Abstract
The use of proteasome inhibitors and immunomodulatory agents in the treatment of myeloma have resulted in significant improvements in patient outcomes over the last decade. Although these agents now form the backbone of current myeloma treatment regimens both in the frontline and in a relapsed setting, drug resistance remains an inevitable challenge that most patients will encounter during their disease course. Hence, new treatment strategies continue to be explored, and the recent regulatory approvals of the monoclonal antibodies (mAbs) daratumumab (DARA) and elotuzumab (ELO), which target the plasma cell surface proteins CD38 and signaling lymphocytic activation molecule F7 (SLAMF7), respectively, have heralded the long-awaited era of antibody-based approaches in the treatment of myeloma. Hoping to build on these advances, a number of other mAbs are in various stages of clinical development, including those targeting myeloma cell surface antigens, the bone marrow microenvironment, and immune effector T cells such as anti-programmed cell death protein 1 antibodies. In this review, the current landscape and practical use of mAb-based therapy in myeloma will be discussed.
Learning Objectives
Understand the current landscape of mAb-based therapy in myeloma, with a particular focus on the CD38-targeted therapy with DARA and SLAMF7-targeted therapy with ELO
Become aware of practical issues unique to mAb-based therapy in myeloma including red blood cell compatibility testing with anti–CD38-directed therapy and interference with myeloma laboratory response assessments
Introduction
Multiple myeloma (MM) results from the proliferation of a malignant plasma cell and frequently leads to complications such as lytic bone disease, hypercalcemia, renal failure, and impaired immunity. Over the past 2 decades, progression-free survival (PFS) and overall survival for MM have more than doubled, largely due to improvements in therapy with the addition of novel agents such as immunomodulatory agents (IMiDs) and proteasome inhibitors (PIs).1 The understanding of MM pathobiology has undergone a similar period of growth, leading to the discovery of novel targets and pathways that impact proliferation and survival of the malignant clone. Despite these significant improvements, MM remains largely incurable, making new therapies with novel targets essential for continued improvements in clinical end points for patients with this disorder.
Targets for monoclonal antibody (mAb) therapy
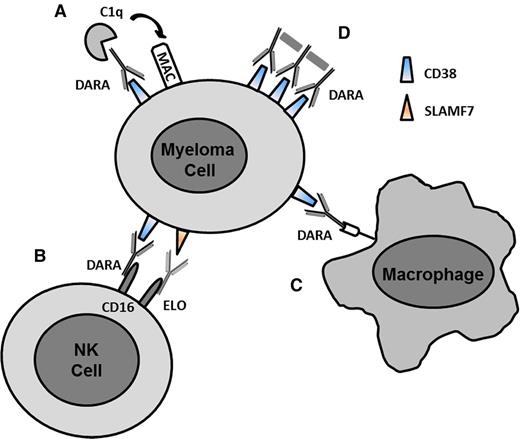
The high expression of a number of surface antigens on malignant plasma cells makes these appealing targets for immune therapy with mAbs. The mechanisms of mAbs are diverse, including directly targeting a receptor and its downstream activity, recruiting effector cells such as natural killer (NK) cells and macrophages to promote antibody-dependent cell-mediated cytotoxicity (ADCC) and antibody-dependent cellular phagocytosis (ADCP), fixing complement for complement-dependent cytotoxicity (CDC), or inducing cell death by delivery of a drug, toxin, or radioisotope to the malignant cell2 (Figure 1). Although the ideal target for mAb therapy would be one that is solely expressed on malignant plasma cells and not on normal cells (including plasma cells), most suitable targets are expressed to some degree on either normal plasma cells, other hematopoietic cells, and/or other cells/tissues. Plasma cell surface targets of mAbs that have already demonstrated significant clinical activity either alone or in combination with other approved myeloma drugs include signaling lymphocytic activation molecule F7 (SLAMF7) (elotuzumab [ELO]) and CD38 (daratumumab [DARA], isatuximab [ISA] [SAR659084], and MOR-202).3-7 Other mAbs directed against MM cellular antigens that have demonstrated at least stable disease include those directed against CD138 (BT062), CD54/ICAM-1 (BI-505), and CD74 (milatuzumab).8-10
Mechanisms of action of mAbs. (A) CDC. C1q binds to the antibody and triggers the complement cascade leading to the formation of the MAC on the surface of the myeloma cell. (B) ADCC. FcγR (CD16) on NK cells or other immune effector cells bind to the Fc region of the antibody leading to cell lysis. (C) ADCP. Fc receptors on macrophages bind to antibody and induce phagocytosis of cell. (D) FcγR-mediated crosslinking of bound antibody on the cell surface leading to apoptosis. MAC, membrane attack complex. Adapted from van de Donk et al32 with permission.
