Abstract
Vitamin K antagonists (VKAs) are commonly used for the prevention and treatment of thrombotic disorders. The response to VKAs is highly variable due to their specific interaction with the vitamin K cycle, and hence interference with hepatic synthesis of vitamin K-dependent coagulation factors. Monitoring the anticoagulant effect of VKAs by assessing the patient’s international normalized ratio (INR) is essential because complications are closely related to the intensity of anticoagulation. Treatment with VKAs contains a substantial risk of bleeding with a high case fatality rate. Reversal of VKAs is required in case of bleeding or a supratherapeutic INR, but also prior to high-risk surgery or interventions. Choice of methods to reverse VKAs depends on whether or not the patient is bleeding or is in need of an urgent procedure, and has to be based on the pharmacokinetic and pharmacodynamic properties of the VKA. Reversal strategies include withholding the VKA, administration of vitamin K1, and substitution of vitamin K-dependent procoagulant factors, and need to be combined with measures according to general bleeding management.
Learning Objectives
To understand how VKAs and their reversal strategies work
To choose the most effective, efficient, and safe method for VKAs reversal in bleeding and nonbleeding patients in daily routine care
Introduction
Around 1920, rumors of cattle bleeding to death in the Midwest of the United States started to spread. Frank Schofield, a Canadian veterinary pathologist, discovered that the disease occurred only in cattle fed with sweet clover that had become moldy. In the end, all it took was a farmer with a milk can full of blood from his bull that had bled to death, and about 100 pounds of sweet clover. Said farmer had fought his way through a blizzard storm into the office of an American Biochemist and his German assistant (by the names of Karl Paul Link and Eugene Wilhelm Schoeffel). They crystallized the anticoagulant that we now know as dicumarol. Only about 20 years later from those early discoveries, dicumarol was used in the clinic for postoperative thromboprophylaxis.1 Finally, in search of a more potent preparation that could be used as a rodenticide, warfarin, which got its name to acknowledge funding by the Wisconsin Alumni Research Foundation, was obtained in 1948. Meanwhile, warfarin is only one of several synthetic dicumarol analogs subsumed as vitamin K antagonists (VKAs). VKAs licensed for humans differ with regard to their chemical structure, come in various strengths, and are substrates of cytochrome P450, all of which influence their pharmacokinetic and dynamic properties.2 Warfarin, the only VKA licensed in the United States, has an elimination half-life of 40 hours. Other VKAs, including acenocoumarol, fluindione, or phenprocoumon are frequently used in Europe and differ substantially regarding their half-lives: acenocoumarol is 9 hours, fluindione is 31 hours, and phenprocoumon is 140 hours. The high protein binding (>90%) is partly responsible for significant drug interactions because the VKA may be displaced from the protein binding site, thereby increasing its free plasma concentration and the risk of toxicity. Tercarfarin, which is not yet available, is a unique VKA because it is not metabolized by cytochrome P450.
VKAs are used for the prevention and treatment of thrombotic disorders, and is ranked among the 20 most frequently mentioned drug names at outpatient department visits in the United States.3 Notably, in a US national surveillance project, warfarin was ranked among the drugs most commonly implicated in adverse events treated in emergency departments.4
How do VKAs work?
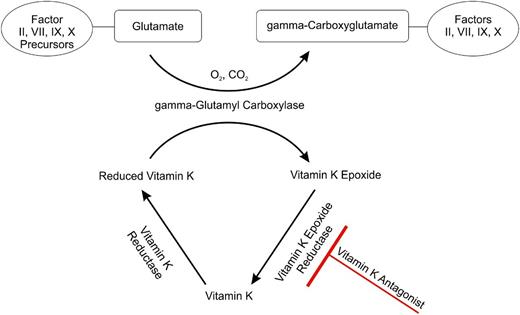
Coagulation factor (F)II, FVII, FIX, and FX require carboxylation of their glutamic acid residues for binding calcium ions thereby gaining full procoagulant activity.5 This γ-carboxylation step involves oxygen, carbon dioxide, and the fully reduced form of vitamin K, which is vitamin K hydrochinone. Vitamin K1 (phylloquinone, phytomenadione, or phytonadione) is found in food and oils derived from plants, and can be converted by animals to vitamin K2 (menaquinone). Because both naturally occurring forms are quinones, they must be reduced by enzymes such as vitamin K epoxide reductase, which is the most important one. VKAs block vitamin K oxide reductase, which results in the hepatic production of partially carboxylated and decarboxylated proteins with reduced coagulant activity (Figure 1).
