Abstract
T-cell–engaging immunotherapies are exciting new approaches to treat patients with acute lymphoblastic leukemia (ALL). These unique agents, which include blinatumomab, a CD3/CD19 bispecific antibody, and chimeric antigen receptor (CAR) modified T cells targeted to CD19 have shown unprecedented remission rates in the relapsed, refractory ALL setting. Cytokine release syndrome (CRS), resulting from the high magnitude of immune activation by these therapies, is the most significant treatment-related toxicity. CRS manifests with fever and malaise and can progress to life-threatening capillary leak with hypoxia and hypotension. The clinical signs of CRS correlate with T-cell activation and high levels of cytokines, including interleukin 6 (IL-6). Tocilizumab, an anti-IL-6 receptor antagonist, is usually effective in the management of severe CRS induced by CAR T cells and has been adopted by most clinical trial programs. With blinatumomab administration, the goal has been to prevent CRS with corticosteroid premedication, disease cytoreduction, and dose adjustments. Collaborative efforts are underway to harmonize the definition and grading system of CRS to allow for better interpretation of toxicities across trials and allow for informed management algorithms.
Learning Objectives
Recognize the key clinical features of CRS
Understand the prevention and management strategies for CRS from blinatumomab and CAR T-cell therapies
Introduction
The goal of cancer immunotherapy has been to harness the antitumor potential of the immune system and translate it into effective therapies for patients. One of the most successful realizations of this approach has been the major histocompatibility complex independent engagement of cytotoxic T cells to target the lymphoid tumor antigen CD19. Both blinatumomab, a bispecific antibody that redirects effector T cells to B cells with its anti-CD3 and anti-CD19 arms, and chimeric antigen receptor (CAR) T cells engineered to target CD19 have been successful in the treatment of patients with relapsed and refractory (r/r) acute lymphocytic leukemia (ALL).1-6 The remarkable demonstrations of efficacy observed with these T-cell engagers are all the more dramatic when considered in the context of the historical plight faced by a patient with r/r ALL. With traditional approaches, adult patients with r/r ALL have a poor prognosis, and almost all will die of their disease.7-14 Children with relapsed disease are traditionally more responsive to initial attempts at salvage than their adult counterparts, but relapsed ALL remains a leading cause of cancer deaths in children, and those with refractory disease have only a 30% chance of long-term survival.15,16
The immune activation critical for the efficacy observed with blinatumomab and CAR T-cell therapy is also responsible for unique treatment-related toxicity. Cytokine release syndrome (CRS) is a potentially life-threatening condition that correlates with the nonphysiologic activation of T cells. Given the potential benefits of these therapies and the promise that immune-based approaches to treat ALL and other malignancies will only become more potent and more prevalent, extensive efforts are underway by several programs to better understand, describe, and manage CRS.
