2.45). Twenty-five percent of the patients with non-catheter-related VTE had recurrent events at a median follow-up of 4.8 years. Thirty patients (7.4%) died during the study period. Adjusting for covariates, non-catheter-related VTE was independently correlated with death (RR, 3.63; 95% CI, 1.66-7.92). The authors suggested that disease-specific prophylaxis and treatment strategies for VTE should be investigated in patients with SCD.
The same group used data from the Cooperative Study of SCD to calculate incidence rates for first VTE. They included 1523 patients with SCD aged 15 years or older with 8862 years of follow-up in this analysis. The incidence rate for first VTE was 5.2 events/1000 person-years (95% CI, 3.8-6.9 events) with a cumulative incidence of 11.3% (95% CI, 8.3%-15.3%) by age 40 years. Individuals with the SS or Sβ0-thalassemia genotype had the highest rate of VTE (7.6 events/1000 person-years [95% CI, 5.3-10.6 events]). The incidence of PE exceeded that of isolated DVT (3.6 [95% CI 2.5-5.1] events/1000 person-years vs 1.6 [95% CI 0.9-2.7] events/1000 person-years), although this difference was not statistically significant. Patients with SCD with VTE had a higher mortality rate (adjusted hazard ratio, 2.32; 95% CI, 1.20-4.46) than those without VTE. They concluded that patients with SCD are at substantial risk for VTE, and individuals with VTE are at higher risk for death than those without VTE.
Pregnancy, SCD, and VTE
The Nationwide Inpatient Sample from the Healthcare Cost and Utilization Project of the Agency for Healthcare Research and Quality for the years 2000 to 2003 was queried for all pregnancy-related discharges with a diagnosis of SCD.36 There were 17 952 deliveries (0.1% of the total) to women with SCD. Cerebral vein thrombosis and deep venous thrombosis were much more common among women with SCD. These results were consistent with a previous study in which the authors observed that SCD was an independent risk factor for pregnancy-related VTE with an odds ratio of 6.7 (95% CI, 4.4-10.1).37
Seaman et al examined inpatient hospital discharge data from 212 hospitalized deliveries in African American women with SCD, and 6 (2.8%; 95% CI, 1.0%-5.9%) had VTE compared with 0.05% to 2.0% in the general population.38 Risk factors for VTE included pneumonia and diabetes mellitus. Overall, the prevalence of VTE among hospitalized deliveries in women with SCD with pneumonia, VOC, and/or acute chest syndrome, at 6.6%, was significantly greater than among those without these conditions, at 2.2% (P < .001). The researchers concluded pregnancy-related VTE in women with SCD appears to be 1.5 to 5 times greater than pregnancy-related VTE in the general population.
Porter et al examined the relationship between sickle cell trait (HbAS) and other sickle hemoglobinopathies and the risk for thromboembolism during pregnancy or the puerperium.39 Of 103 women with HbSS, HbSC, or HbSβ-thalassemia, 3 women (2.9%) experienced thromboembolism. Compared with women with normal hemoglobin status, the RR was 32.2 (95% CI, 9.7-107). The researchers concluded that SCD strongly increased the risk for VTE during pregnancy. One might also speculate that hemostatic activation is relevant to the increased rate of obstetrical complications seen in patients with SCD. The reader is referred to a recent excellent review of the relationship among sickle cell trait, SCD, and VTE.40
The California SCD cohort and VTE
We initiated a large, population-based cohort study to determine the incidence of, and specific risk factors for, the development of VTE among patients with SCD, and the effect of VTE on survival in this population. The State of California Office of Statewide Health Planning and Development maintain records of all patients hospitalized in nonfederal hospitals in the state, called the Patient Discharge Database. Since 2005, an Emergency Department Utilization database has also been available. An encrypted social security number called the record linkage number is used to identify serial admissions for individual patients during the 23 years of discharge data.
We identified all admissions with an International Classification of Diseases–9 code for SCD (282.60, 282.61, 282.62, 282.63, 282.68, 282.69, 282.41, or 282.42) in any of the 25 diagnosis fields. Through an iterative process, we refined the search criteria and again reviewed case listings. To be considered a SCD case, the final case definition included at least 2 separate admissions with SCD coded as the principal diagnosis, or at least 1 admission with a SCD code in the principal position and a SCD code in a secondary position in at least 2 additional admissions. All cases had to be younger than 65 years of age at entry into the registry. Although not validated by source documentation, the cases enumerated here are very similar to that derived by an independent analysis in California.41 Potential limitations include ascertainment bias for patients with SCD never hospitalized in California and for outmigration. There is also no information on anticoagulation therapy.