The vitamin K cycle and the anticoagulant effect of VKAs. FII, FVII, FIX, and FX gain full procoagulant activity after conversion of their glutamate residues into γ-carboxyglutamate residues through conversion of reduced vitamin K, to vitamin K epoxide by γ-glutamyl carboxylase. Vitamin K epoxide is recycled by vitamin K epoxide reductase, such that it can be reused. This step is blocked by VKAs because they inhibit vitamin K epoxide reductase.
The vitamin K cycle and the anticoagulant effect of VKAs. FII, FVII, FIX, and FX gain full procoagulant activity after conversion of their glutamate residues into γ-carboxyglutamate residues through conversion of reduced vitamin K, to vitamin K epoxide by γ-glutamyl carboxylase. Vitamin K epoxide is recycled by vitamin K epoxide reductase, such that it can be reused. This step is blocked by VKAs because they inhibit vitamin K epoxide reductase.
VKAs also interfere with the synthesis of the regulatory anticoagulant proteins C and S because they are also dependent on carboxylation.
How to monitor VKAs?
Response to VKAs is highly variable and depends on dose, genetics, diet, co-medications, comorbidities, liver synthesis capacity, and probably also microbial composition in the gut. Close monitoring of the anticoagulant effect of VKAs is essential because bleeding or thrombotic complications during VKA treatment are closely related to the intensity of anticoagulation.6
Monitoring is accomplished by measuring the prothrombin time (PT) because it responds to a reduction of 3 (FII, FVII, and FX) of the 4 vitamin K-dependent procoagulant clotting factors. The ratio of the patient’s PT and the PT of normal plasma raised to the power of the international sensitivity index (ISI) of the thromboplastin, results in the international normal ratio (INR).7 The ISI reflects the responsiveness of a given thromboplastin to the reduction of the vitamin K-dependent coagulation factors in comparison with the primary World Health Organization international reference preparations, so that the more responsive the reagent, the lower the ISI value.
An INR range from 2 to 3 confers the lowest rate of a composite outcome of major bleeding and symptomatic thromboembolism, and is thus the target for most indications.8 Patients at high thrombotic risk including those with a mechanical mitral valve, a mechanical aortic valve in combination with additional risk factors, or caged-ball or -disk valve require more intense anticoagulation with an INR of 2.5 to 3.5.
How to reverse VKAs?
Reversing the action of VKAs is needed in case of bleeding, surgery or interventions with a high bleeding risk, or VKA-coagulopathy as indicated by an INR above the therapeutic range. The relationship between bleeding and the INR increases exponentially once the INR rises above 4.5.9 It has to be kept in mind that compared with individuals without anticoagulation, the risk of bleeding is already increased at an INR in the therapeutic range.9
Withholding the VKA
The inhibition of the pathways involved in vitamin K metabolism by VKAs is in essence irreversible. If VKA intake is stopped, the anticoagulant effect tapers off in relation to the half-life of the drug, the liver capacity to synthesize coagulation factors, and the patient’s vitamin K intake and reserve. In asymptomatic patients with a moderate increase of the INR (up to 5), simply withholding VKAs will suffice to lower the INR into therapeutic values within 24 to 48 hours, but may take longer in patients who are older, those with heart failure, cancer, low-maintenance VKA dose, or longer-acting VKAs.10
Administration of vitamin K
In patients with substantially elevated INRs (above 5), supplementation of vitamin K1 leads to a more rapid decline of the INR than just withholding VKAs.11 Depending on the clinical situation, vitamin K1 may be given as an oral or IV preparation. Anaphylactoid infusion reactions are extremely rare and may occur despite the recommended infusion time of 20 to 30 minutes.12 Subcutaneous or intramuscular applications are not recommended because of an unpredictable response and increased bleeding risk at the injection site.11,13
Low doses of vitamin K1 (1 mg to 2.5 mg) given orally to nonbleeding patients with INR values >4.5 lower the INR to levels between 1.8 and 4.0.11 Most studies did not differentiate patient groups with excessively high INRs (>10).14 Single reports indicate that low-dose vitamin K1 (2.5 mg) is a safe and effective treatment also for severely over-anticoagulated nonbleeding patients.15-17 The optimal dose and route of vitamin K1 supplementation have not been evaluated in controlled studies powered for bleeding and thrombotic outcomes. In those studies, which reported clinical end points, thrombotic events were scarce and bleeding complications were similar or only marginally reduced.18 Response to vitamin K1 is on average about 4 hours faster after IV compared with oral application, albeit with similar INR levels after 24 hours.19
Substitution of vitamin K-dependent procoagulant factors
Plasma.