Acute lymphocytic leukemia-targeted agents associated with CRS
CAR T cells
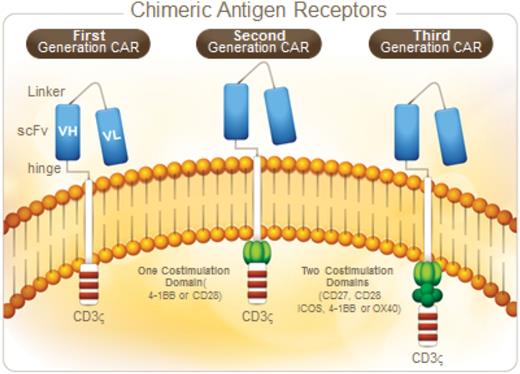
CAR T cells (CARs) are engineered to combine an extracellular antigen recognition domain (usually the variable regions of a specific monoclonal antibody [scFv]) with 1 or more intracellular T cell signaling domains. With the use of gene transfer techniques, CARs can then be introduced into normal T cells, redirecting them to target new antigens (such as CD19) in a manner independent of major histocompatibility complex.17,18 Transduction of T cells with CARs encoded in lenti- or retroviral vectors results in permanent modification of the genome, and thus the potential for ongoing expression of the CAR protein for the life of the T cell. The components of the CAR signaling domain are critical for maximal activation, expansion, and persistence of CAR T cells, and therefore are a key target for manipulation. The so-called “first-generation CARs” included only the antigen recognition domain with an intracellular CD3ζ signaling domain, which resulted in limited clinical activity.19-21 Several groups worked to improve efficacy by developing “second-generation CARs,” which included an additional CD28 or CD137(4-1BB)-derived co-stimulatory domain in addition to the CD3ζ domain (Figure 1). The preclinical evidence that second-generation CARs would provide more potent antitumor activity22,23 has now been proven by dramatic clinical outcomes for patients with relapsed and refractory CD19+ malignancies.1,3,6,24,25
At the time of this report, CAR T cells are available only in the context of a clinical trial. The anti-CD19 CAR T cells used across different clinical trial programs may differ in regard to the design of the CAR molecule, the method of CAR transfer into T cells, and other aspects of the CAR T-cell culture environment. The basic steps involved in the manufacture of anti-CD19 CAR T cells can be summarized as follows. First, patients undergo an apheresis procedure to collect autologous T cells. Once collected, T cells are genetically modified with the CAR construct (often using lenti- or retroviral techniques) and expanded ex vivo for clinical use. Before anti-CD19 CAR T-cell infusion, patients typically receive chemotherapy in an effort to induce lymphodepletion to enhance CAR T-cell expansion and persistence in vivo.26 Lymphodepletion may have the additional benefit of tumor cytoreduction, which may improve efficacy and minimize toxicity of the CAR T cells.
Unprecedented remission rates of 67% to 90% have been observed in adult and pediatric patients with r/r ALL treated with second-generation anti-CD19 CAR T cells (Table 1).1,3,6 In our program (Hospital of University of Pennsylvania/Children’s Hospital of Philadelphia [PENN/CHOP]), we reported a 90% complete remission (CR) rate in 30 pediatric (n = 25) and adult (n = 5) patients treated with anti-CD19 CAR T cells incorporating the 4-1BB costimulatory domain, referred to as CTL019 cells.6 Flow cytometry to assess for minimal residual disease (MRD) was negative in 22 patients, positive in 3 patients (0.1%, 0.09%, and 0.22%), and not performed in 2 patients who achieved a CR. Eighteen of the 30 patients treated had relapsed after a prior allogeneic stem cell transplantation (SCT), with T cells successfully collected and manufactured from the recipient and no postinfusion graft-versus-host disease observed. Of the 27 responding patients, 7 developed relapsed disease (range, 6 weeks-8.5 months after infusion), 3 with CD19-negative leukemia. Of importance, remissions were sustained from 2 to 24+ months in the remaining 19 patients. The durable remissions observed in patients not bridged to allogeneic SCT correlated with CAR T-cell persistence and the biological correlate of ongoing CAR T-cell activity, B-cell aplasia.6
Relapsed/refractory acute lymphoblastic leukemia outcomes after second-generation anti-CD19 chimeric antigen receptor T cells and blinatumomab
| Reference . | T-cell engager . | Population . | Response . | CRS . | Neurologic toxicity . |
|---|---|---|---|---|---|
| Anti-CD19 CAR T cells | |||||
| 6 | Anti-CD19 CAR T | N = 30 (r/rALL) | CR = 90% | 100% CRS | 43% total |
| CD3z-4-1BB | Pediatric and adults | 27% severe | Encephalopathy | ||