The primary outcome measured was incident acute VTE (PE or DVT, excluding upper extremity DVT). We identified VTE cases using specific International Classification of Diseases–9 codes validated in previous work,42 albeit not specifically in the SCD population. To be defined as acute VTE, a VTE code had to be in the principle or second diagnosis position and be present on admission (POA = yes). To capture VTE as a complication of a procedure, we also included patients with a principal diagnosis code of 997.2 or 997.3 plus the corresponding DVT or PE in the second position (POA = yes). Hospital-acquired VTE events were included (POA = no).
There were 6237 unique cases of SCD identified in the discharge data set (Table 3). In this SCD cohort, 696 unique patients (11.2%) developed an incident acute VTE. Three hundred fifty-nine (51.6%) were PE (± DVT), and 158 (22.7%) were upper extremity only. Seventy-five percent were present on admission or not diagnosed during a hospitalization. However, 60% of the VTE occurred 90 days or less before hospital discharge. The incidence of VTE per hospitalization (occurring during or 90 days or less from discharge) was 0.45%. The overall incidence density rate of VTE was 8.4 per 1000 patient years, but this was 14 per 1000 patient-years for the group, with an average of 3 or more annual hospital visits vs 4.6 per 1000 patient-years for the group with less frequent visits. The median age at diagnosis of VTE was 31 years. Eighteen (4.3%) of 414 women with VTE had a pregnancy code at the time of or within 6 weeks of their thrombotic event. Of the 158 patients with upper extremity thrombosis, 59 (37.3%) had a central venous catheter placed before the VTE.
California sickle cell cohort
| . | All . | Thrombosis . | No thrombosis . | |||
|---|---|---|---|---|---|---|
| Variables . | N . | % . | N . | % . | N . | % . |
| All | 6237 | 100.0% | 696 | 100.0% | 5541 | 100.0% |
| Sex | ||||||
| Male | 2948 | 47.3% | 282 | 40.5% | 2666 | 48.1% |
| Female | 3283 | 52.6% | 414 | 59.5% | 2869 | 51.8% |
| Race/ethnicity | ||||||
| Non-Hispanic white | 176 | 2.8% | 13 | 1.9% | 163 | 2.9% |
| African American | 5615 | 90.0% | 648 | 93.1% | 4967 | 89.6% |
| Hispanic | 252 | 4.0% | 17 | 2.4% | 235 | 4.2% |
| Asian/Pacific Islander | 34 | 0.5% | 4 | 0.6% | 30 | 0.5% |
| Other/unknown | 160 | 2.6% | 14 | 2.0% | 146 | 2.6% |
| Average visits/year* | ||||||
| <3 visits per year | 3583 | 57.4% | 225 | 32.3% | 3358 | 60.6% |
| ≥3 visits per year | 2654 | 42.6% | 471 | 67.7% | 2183 | 39.4% |
| Type of thrombosis | ||||||
| PE (±DVT) | 359 | 5.8% | 359 | 51.6% | — | — |
| LE proximal DVT | 132 | 2.1% | 132 | 19.0% | — | — |
| LE distal DVT | 28 | 0.4% | 28 | 4.0% | — | — |
| LE DVT NOS | 15 | 0.2% | 15 | 2.2% | — | — |
| Other NOS | 4 | 0.1% | 4 | 0.6% | — | — |
| Upper extremity | 158 | 2.5% | 158 | 22.7% | — | — |
| . | All . | Thrombosis . | No thrombosis . | |||
|---|---|---|---|---|---|---|
| Variables . | N . | % . | N . | % . | N . | % . |
| All | 6237 | 100.0% | 696 | 100.0% | 5541 | 100.0% |
| Sex | ||||||
| Male | 2948 | 47.3% | 282 | 40.5% | 2666 | 48.1% |
| Female | 3283 | 52.6% | 414 | 59.5% | 2869 | 51.8% |
| Race/ethnicity | ||||||
| Non-Hispanic white | 176 | 2.8% | 13 | 1.9% | 163 | 2.9% |
| African American | 5615 | 90.0% | 648 | 93.1% | 4967 | 89.6% |
| Hispanic | 252 | 4.0% | 17 | 2.4% | 235 | 4.2% |
| Asian/Pacific Islander | 34 | 0.5% | 4 | 0.6% | 30 | 0.5% |
| Other/unknown | 160 | 2.6% | 14 | 2.0% | 146 | 2.6% |
| Average visits/year* | ||||||
| <3 visits per year | 3583 | 57.4% | 225 | 32.3% | 3358 | 60.6% |
| ≥3 visits per year | 2654 | 42.6% | 471 | 67.7% | 2183 | 39.4% |
| Type of thrombosis | ||||||
| PE (±DVT) | 359 | 5.8% | 359 | 51.6% | — | — |
| LE proximal DVT | 132 | 2.1% | 132 | 19.0% | — | — |
| LE distal DVT | 28 | 0.4% | 28 | 4.0% | — | — |
| LE DVT NOS | 15 | 0.2% | 15 | 2.2% | — | — |
| Other NOS | 4 | 0.1% | 4 | 0.6% | — | — |
| Upper extremity | 158 | 2.5% | 158 | 22.7% | — | — |
The cohort was derived from the State of California Office of Statewide Health Planning and Development Patient Discharge and Emergency Department Utilization Datasets, as described in the text.