Fresh frozen plasma (FFP), plasma frozen within 24 hours after phlebotomy (PF24), and thawed plasma derived from FFP or PF24, contain labile and nonlabile plasma proteins and are sources of all vitamin K-dependent coagulation factors. FFP and PF24 are prepared from a whole blood or apheresis collection and frozen at –18°C or colder. Both preparations should be infused immediately after thawing or stored at 1° to 6°C, and must be discarded after 24 hours unless collected in a functionally closed system.20
Efficacy of plasma for VKA reversal has not been systematically established. Coagulation factor content in plasma preparations is variable.20 Minimum coagulation factor level for sufficient hemostasis is in the order of ∼30%, but the relative contribution of individual clotting factors is unknown. The minimum amount of plasma for effective restoration of hemostasis is ∼15 mL/kg bodyweight (which translates into at least 4 units with a total volume of 1000 mL for an average weight adult). Despite the administration of an adequate dose, the correction of the coagulation defect may not be complete and FIX levels may only reach 20%. Because of the short half-lives of some coagulation factors, in particular FVII, repeat transfusions are often necessary to keep up with the required coagulation factor levels.
In its latest update of “Circular of Information,” the American Association of Blood Banks states that neither FFP nor PF24 should be used when the coagulopathy can be corrected more effectively with a specific therapy, such as vitamin K or prothrombin complex concentrates (PPCs) to reverse VKA.20
PPCs.
PCCs are purified concentrates of vitamin K-dependent coagulation factors and are available in a non-activated and an activated form.
Although head-to-head comparisons between PCCs are lacking, non-activated 4-factor concentrates, which contain all vitamin K-dependent procoagulant factors (FII, FVII, FIX, and FX), can be considered as the preparations of choice for vitamin K reversal. Three-factor concentrates (licensed in the United States for the treatment of hemophilia B) may be inferior because they have no or little FVII. In a systematic review of 18 studies including 654 patients, the INR decreased to ≤1.5 within 1 hour of infusion in 6 of 9 studies in the 3-factor group and in 12 of 13 studies in the 4-factor group.21 Activated PCCs (eg, FEIBA, also referred to as FVIII inhibitor bypassing activity) harbor a noteworthy prothrombotic risk because they contain FVII in mainly activated form.