| Aphasia | |||||
| Seizure (1) | |||||
| 1 | Anti-CD19 CAR T | N = 16 (r/rALL) | CR = 88% | 43% severe | 25% grade 3-grade 4 |
| CD3z-CD28 | Adults | Encephalopathy | |||
| Seizure | |||||
| 3 | Anti-CD19 CAR T | N = 21 (r/rALL) | CR = 67% | 76% CRS | 29% total |
| CD3z-CD28 | Pediatric and young adults | 28% severe | Hallucinations | ||
| Dysphasia | |||||
| Encephalopathy | |||||
| Blinatumomab | |||||
| 5 | Blinatumomab | N = 189 (r/rALL) | CR/CRh = 43% | 60% pyrexia | 52% total |
| Adults | 28% febrile neutropenia | 11% grade 3 | |||
| 2% grade 3 CRS | 2% grade 4 |
| Reference . | T-cell engager . | Population . | Response . | CRS . | Neurologic toxicity . |
|---|---|---|---|---|---|
| Anti-CD19 CAR T cells | |||||
| 6 | Anti-CD19 CAR T | N = 30 (r/rALL) | CR = 90% | 100% CRS | 43% total |
| CD3z-4-1BB | Pediatric and adults | 27% severe | Encephalopathy | ||
| Aphasia | |||||
| Seizure (1) | |||||
| 1 | Anti-CD19 CAR T | N = 16 (r/rALL) | CR = 88% | 43% severe | 25% grade 3-grade 4 |
| CD3z-CD28 | Adults | Encephalopathy | |||
| Seizure | |||||
| 3 | Anti-CD19 CAR T | N = 21 (r/rALL) | CR = 67% | 76% CRS | 29% total |
| CD3z-CD28 | Pediatric and young adults | 28% severe | Hallucinations | ||
| Dysphasia | |||||
| Encephalopathy | |||||
| Blinatumomab | |||||
| 5 | Blinatumomab | N = 189 (r/rALL) | CR/CRh = 43% | 60% pyrexia | 52% total |
| Adults | 28% febrile neutropenia | 11% grade 3 | |||
| 2% grade 3 CRS | 2% grade 4 |
NR, not reported.
Similarly high response rates have also been observed by the Memorial Sloan-Kettering Cancer Center, using anti-CD19-directed CAR T cells containing a CD28 costimulatory domain.1 In 16 adults with r/r ALL, 14 (88%) achieved a CR. Four patients had relapsed after allogeneic SCT, and no patient developed graft-versus-host disease. Of the 16 patients treated, 7 underwent allogeneic SCT, with 2 deaths related to SCT complications and the remaining 5 in continued clinical remission (follow up, 2-24 months). Persistence of CAR T cells in this study was limited to 1-3 months after infusion.1
The National Cancer Institute (NCI) observed a CR rate of 67% in a phase 1 intent-to-treat analysis of 20 children and young adults treated with anti-CD19-directed CAR T cells containing a CD28 domain.3 Ten of the 14 responding patients were then treated with a consolidative SCT. Two patients not consolidated with SCT developed CD19− relapse, similar to the PENN/CHOP reports. Three patients who did not respond to initial treatment were reinfused with CAR T cells, but did not respond. CAR T cells did not persist beyond 68 days. Similar to the experience described earlier, no one treated with CAR T cells after allogeneic SCT (n = 8) developed graft-versus-host disease.3
Blinatumomab
Blinatumomab is a bispecific T-cell-engaging single-chain antibody construct that links CD3+ T cells with CD19+ B cells. Blinatumomab was first explored in patients in first morphologic remission with MRD-positive ALL and successfully converted the majority of patients to an MRD-negative state.27,28 Follow-up studies in patients with r/r Philadelphia chromosome–negative (Ph−) B-cell ALL have been very promising, leading to the drug’s accelerated approval by the US Food and Drug administration on December 3, 2014, and by the European Medicines Agency in November 2015.5,29 A multicenter, single-group study treated 189 adult patients with r/r Ph− B-cell ALL with single-agent blinatumomab. Blinatumomab was administered as a 28-day continuous infusion (9 µg/day for days 1-7; 28 µg/day thereafter) followed by 2 weeks of rest for up to 5 cycles. Sixty-four of these subjects had relapsed after a prior allogeneic SCT. CR and CRh (complete remission without full hematologic recovery) occurred in 43% of patients within the first 2 cycles. Of note, 82% of patients achieving CR/CRh were also MRD-negative, as determined by allele-specific quantitative PCR. Of responding patients without prior SCT, 40% were bridged successfully to SCT.5 Durable remissions were dependent on subsequent allogeneic SCT, with a median OS of 6.1 months. Observed treatment-related adverse events with blinatumomab usually occur in the first cycle and include fever, CRS, and neurologic toxicity.