Mechanisms of action of mAbs. (A) CDC. C1q binds to the antibody and triggers the complement cascade leading to the formation of the MAC on the surface of the myeloma cell. (B) ADCC. FcγR (CD16) on NK cells or other immune effector cells bind to the Fc region of the antibody leading to cell lysis. (C) ADCP. Fc receptors on macrophages bind to antibody and induce phagocytosis of cell. (D) FcγR-mediated crosslinking of bound antibody on the cell surface leading to apoptosis. MAC, membrane attack complex. Adapted from van de Donk et al32 with permission.
The development of therapeutic mAbs for the treatment of MM has also been directed against growth factors and their receptors (eg, interleukin-6, interleukin-6 receptor, insulin-like growth factor-1 or -2, vascular endothelial growth factor, and B-cell activating factor). Antibodies that augment the host immune response through immune checkpoint inhibitors targeting programmed cell death protein 1 (PD-1) and PD ligand 1 (PD-L1) are also in development.2,11 The role of mAbs continues to expand in MM as the number of targets and subsequent successful clinical applications continue to grow (Table 1).3-10,12-19
Select studies with mAbs in myeloma
| . | . | . | . | . | . | Response rate (%) . | . | ||
|---|---|---|---|---|---|---|---|---|---|
| . | Reference . | Phase . | Regimen . | N . | Study population . | ORR . | VGPR . | CR . | PFS (mo) . |
| CD38 | 4 | 1/2 | DARA 16 mg/kg | 42 | R/R, ≥2 lines of therapy including IMiD and PI | 36 | 5 | 5 | 5.6 |
| 5 | 2 | DARA 16 mg/kg | 106 | R/R, ≥3 lines therapy or refractory to IMiD and PI | 29 | 9 | 3 | 3.7 | |
| 15 | 1b | DARA 16 mg/kg | 77 | R/R, ≥2 lines of therapy | 59 | 16 | 5 | N/A | |
| POM 4 mg | |||||||||
| DEX 40 mg | |||||||||
| 14 | 3 | DARA 16 mg/kg | 251 | R/R, ≥1 line of therapy | 83 | 40 | 19 | N/R | |
| BOR 1.3 mg/m2 | |||||||||
| DEX 20 mg* | |||||||||
| BOR 1.3 mg/m2 | 247 | 63 | 20 | 9 | 7.2 | ||||
| DEX 20 mg* | |||||||||
| 30 | 3 | DARA 16 mg/kg | 286 | R/R, ≥1 line of therapy | 93 | 33 | 38 | N/R | |
| LEN 25 mg | |||||||||
| DEX 20 mg | |||||||||
| LEN 25 mg | 283 | 76 | 25 | 19 | 18.4 | ||||
| DEX 40 mg | |||||||||
| 7 | 2 | ISA 10-20 mg/kg | 74 | R/R, ≥3 lines of therapy or refractory to IMiD and PI | 24 | 14 | 0 | 3.7 | |
| 16 | 1b | ISA 10 mg/kg | 46 | R/R, ≥2 lines of therapy | 57 | 33 | 4 | N/A | |
| LEN 25 mg | |||||||||
| DEX 40 mg | |||||||||
| 6 | 1/2a | MOR202 4-16 mg/kg | 15 | R/R, ≥2 lines of therapy | 27 | 7 | 0 | N/A | |
| DEX 40 mg | |||||||||
| SLAMF7 | 12 | 1 | ELO 0.5-20 mg/kg | 34 | R/R, ≥2 lines of therapy | 0 | 0 | 0 | N/A |
| 13 | 2 | ELO 10 mg/kg | 77 | R/R, 1-3 lines prior therapy | 66 | 33 | 4 | 9.7 | |
| BOR 1.3 mg/m2 | |||||||||
| DEX 20 mg† | |||||||||
| BOR 1.3 mg/m2 | 55 | 63 | 23 | 4 | 6.9 | ||||
| DEX 20 mg | |||||||||
| 3 | 3 | ELO 10 mg/kg | 321 | R/R, 1-3 lines prior therapy | 79 | 28 | 4 | 19.4 | |