Several 4-factor PCCs are available that differ slightly with regard to their factor concentration. Most preparations contain heparin, which needs to be considered in patients with a history of heparin-induced thrombocytopenia. Beriplex B/N has been on the market in Europe for many years and was approved by the Food and Drug Administration in April 2013 under the name Kcentra for urgent reversal of acquired coagulation factor deficiency induced by VKAs in adult patients with acute major bleeding or need for an urgent surgery/invasive procedure. Kcentra is a sterile, heat-treated, non-activated, 35 nm nano-filtered, and lyophilized product, manufactured from a pool of human US source plasma.22
Efficacy and safety of Kcentra have been evaluated in 2 prospective, open-label, randomized, plasma-controlled trials. In 202 patients who required VKA reversal for major bleeding, the 4-factor PCC was superior to plasma in achieving rapid INR reduction in 62.2% patients as compared with 9.6% of patients (difference, 52.6%; 95% confidence interval [CI], 39.4-65.9) and was non-inferior in achieving effective hemostasis in 72.4% as compared with 65.4% of patients (difference, 7.1%; 95% CI, –5.8-19.9).23 In 181 VKA-treated patients in need of urgent surgery, effective hemostasis was achieved in 90% of patients in the 4-factor PCC group compared with 75% of patients in the plasma group, demonstrating superiority of 4-factor PCC over plasma (difference, 14.3%; 95% CI, 2.8-25.8).24 Rapid INR reduction was achieved in 55% of patients in the 4-factor PCC group compared with 10% in the plasma group (difference, 45.3%; 95% CI, 31.9-56.4). Table 1 summarizes the characteristics and major outcomes of randomized controlled trials comparing 4-factor PCCs and FFP.23-25
Characteristics and major outcomes of randomized controlled trials on use of 4-factor PCC vs FFP
| . | Demeyere et al25 . | Sarode et al23 . | Goldstein et al24 . |
|---|---|---|---|
| Patients, n | 40 | 216 | 181 |
| Clinical setting | Cardiopulmonary bypass | Major bleeding | Urgent surgical/invasive procedure |
| Intervention | Cofact vs FFP | Kcentra vs FFP | Kcentra vs FFP |
| Primary end point, n (%) | Patients reaching INR ≤1.5 | 24-h hemostatic efficacy | Effective hemostasis |
| 7 (44) vs 0; P = .007 | 71 (72) vs 68 (65)* | 78 (90) vs 61 (75)† | |
| Mortality (d 45) | Data not provided | 10 (9.7) vs 5 (4.6) | 3 (3) vs 8 (9) |
| Thrombotic events, n (%) | Data not provided | 8 (7.8) vs 7 (6.4) | 6 (7) vs 7 (8) |
| Fluid overload, n (%) | Data not provided | 0 vs 7 (6.4) | 3 (3) vs 11 (13) |
| . | Demeyere et al25 . | Sarode et al23 . | Goldstein et al24 . |
|---|---|---|---|
| Patients, n | 40 | 216 | 181 |
| Clinical setting | Cardiopulmonary bypass | Major bleeding | Urgent surgical/invasive procedure |
| Intervention | Cofact vs FFP | Kcentra vs FFP | Kcentra vs FFP |
| Primary end point, n (%) | Patients reaching INR ≤1.5 | 24-h hemostatic efficacy | Effective hemostasis |
| 7 (44) vs 0; P = .007 | 71 (72) vs 68 (65)* | 78 (90) vs 61 (75)† | |
| Mortality (d 45) | Data not provided | 10 (9.7) vs 5 (4.6) | 3 (3) vs 8 (9) |
| Thrombotic events, n (%) | Data not provided | 8 (7.8) vs 7 (6.4) | 6 (7) vs 7 (8) |
| Fluid overload, n (%) | Data not provided | 0 vs 7 (6.4) | 3 (3) vs 11 (13) |
Primary end point was 4-factor PCC non-inferior to plasma: P value for non-inferiority = .0045.
P = .0142 for superiority of 4-factor PCC to plasma.
Four-factor PCCs have been evaluated in several other controlled and uncontrolled trials for VKA reversal in patients with major bleeding or emergencies.26 By use of 4-factor PCC, the coagulation defect is corrected more rapidly compared with plasma. Some studies suggest reduced progression of intracerebral hemorrhage.26 However, none of these studies was large enough to assess differences in clinical outcomes with adequate statistical power. In a systematic review of 4 randomized controlled trials, mortality or transfusion requirements did not seem to be reduced by PCCs compared with plasma.26 In an integrated analysis of the 2 phase 3b clinical trials, Kcentra had a similar safety profile to plasma but was associated with fewer fluid overload events (4.7% vs 12.7%).27