CRS after CAR T-cell therapy and blinatumomab
CRS is a systemic inflammatory response that correlates with the in vivo activation and proliferation of CAR T cells. The clinical features of the syndrome are associated with high levels of inflammatory markers and cytokines, including C-reactive protein, ferritin, interferon-ɤ, and interleukin-6 (IL-6). The first clinical sign of CRS is fever, which often starts low but escalates to levels as high as 105°F/40.5°C. In the vast majority of patients, CRS occurs within 1 to 14 days of anti-CD19 CAR T-cell infusion. Unfortunately, this syndrome can progress beyond fevers and malaise to life-threatening vasodilatory shock and capillary leak with hypoxic respiratory failure. Depending on its severity, CRS can either be self-limited (requiring only supportive care with antipyretics and intravenous fluids) or may require intervention with anticytokine-directed therapy. The duration of CRS is variable and dependent on intervention, with resolution typically by 2 to 3 weeks after CAR T-cell infusion.
In our first 30 adult and pediatric patients with ALL, all experienced some degree of CRS. This was defined as severe in 8 patients who required intensive care unit–level care for vasopressor support and supplemental oxygen.6 Other programs, as summarized in Table 1, have noticed a similar incidence, duration, and severity of CRS. Given the life-threatening nature of CRS, it is helpful to try and identify disease-, patient-, or therapy-related factors that may predict the severity of CRS. Disease burden in ALL strongly correlates with the severity of CRS.1,6,30 Unlike more traditional agents, there is not an obvious dose–toxicity relationship with anti-CD19 CAR T-cell therapy, as the infusion dose grossly underestimates the final expanded active dose, and dose is only 1 of many factors that may correlate with peak in vivo expansion. However, there is a suggestion that the infusion dose of anti-CD19 CAR T cells may have some effect on the severity of CRS. In the phase 1 portion of their ALL study, the NCI found an increase in the severity of CRS with escalating dose levels.3
Clinically available laboratory markers of inflammation, including C-reactive protein and ferritin, are universally elevated in patients with CRS from CAR T cells.6,25 We and other groups have also noted investigational cytokine activation profiles that correlate with the clinical syndrome of CRS.1-3,6,24,25 Effector cytokines such as interferon-ɤ and soluble IL-2 receptor α (sIL2Ra) are elevated, but so are cytokines traditionally associated with macrophage activation, such as IL-6 and IL-10. Indeed, many of the clinical manifestations of CRS overlap with those of macrophage activation syndrome/hemophagocytic lymphohistiocytosis.31 An area of ongoing investigation is whether cytokine profiles can be used to predict severity of CRS and be used to guide preemptive anticytokine directed treatment.1,30
The clinical signs and symptoms of CRS with blinatumomab are similar to those observed with anti-CD19 CAR T cells. Risk factors for CRS include disease burden and initial starting dose of blinatumomab. In general, signs and symptoms of CRS are limited to the first cycle of the drug. In the study of 189 patients with r/r ALL discussed earlier, only 2% developed grade 3 CRS, whereas 60% developed pyrexia. In this study, cytoreduction was performed for patients with high disease burdens, and CRS prophylaxis with dexamethasone was performed on day 1 and with dose escalation.5
Grading of CRS