| LEN 25 mg | |||||||||
| DEX 40 mg‡ | |||||||||
| LEN 25 mg DEX 40 mg | 325 | 66 | 21 | 7 | 14.9 | ||||
| PD-1 | 17 | 1 | Nivolumab 1-3 mg/kg | 27 | R/R, 78% ≥3 lines of therapy | 0 | 0 | 0 | N/A |
| 18 | 1 | PEMBRO 2 mg/kg or 200 mg fixed dose | 40 | R/R, ≥ 2lines of therapy | 50 | 13 | 3 | N/A | |
| LEN 10 or 25 mg | |||||||||
| DEX 40 mg | |||||||||
| 19 | 2 | PEMBRO 200 mg fixed dose | 24 | R/R, ≥2 lines of therapy | 50 | 9 | 14 | N/A | |
| POM 4 mg | |||||||||
| DEX 40 mg | |||||||||
| CD138 | 8 | 1 | BT062 80-120 mg/m2 | 36 | R/R, ≥1 line of therapy | 78 | 28 | 8 | N/A |
| LEN 25 mg | |||||||||
| DEX 40 mg | |||||||||
| ICAM-1 | 9 | 1 | BI-505 0.09-20 mg/kg | 29 | R/R, ≥2 lines of therapy | 0 | 0 | 0 | N/A |
| CD74 | 10 | 1 | Milatuzumab 1.5-16 mg/kg | 25 | R/R, ≥2 lines of therapy | 0 | 0 | 0 | N/A |
| . | . | . | . | . | . | Response rate (%) . | . | ||
|---|---|---|---|---|---|---|---|---|---|
| . | Reference . | Phase . | Regimen . | N . | Study population . | ORR . | VGPR . | CR . | PFS (mo) . |
| CD38 | 4 | 1/2 | DARA 16 mg/kg | 42 | R/R, ≥2 lines of therapy including IMiD and PI | 36 | 5 | 5 | 5.6 |
| 5 | 2 | DARA 16 mg/kg | 106 | R/R, ≥3 lines therapy or refractory to IMiD and PI | 29 | 9 | 3 | 3.7 | |
| 15 | 1b | DARA 16 mg/kg | 77 | R/R, ≥2 lines of therapy | 59 | 16 | 5 | N/A | |
| POM 4 mg | |||||||||
| DEX 40 mg | |||||||||
| 14 | 3 | DARA 16 mg/kg | 251 | R/R, ≥1 line of therapy | 83 | 40 | 19 | N/R | |
| BOR 1.3 mg/m2 | |||||||||
| DEX 20 mg* | |||||||||
| BOR 1.3 mg/m2 | 247 | 63 | 20 | 9 | 7.2 | ||||
| DEX 20 mg* | |||||||||
| 30 | 3 | DARA 16 mg/kg | 286 | R/R, ≥1 line of therapy | 93 | 33 | 38 | N/R | |
| LEN 25 mg | |||||||||
| DEX 20 mg | |||||||||
| LEN 25 mg | 283 | 76 | 25 | 19 | 18.4 | ||||
| DEX 40 mg | |||||||||
| 7 | 2 | ISA 10-20 mg/kg | 74 | R/R, ≥3 lines of therapy or refractory to IMiD and PI | 24 | 14 | 0 | 3.7 | |
| 16 | 1b | ISA 10 mg/kg | 46 | R/R, ≥2 lines of therapy | 57 | 33 | 4 | N/A | |
| LEN 25 mg | |||||||||
| DEX 40 mg | |||||||||
| 6 | 1/2a | MOR202 4-16 mg/kg | 15 | R/R, ≥2 lines of therapy | 27 | 7 | 0 | N/A | |
| DEX 40 mg | |||||||||
| SLAMF7 | 12 | 1 | ELO 0.5-20 mg/kg | 34 | R/R, ≥2 lines of therapy | 0 | 0 | 0 | N/A |
| 13 | 2 | ELO 10 mg/kg | 77 | R/R, 1-3 lines prior therapy | 66 | 33 | 4 | 9.7 | |
| BOR 1.3 mg/m2 | |||||||||
| DEX 20 mg† | |||||||||
| BOR 1.3 mg/m2 | 55 | 63 | 23 | 4 | 6.9 | ||||
| DEX 20 mg | |||||||||
| 3 | 3 | ELO 10 mg/kg | 321 | R/R, 1-3 lines prior therapy | 79 | 28 | 4 | 19.4 | |
| LEN 25 mg | |||||||||
| DEX 40 mg‡ | |||||||||
| LEN 25 mg DEX 40 mg | 325 | 66 | 21 | 7 | 14.9 | ||||
| PD-1 | 17 | 1 | Nivolumab 1-3 mg/kg | 27 | R/R, 78% ≥3 lines of therapy | 0 | 0 | 0 | N/A |
| 18 | 1 | PEMBRO 2 mg/kg or 200 mg fixed dose | 40 | R/R, ≥ 2lines of therapy | 50 | 13 | 3 | N/A | |
| LEN 10 or 25 mg | |||||||||
| DEX 40 mg | |||||||||