Four-factor PCCs are considered the preferred method for rapid, complete, and efficient restitution of the deficient vitamin K-dependent coagulation factors because they have substantial advantages over plasma preparations: there is no need to thaw the product; no time delay for blood typing; no risk of transfusion-associated lung injury or circulatory overload; short infusion times; administration of a standardized dose; minimization of the risk of viral transmission due to viral removal and inactivation procedures in the manufacturing process; and the potential to reduce transfusion reactions (Table 2). In fact, the American College of Chest Physicians recommends the use of PCCs over FFP in patients with warfarin-associated coagulopathy.6,14
Comparison of FFP and 4-factor PCCs
| . | FFP . | 4-Factor PCCs* . |
|---|---|---|
| Coagulation factor contained | All | FII, FVII, FIX, and FX (in all preparations); PC, PS, antithrombin (in some preparations) |
| ABO typing | Required | Not required |
| Preparation time | 30-60 min (requires thawing) | Minutes (reconstitution of lyophilized powder) |
| Administration time | Hours | 15 min per 2000 IU |
| Initial effective volume | 1000 mL | ∼40 mL |
| Duration of action | ∼6 h (dependent on half-life of coagulation factors) | ∼6 h (dependent on half-life of coagulation factors) |
| Virus inactivated | No | Yes |
| . | FFP . | 4-Factor PCCs* . |
|---|---|---|
| Coagulation factor contained | All | FII, FVII, FIX, and FX (in all preparations); PC, PS, antithrombin (in some preparations) |
| ABO typing | Required | Not required |
| Preparation time | 30-60 min (requires thawing) | Minutes (reconstitution of lyophilized powder) |
| Administration time | Hours | 15 min per 2000 IU |
| Initial effective volume | 1000 mL | ∼40 mL |
| Duration of action | ∼6 h (dependent on half-life of coagulation factors) | ∼6 h (dependent on half-life of coagulation factors) |
| Virus inactivated | No | Yes |
IU, international units; PC, protein C; PS, protein S.
Beriplex N/P/Kcentra (CSL Behring), Cofact (Sanquin), Kaskadil (LFB), Octaplex (Octapharma), and Prothromplex T(otal) (Baxter).
A potential safety concern of 4-factor PCCs is an increased thrombotic risk. Overall, the risk of arterial or venous thrombosis was low in the individual trials. In a meta-analysis of 18 studies, the frequency of thromboembolic events was 1.8% (95% CI, 1.0%-3.0%) in patients treated with 4-factor PCCs.28 The analysis was based on retrospective and prospective observational studies with heterogeneity in size, inclusion, and exclusion criteria as well as follow-up times, which implies the risk of bias and random effects. The mortality rate of VKA-associated bleeding in this analysis was similar to those reported in other studies, but the authors were unable to assess the impact of PCCs on death rates. In a more recent integrated analysis of safety data from 2 randomized controlled trials in patients requiring rapid VKA reversal for acute major bleeding or prior to an urgent surgical procedure, the proportion of patients with thromboembolic events or deaths was similar between the 4-factor PCC (7.3% and 6.8%) and the plasma (7.1% and 6.6%) group.27,29 Because patients treated with VKAs have underlying disease states that predispose them to thromboembolic events if the anticoagulant is stopped, such adverse events cannot necessarily be isolated to either the use of PCCs or plasma.
Practical considerations for VKA reversal in routine care
Choice of methods to reverse VKAs depends on whether or not the patient is bleeding or on the urgency of a procedure and has to be based on the pharmacodynamic and kinetic properties of the respective VKA. Of note, most studies were performed with warfarin and extrapolation of these results to other VKAs with different half-lives may not be valid. Underlying coagulopathies as may be seen in patients with severe liver disease or thrombocytopenia, must be considered. Concurrent medications, in particular platelet function inhibitors or nonsteroidal antirheumatic drugs, and comorbidities should be checked and treatment be optimized accordingly. As an example, transfusion of platelets in a patient with dual or triple antithrombotic treatment may be an option to attenuate the coagulopathy.