A CRS grading system allows for an objective assessment and reporting of clinical severity that can also help guide anticytokine treatment algorithms. The NCI Common Terminology Criteria for Adverse Events (CTCAE v 4.0) description for CRS has been used in describing outcomes in recipients of blinatumomab and other therapeutic antibodies (Table 2). The grading system, however, is written with an infusional antibody medication in mind, with grading linked not only to severity of clinical signs and symptoms but also to whether the infusion of drug needs to be held and/or intervention occurs. Given the unique nature of cellular therapy, several alternative CRS grading systems have been developed to better capture this adverse event after CAR T-cell therapy. A consensus grading scale with input from several programs was presented in 2014 and has been used by the NCI to grade CRS in their anti-CD19 CAR T-cell programs.32 In the PENN/CHOP clinical trial programs, we have been using a different modified grading scale25 (Table 2). It is important to note that a patient with CRS would receive a different grade, depending on which scale is used. As an example, a patient receiving anti-CD19 CAR T cells for ALL with subsequent hypotension requiring low-dose pressors for hemodynamic support would have grade 2 CRS on the NCI scale, grade 3 CRS on the UPENN/CHOP scale, and grade 4 CRS on the CTCAEv4.0 scale.
Grading schemes for cytokine release syndrome
| Grading scale . | Grade 1 . | Grade 2 . | Grade 3 . | Grade 4 . |
|---|---|---|---|---|
| CTCAEv4 (blinatumomab) | Mild | Infusion interruption indicated but responds promptly to symptomatic treatment (eg, antihistamines, nonsteroidal anti-inflammatory drug, narcotics, intravenous fluids); prophylactic medications indicated for ≤ 24 h | Prolonged (eg, not rapidly responsive to symptomatic medications and/or brief interruption of infusion); recurrence of symptoms after initial improvement; hospitalization indicated for clinical sequelae (eg renal impairment, pulmonary infiltrates) | Life-threatening consequences; pressor or ventilator support |
| No infusion interruption | ||||
| No intervention | ||||
| 2014 NCI Consensus | Symptoms are not life-threatening and require symptomatic treatment only; eg fever, nausea, fatigue, headache, myalgias, malaise | Symptoms require and respond to moderate intervention | Symptoms require and respond to aggressive intervention | Life-threatening symptoms; requirement for ventilator support or grade 4 organ toxicity (excluding transaminitis) |
| Oxygen requirement < 40% or hypotension responsive to fluids or low-dose pressors or grade 2 organ toxicity | Oxygen requirement < 40% or hypotension requiring high-dose or multiple pressors or grade 3 organ toxicity or grade 4 transaminitis | |||
| UPENN/CHOP | Mild reaction treated with supportive care only | Moderate reaction requiring intravenous therapies or parenteral nutrition; mild signs of organ dysfunction (creatinine ≤grade 2 or liver function tests ≤grade 3) | More severe reaction, requiring hospitalization; moderate signs of organ dysfunction (grade 3 creatinine or grade 4 liver function tests) related to CRS; hypotension treated with intravenous fluids or low-dose pressors; hypoxemia requiring oxygenation, bilevel positive airway pressure, or continuous positive airway pressure | Life-threatening complications, including hypotension requiring high-dose vasoactives or hypoxemia requiring mechanical ventilation |
| Hospitalization for CRS or febrile neutropenia | ||||
| Comparisons across scales | CTCAE: linked to infusion of drug, not applicable to cellular therapy. | CTCAE: linked to infusion/withholding of a drug, not applicable to cellular therapy. | Grade 3 NCI permits more severe hypotension compared with a UPENN/CHOP grade 3. | Life-threatening hypoxia (mechanical ventilation) similar across scales. |
| NCI and UPENN/CHOP allow for symptom management; CTCAE does not | Grade 2 NCI permits more severe hypoxia and hypotension compared with a UPENN/CHOP grade 2 |
| Grading scale . | Grade 1 . | Grade 2 . | Grade 3 . | Grade 4 . |
|---|---|---|---|---|