LE = lower extremity; NOS = not otherwise specified.
Average number of inpatient admissions or emergency department visits during the study period.
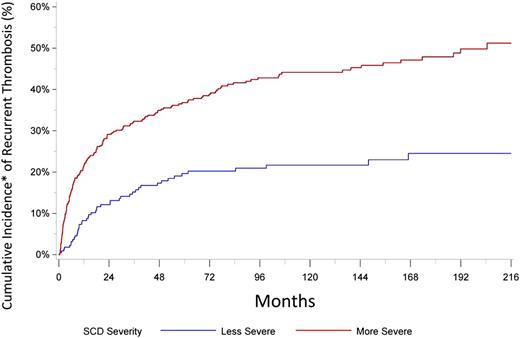
We next looked at VTE recurrence. A total of 232 recurrences occurred after the incident 696 events, for an overall cumulative incidence of 31.3% at 5 years, adjusted for competing risk for death. The recurrence was greater in those more frequently hospitalized (Figure 1). The median time to recurrence was 359 days. It is worth noting that even for those hospitalized less frequently, the rate of recurrence is higher than that associated with provoked VTE in the general population43 and is similar to those with unprovoked VTE.44 We are presently analyzing multivariable models to determine the risk factors for incident VTE and the effect of VTE on mortality in patients with SCD.
Recurrent VTE in patients with SCD. More severe = average number of annual admissions and/or emergency department visits ≥3, less severe = average number of annual admissions and/or emergency department visits <3. *Cumulative incidence of recurrent thrombosis is adjusted for competing risk for death.
Recurrent VTE in patients with SCD. More severe = average number of annual admissions and/or emergency department visits ≥3, less severe = average number of annual admissions and/or emergency department visits <3. *Cumulative incidence of recurrent thrombosis is adjusted for competing risk for death.
Diagnosis and therapy of VTE in patients with SCD
There are no studies that directly address the diagnosis algorithm for VTE in patients with SCD; therefore, the diagnostic approach to a patient with SCD suspected of DVT or PE is no different than for that in other patients. Our approach is summarized in Table 4. Special considerations include the possibility that small peripheral filling defects on CT angiogram may represent in situ sickling, rather than a classic fibrin-rich clot, and the lack of utility of D-dimer in diagnostic algorithms for VTE resulting from persistent elevations in many patients. There is also concern about contrast-induced acute kidney injury with CT angiogram, although we have not seen this with the newer nonionic low-osmolality contrast agents.