According to evidence-based recommendations developed by the American Society of Hematology, plasma or PCCs should not be administered for non-emergent reversal of VKAs (ie, outside of the setting of major bleeding, intracranial hemorrhage, or anticipated emergent surgery).30
Nonbleeding patients with elevated INR
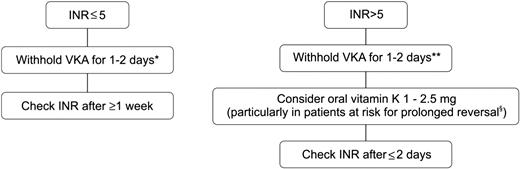
Fluctuations in INR with supratherapeutic values conferring an inherent increased bleeding risk are common. For previously stably anticoagulated patients with a single INR of ≤0.5 above the therapeutic range, the VKA dose should remain unchanged and INR testing performed within 1 to 2 weeks.31 Figure 2 shows an algorithm for the management of a nonbleeding patient with an INR above the therapeutic range. If the INR is only moderately increased (up to 5), simply withholding VKAs will suffice and a strategy of “watchful stopping” the VKA for at least 1 day can be pursued. The risk of bleeding is also a function of time and complications during short-term supratherapeutic anticoagulation are infrequent.32 If the INR is substantially above the therapeutic range (>5) and particularly when long-acting VKAs are used, oral administration of vitamin K1 can be advocated. However, reversal of VKAs by vitamin K1 has a slow onset. Although some correction of the INR can be seen within 6 hours, full correction will not occur before 24 hours. Because these patients are to remain on VKAs, limiting the dose of vitamin K1 to 1 mg to 2.5 mg (split tablets or orally administered IV preparations) will avoid overcorrection of the INR.33,34
Suggested algorithm for reversing VKAs in nonbleeding patients with an INR above the therapeutic range. For patients with a single INR of ≤0.5 above the therapeutic range, the VKA dose should remain unchanged. *With acenocoumarol, consider merely reducing the dose because stopping may result in INR overcorrection. **With acenocoumarol, stopping for more than 1 day may result in INR overcorrection. §Patients with older age, heart failure, cancer, or longer acting VKA.
Suggested algorithm for reversing VKAs in nonbleeding patients with an INR above the therapeutic range. For patients with a single INR of ≤0.5 above the therapeutic range, the VKA dose should remain unchanged. *With acenocoumarol, consider merely reducing the dose because stopping may result in INR overcorrection. **With acenocoumarol, stopping for more than 1 day may result in INR overcorrection. §Patients with older age, heart failure, cancer, or longer acting VKA.
Bleeding patients
The annual incidence of VKA-associated major bleeding is estimated at 1% to 3% with a case fatality rate of ∼11%.35 Efficient management of bleeding is, thus, an integral part in medical care of the VKA patient.
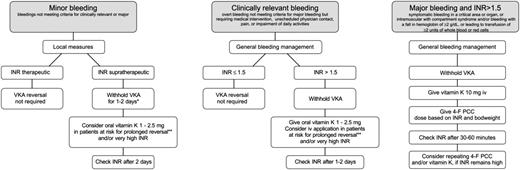
Figure 3 shows algorithms for the management of patients with nonmajor or major bleeding during VKA treatment. A management strategy for bleeding in general that implies a multidisciplinary approach should be followed according to guidelines of national and international societies and local institutions.36-38 In case of major bleeding, this will include location of the bleeding source, volume resuscitation, hemodynamic support, mechanical or surgical hemostatic measures, and blood cell transfusions. Laboratory tests will help to determine the degree of bleeding and other potential risk factors.
Suggested algorithm for reversing VKA in bleeding patients. *With acenocoumarol, consider merely reducing the dose because stopping may result in INR overcorrection. **Patients with older age, heart failure, cancer, or longer-acting VKAs. 4-F PCC, 4-factor prothrombin complex concentrate.
Suggested algorithm for reversing VKA in bleeding patients. *With acenocoumarol, consider merely reducing the dose because stopping may result in INR overcorrection. **Patients with older age, heart failure, cancer, or longer-acting VKAs. 4-F PCC, 4-factor prothrombin complex concentrate.
In case of minor bleedings (eg, epistaxis, ecchymosis, and menorrhagia), local measures sometimes may be all that is required.
The INR needs to be checked and VKA treatment must be stopped in case of an elevated INR. In case of major bleeding, the VKA-coagulopathy requires immediate reversal by IV infusion of a 4-factor PCC at an individualized dose based on the patient’s baseline INR and bodyweight (Table 3). The reconstituted 4-factor PCC should be administered at a rate of 0.12 mL/kg per minute (∼3 units/kg per minute) up to a maximum rate of 8.4 mL/min (∼210 units/min), which translates into ∼15 minutes for 2000 units. The dose should be rounded to the nearest multiple of 500 units.