| CTCAEv4 (blinatumomab) | Mild | Infusion interruption indicated but responds promptly to symptomatic treatment (eg, antihistamines, nonsteroidal anti-inflammatory drug, narcotics, intravenous fluids); prophylactic medications indicated for ≤ 24 h | Prolonged (eg, not rapidly responsive to symptomatic medications and/or brief interruption of infusion); recurrence of symptoms after initial improvement; hospitalization indicated for clinical sequelae (eg renal impairment, pulmonary infiltrates) | Life-threatening consequences; pressor or ventilator support |
| No infusion interruption | ||||
| No intervention | ||||
| 2014 NCI Consensus | Symptoms are not life-threatening and require symptomatic treatment only; eg fever, nausea, fatigue, headache, myalgias, malaise | Symptoms require and respond to moderate intervention | Symptoms require and respond to aggressive intervention | Life-threatening symptoms; requirement for ventilator support or grade 4 organ toxicity (excluding transaminitis) |
| Oxygen requirement < 40% or hypotension responsive to fluids or low-dose pressors or grade 2 organ toxicity | Oxygen requirement < 40% or hypotension requiring high-dose or multiple pressors or grade 3 organ toxicity or grade 4 transaminitis | |||
| UPENN/CHOP | Mild reaction treated with supportive care only | Moderate reaction requiring intravenous therapies or parenteral nutrition; mild signs of organ dysfunction (creatinine ≤grade 2 or liver function tests ≤grade 3) | More severe reaction, requiring hospitalization; moderate signs of organ dysfunction (grade 3 creatinine or grade 4 liver function tests) related to CRS; hypotension treated with intravenous fluids or low-dose pressors; hypoxemia requiring oxygenation, bilevel positive airway pressure, or continuous positive airway pressure | Life-threatening complications, including hypotension requiring high-dose vasoactives or hypoxemia requiring mechanical ventilation |
| Hospitalization for CRS or febrile neutropenia | ||||
| Comparisons across scales | CTCAE: linked to infusion of drug, not applicable to cellular therapy. | CTCAE: linked to infusion/withholding of a drug, not applicable to cellular therapy. | Grade 3 NCI permits more severe hypotension compared with a UPENN/CHOP grade 3. | Life-threatening hypoxia (mechanical ventilation) similar across scales. |
| NCI and UPENN/CHOP allow for symptom management; CTCAE does not | Grade 2 NCI permits more severe hypoxia and hypotension compared with a UPENN/CHOP grade 2 |
As antibody and cellular immune-based therapies continue to expand, it will be important to establish a defined grading scale for CRS to be adopted universally. This would allow for better interpretation of adverse events across studies and help guide initial treatment algorithms for agents in clinical trial development. In an effort to harmonize a CRS definition and grading scale, the National Institutes of Health Office of Biotechnology Activities hosted a conference with representatives from several institutions in June 2015. A consensus report from this meeting is in development.
Prevention and management of CRS
The management of CRS in patients receiving blinatumomab vs anti-CD19 CAR T cells differs in several critical ways. Blinatumomab is an off-the-shelf medication that can be stopped and restarted with intervening dose adjustments, as needed, in response to toxicity. With CAR T cells, that luxury is lost. Each autologous CAR T-cell product is uniquely manufactured for each patient and infused at a singular point in time. The CAR T cells are “living” drugs capable of in vivo expansion of several log-fold after infusion, with persistence of CAR T cells for months, or even years, after infusion.4 Traditional pharmacologic dose adjustment strategies in response to toxicity are thus impossible for CAR T cells. In addition, there is concern that agents used to mitigate signs and symptoms of CRS may abrogate the antitumor response of the T cells. We therefore describe CRS management approaches for the 2 therapeutics separately (Table 3).