How I evaluate and treat patients with SCD with VTE
| Diagnosis | Little utility to D-dimer |
| Compression ultrasonography (±Doppler) for deep venous thrombosis | |
| Computed tomography angiography with nonionic low-osmolality contrast media | |
| I do not routinely give red cell transfusion before contrast | |
| As with the general population, V/Q scans now rarely performed | |
| Therapy | Treatment as per American College of Chest Physicians 2016 guidelines with full-dose anticoagulation |
| Caution in patients with history of ischemic stroke at risk for moyamoya syndrome | |
| Consider extended anticoagulation in those with low bleeding risk even if the event was provoked by hospitalization for medical illness | |
| Continue anticoagulation for catheter-associated upper extremity thrombosis until catheter removal | |
| No convincing evidence to support primary prophylaxis for those with a catheter and no history of thrombosis | |
| I do not recommend primary thromboprophylaxis for pregnant patients with sickle cell who have never had a VTE | |
| Low molecular weight heparin during pregnancy and 6 wk postpartum for a patient with any prior VTE (if catheter-related, only if the catheter in still in place) |
| Diagnosis | Little utility to D-dimer |
| Compression ultrasonography (±Doppler) for deep venous thrombosis | |
| Computed tomography angiography with nonionic low-osmolality contrast media | |
| I do not routinely give red cell transfusion before contrast | |
| As with the general population, V/Q scans now rarely performed | |
| Therapy | Treatment as per American College of Chest Physicians 2016 guidelines with full-dose anticoagulation |
| Caution in patients with history of ischemic stroke at risk for moyamoya syndrome | |
| Consider extended anticoagulation in those with low bleeding risk even if the event was provoked by hospitalization for medical illness | |
| Continue anticoagulation for catheter-associated upper extremity thrombosis until catheter removal | |
| No convincing evidence to support primary prophylaxis for those with a catheter and no history of thrombosis | |
| I do not recommend primary thromboprophylaxis for pregnant patients with sickle cell who have never had a VTE | |
| Low molecular weight heparin during pregnancy and 6 wk postpartum for a patient with any prior VTE (if catheter-related, only if the catheter in still in place) |
Most of the patients with SCD in the world live in low- and middle-income countries, where the routine tools for diagnosis of VTE in other parts of the world (compression ultrasound, CT angiography) may not be available. The inaccuracy of clinical impression and physical examination for VTE is well documented and improved by the use of validated clinical decision tools.45,46 The development of small, portable, and affordable point-of-care ultrasound devices will hopefully facilitate diagnosis of DVT in lower-resource countries.
For the primary or secondary prevention of VTE, there is no clinical trial evidence that the use of anticoagulant medications should be any different than in other medically ill patients with regard to indications, dose, intensity, or duration of therapy. This includes the current recommendation against primary pharmacological prophylaxis to prevent catheter-related thrombosis. This extends to the most recent recommendation for using the newer direct oral anticoagulants as first-line agents for acute VTE in patients without cancer.43 However, we note anecdotal experience with 3 recent patients with SCD who had recurrence or progression of venous thrombosis while receiving anti-Xa drugs. It is possible the marked hypercoagulable state in sickle cell patients with venous thrombosis may require more tailored therapy. Hospitalized sickle cell patients without contraindication should receive pharmacological thromboprophylaxis. In patients with history of ischemic stroke at risk for moyamoya syndrome, there may be hesitation to initiate full-dose therapeutic anticoagulation and/or thrombolytic therapy before assessment of cerebral vasculature.
Extended anticoagulation after unprovoked events for all patients with low risk of bleeding should be considered. This suggestion is based on the rate of recurrence noted earlier, which is similar to that for unprovoked VTE in the general population (where extended anticoagulation is recommended). Perhaps a less straightforward situation is the patient with SCD who has VTE within 90 days of hospital discharge for medical illness, which made up 60% of the incident events in the California cohort. This would be considered a provoked event in the general patient population, and typically extended anticoagulation is not recommended. We consider SCD to be a potent and persistent hypercoagulable state, and an argument for extended secondary prophylaxis after a first medical illness hospitalization-provoked event, with the exception of catheter-related upper extremity thrombosis, as long as the catheter is removed. As with any patient in whom extended anticoagulation is considered, individualized risk/benefit assessment and discussion are mandatory.43,44
Summary
There is overwhelming evidence for activation of the hemostatic system in patients with SCD. The contribution of this activation to sickle cell pathophysiology is less certain. Clinical trials of anticoagulation or platelet inhibition have demonstrated the ability to alter biomarkers of hemostatic activation, but this has rarely resulted in improvement in clinically relevant outcomes. Clinically overt VTE has been an underappreciated complication of SCD until recently, with event and recurrence rates consistent with strong thrombophilia. With current knowledge, management should not differ than for other patients with VTE, with the possible exception that secondary prophylaxis be extended regardless of provocation, given the persistent hypercoagulable state.
Correspondence
Ted Wun, Division of Hematology Oncology, UC Davis Comprehensive Cancer Center, 4501 X St, Sacramento, CA 95817; e-mail: twun@ucdavis.edu.
References
Competing Interests
Conflict-of-interest disclosures: T.W. has received research funding from Pfizer and Janssen and has consulted for Janssen. A.B. declares no competing financial interests.
Author notes
Off-label drug use: Anticoagulant for sickle cell disease.