Dosing of a 4-factor PCC for normalization of the INR
| Pretreatment INR . | 2-<4 . | 4–6 . | >6 . |
|---|---|---|---|
| Dose of 4-factor PCC (units of FIX)/kg.bw | 25 | 35 | 50 |
| Maximum dose* (units of FIX) | 2500 | 3500 | 5000 |
| Pretreatment INR . | 2-<4 . | 4–6 . | >6 . |
|---|---|---|---|
| Dose of 4-factor PCC (units of FIX)/kg.bw | 25 | 35 | 50 |
| Maximum dose* (units of FIX) | 2500 | 3500 | 5000 |
Dose is based on body weight up to but not exceeding 100 kg. For patients weighing more than 100 kg, the dose should not exceed this maximum single dose.
In most cases, this will be initially sufficient for providing time for concomitant vitamin K1 administration at a dose of 5 mg to 10 mg to exert its action. Vitamin K1 should be given IV to expedite its effect. Larger and repeat vitamin doses should be avoided because they may subsequently make the patient “resistant” to VKA for many days. The INR must be checked after 30 to 60 minutes to assure adequate correction of the coagulation defect. As with plasma preparations, the coagulation defect may not completely be restored after the initial administration because of the short half-lives of certain coagulation factors. The safety and effectiveness of repeat dosing of 4-factor PCCs has not been established. However, in case of massive hemorrhage resulting in a bleeding coagulopathy or in case of prolonged effects of the VKA (as indicated by continuously elevated INRs, which can be seen with long-acting VKAs or massive overdose), repeat dosing of the 4-factor PCC may be justified. Because infusion of a 4-factor PCC may exacerbate an underlying hypercoaguable state, the potential benefit of treatment should be weighed against the potential risk of such a complication.
Elective and semi-urgent interventions
Interventions with a low-bleeding risk should be performed at an INR in the lower part of the therapeutic range without stopping VKA.
For elective interventions with a higher bleeding risk, stopping VKAs 3 to 8 days in advance (depending on the half-life of the substance) will usually suffice to achieve a preoperatively required INR of <1.5 (except in patients with supratherapeutic INRs at the time of interruption).39,40 The INR needs to be checked the day before surgery. If the INR is still >1.5 or in case of semi-urgent situations, low-dose oral vitamin K1 can be given the day before the intervention. Despite vitamin K1 substitution, ∼10% of the patients will have INR levels >1.5 the next day, in which case elective interventions need to be further postponed. If the intervention is required within the next 6 to 12 hours, infusion of a 4-factor PCC may be considered to ascertain immediate and complete correction of the coagulation defect. Because under these circumstances, “semicorrection” of the coagulation system has already been achieved by vitamin K1, only small doses of PCC (25 IU/kg) are usually required.
Urgent and emergency interventions
Stopping VKA and IV administration of vitamin K will normalize the INR, but not before 12 to 24 hours. For surgery, which cannot be delayed, the INR can be rapidly corrected by giving 4-factor PCCs in addition to parenteral vitamin K1. The INR should be measured as close as possible to the time the concentrate is given in order to calculate the appropriate dose of the 4-factor PCC. FFP should not be used to correct a mildly elevated INR before procedures, because a mildly elevated INR is not predictive of an increased risk of bleeding and transfusion of plasma has not been shown to change the value significantly.30,40,41
Conclusion
Despite declining use of VKAs in the era of novel oral anticoagulants, VKAs remain highly frequently used antithrombotic agents worldwide. The risk of bleeding remains the major concern during VKA therapy. Close monitoring of anticoagulant intensity and a management strategy of VKA reversal will improve the safety of treatment. Options to reverse the effect of VKAs range from simply withholding the drug over vitamin K1 administration to substitution of vitamin K-dependent coagulation factors. Because all VKA-treated patients harbor an intrinsic thrombotic risk, choosing the reversal strategy with minimal correction of the coagulation defect that provides maximal hemostatic effect represents a challenge. Although withholding VKAs in nonbleeding patients with supratherapeutic INR may be enough, rapid correction of the hemostatic defect by infusion of a 4-factor PCC is required in patients with life-threatening bleeding.
Correspondence
Sabine Eichinger, Department of Medicine I, Medical University of Vienna, Vienna, Austria; e-mail: sabine.eichinger@meduniwien.ac.at.
References
Competing Interests
Conflict-of-interest disclosure: The author declares no competing financial interests.
Author notes
Off-label drug use: None disclosed.