Risk factors, prevention, and management of CRS
| . | Risk factors . | Prevention . | Treatment . |
|---|---|---|---|
| CAR T cells | Disease burden | Pretreatment cytoreduction | Fever: symptom management, acetaminophen |
| High dose Degree of lymphodepletion | Future directions: Dose adjustment by disease burden Fractionated dosing schemes | CRS: protocol-dependent anticytokine intervention with tocilizumab ± corticosteroids | |
| Blinatumomab | Disease burden Starting dose | Pretreatment cytoreduction Lower dose week 1 | Fever/CRS: paracetamol/ acetaminophen and/or dexamethasone. |
| 20 mg dexamethasone day 1, with dose escalation and with restarting drug | Grade 3 CRS: hold drug until resolution then restart at 9 µg/day with escalation to 28 µg/day after 7 d if toxicity does not recur | ||
| Grade 4 CRS: consider discontinuation | |||
| Tocilizumab has been successful in cases of CRS refractory to holding agent and giving corticosteroids |
| . | Risk factors . | Prevention . | Treatment . |
|---|---|---|---|
| CAR T cells | Disease burden | Pretreatment cytoreduction | Fever: symptom management, acetaminophen |
| High dose Degree of lymphodepletion | Future directions: Dose adjustment by disease burden Fractionated dosing schemes | CRS: protocol-dependent anticytokine intervention with tocilizumab ± corticosteroids | |
| Blinatumomab | Disease burden Starting dose | Pretreatment cytoreduction Lower dose week 1 | Fever/CRS: paracetamol/ acetaminophen and/or dexamethasone. |
| 20 mg dexamethasone day 1, with dose escalation and with restarting drug | Grade 3 CRS: hold drug until resolution then restart at 9 µg/day with escalation to 28 µg/day after 7 d if toxicity does not recur | ||
| Grade 4 CRS: consider discontinuation | |||
| Tocilizumab has been successful in cases of CRS refractory to holding agent and giving corticosteroids |
CRS management after CAR T-cell therapy
Early in the CHOP clinical trial program using anti-CD19 CAR T cells for children with r/r ALL, a child was critically ill with life-threatening hypoxia and hypotension attributed to CRS. The patient’s clinical status did not improve, despite administration of high-dose steroids and anti–tumor necrosis factor directed therapy. It was noted that the patient’s IL-6 level was markedly elevated, and tocilizumab, an antibody against the IL-6 receptor (approved by the US Food and Drug Administration for the treatment of rheumatoid arthritis), was administered with rapid improvement in her clinical status.2
The use of tocilizumab to manage CRS has now become standard after CAR T-cell therapy and remains an attractive approach for 2 reasons. The first is quite simple, in that it continues to be effective for most patients and has limited inherent toxicity. The other is based on the hypothesis that by targeting IL-6, which is most likely released by macrophages, one could provide toxicity management of the CRS with less effect on CAR T-cell efficacy compared with targeting other cytokines or using T-cell toxic agents such as corticosteroids. Memorial Sloan-Kettering Cancer Center has shown differential survival of anti-CD19 CAR T cells in the bone marrow at day 28, based on whether patients’ severe CRS was managed with steroid- or tocilizumab-based approaches.1 The optimal time to intervene with anticytokine directed therapy is unknown and is the subject of ongoing clinical trials. One could envision a prophylactic approach (such as giving tocilizumab the day after CAR T-cell infusion) or a preemptive approach guided by clinical factors such as first fever or early cytokine profiles. At this time in our CRS management algorithm, we wait to treat patients with tocilizumab until they develop more severe signs and symptoms of CRS. This is based on the hypothesis that IL-6 may represent part of a cytokine feedback loop that enhances T-cell proliferation; therefore, targeting IL-6 too early in the response could inhibit CAR T-cell efficacy.
CRS management with blinatumomab therapy
In large part, the guidelines for administering blinatumomab for patients with r/r ALL were generated with the intent of limiting the incidence and severity of CRS. In the r/r ALL setting, patients initiating blinatumomab are admitted to the hospital to monitor for CRS and neurologic toxicities. Because of the high correlation of disease burden with CRS, a pretreatment attempt to cytoreduce patients with more than 50% bone marrow blasts or a peripheral blast count more than 15 × 109/L with dexamethasone and/or cyclophosphamide is recommended. Blinatumomab dose also correlates with severity of CRS. During the first week of the first cycle of treatment, patients with r/r ALL receive 9 µg/day of drug before escalating to 28 µg/day for the remaining 3 weeks. In the event of grade 3 CRS, blinatumomab should be held until resolution, and then restarted at 9 µg/day with escalation to 28 µg/day after 7 days if toxicity does not recur. The package insert recommends discontinuing blinatumomab permanently if grade 4 CRS occurs.
Corticosteroids are known to be efficacious in the treatment of T-cell–mediated inflammatory states, including autoimmune disease and graft-versus-host disease. As discussed earlier, although steroids have a potential role in managing CRS, their potential to negatively affect the antitumor effects of cellular therapy limits their liberal use for CAR T-cell–induced CRS. However, steroids are currently the mainstay of prevention and treatment of blinatumomab-induced CRS. Blinatumomab’s package insert, approved by the US Food and Drug Administration, recommends pretreatment with 20 mg intravenous dexamethasone before the first dose of each cycle, before each intracycle dose escalation, or when restarting an infusion after treatment interruption. The full effect of steroids on efficacy of blinatumomab is not known, but preclinical studies suggest intact T-cell activation with a reduction in cytokine production.33 Tocilizumab has also been shown to mitigate blinatumomab-induced CRS in a patient with no improvement after drug cessation and corticosteroids.34
Neurologic events
Both blinatumomab and anti-CD19 CAR T-cell therapy have treatment-related neurologic events (Table 1).1,3,5,6 It is not clear at this time whether neuropsychiatric symptoms observed with these agents are independent of systemic CRS. In our experience, the onset and resolution of CNS symptoms after CAR T-cell therapy do not correlate precisely with the clinical course of systemic CRS and do not respond to tocilizumab intervention. CNS events are therefore not currently part of the definition or grading scale for CRS.
The etiology of CNS toxicity with these agents remains unclear. It should be noted that tocilizumab’s inability to control neurologic toxicity does not necessarily imply that these events are not cytokine mediated. Similar to most other monoclonal antibodies, tocilizumab does not typically cross the blood–brain barrier, and thus would be unlikely to rapidly control inflammation in the CNS compartment. Investigations are underway to better understand the pathophysiology and management of neurologic adverse effects.
Conclusion
The successes of blinatumomab and anti-CD19 CAR T-cell therapy for patients with relapsed and refractory chemotherapy-resistant ALL have been dramatic. Blinatumomab is now approved in the relapsed ALL setting and is being explored in the upfront setting in cooperative group trials. Large multicenter studies using anti-CD19 CAR T-cell therapy to assess the feasibility of expanding this therapy beyond just a few highly specialized centers are now ongoing. The success of blinatumomab and second-generation anti-CD19 CARs also inspires the development of T-cell–engaging therapies to target new antigens for ALL and other tumor types. A better understanding of the unique toxicities of these agents and the optimal management approaches to maintain high levels of efficacy and safety is a key component to moving the field forward.
Correspondence
Noelle V. Frey, Hospital of the University of Pennsylvania, 12 PCAM South, Philadelphia, PA 19104; e-mail: noelle.frey@uphs.upenn.edu.
References
Competing Interests
Conflict-of-interest disclosures: N.V.F. has received research funding from Novartis and has consulted for Amgen. D.L.P. has a spouse employed at Genentech and has received research funding and patents and royalties from Novartis.
Author notes
Off-label drug use: CAR T cells for B-cell malignancies, tocilizumab for cytokine release syndrome.