| 19 | 2 | PEMBRO 200 mg fixed dose | 24 | R/R, ≥2 lines of therapy | 50 | 9 | 14 | N/A | |
| POM 4 mg | |||||||||
| DEX 40 mg | |||||||||
| CD138 | 8 | 1 | BT062 80-120 mg/m2 | 36 | R/R, ≥1 line of therapy | 78 | 28 | 8 | N/A |
| LEN 25 mg | |||||||||
| DEX 40 mg | |||||||||
| ICAM-1 | 9 | 1 | BI-505 0.09-20 mg/kg | 29 | R/R, ≥2 lines of therapy | 0 | 0 | 0 | N/A |
| CD74 | 10 | 1 | Milatuzumab 1.5-16 mg/kg | 25 | R/R, ≥2 lines of therapy | 0 | 0 | 0 | N/A |
N/A, not available; N/R, not reached; R/R, relapsed and/or refractory.
DEX 20 mg PO or IV on day 1, 2, 3, 4, 8, 9, 11, and 12 on 21-day cycle for C1-C8.
DEX 8 mg IV and 8 mg PO given on days of ELO infusions.
DEX 8 mg IV and 28 mg PO given on days of ELO infusions.
United States Food and Drug Administration (FDA)-approved mAbs for myeloma
ELO (anti-SLAMF7)
SLAMF7 (CS1), a cell surface glycoprotein and a member of the signaling lymphocyte activating-molecule–related receptor family, was first identified as a plasma cell–specific target using a complementary DNA subtraction library that included genes preferentially expressed in memory B cells and plasma cells compared with naïve B cells. Overexpression of SLAMF7 on normal and malignant plasma cells, and to a lesser extent on NK and CD8+ T cells, was confirmed through gene expression profiling studies on primary patient samples, whereas expression was largely absent in other normal human tissue.20,21 Moreover, small interfering RNA-mediated knockdown of SLAMF7 expression decreased MM cell adhesion to bone marrow stromal cells, suggesting a mechanistic role of SLAMF7 in MM pathobiology.20
Based on these studies, a humanized immunoglobulin G1-κ (IgG1-κ) mAb HuLuc63, later known as ELO, was developed to target SLAMF7 in MM. ELO demonstrated potent in vitro and in vivo activity in MM preclinical models, primarily mediated through NK-dependent ADCC but not CDC.20 Moreover, ELO also induces activation of NK cells through SLAMF7 ligation, enhancing the cytotoxic effects of NK cells against MM cells.22
Clinically, this has not translated into activity of ELO as a single agent, illustrated by the lack of any objective responses in a phase 1 trial in patients with relapsed and/or refractory MM (RRMM).12 Preclinical data, however, suggested that the activity of ELO may be enhanced by the addition of IMiDs or bortezomib (BOR) through activation of immune effector cells involved in ADCC,23,24 providing rationale that such combinations with ELO may be effective therapeutic approaches in MM despite the lack of ELO single-agent activity.
The addition of BOR and dexamethasone (DEX) to ELO failed to demonstrate a benefit in overall response rate (ORR, ≥ partial response) in a randomized phase 2 study but did demonstrate a short, yet significant improvement in PFS for the ELO/BOR/DEX combination (9.7 months vs 6.9 months).13 A phase 1 trial with ELO/lenalidomide (LEN)/DEX demonstrated an impressive 82% ORR in RRMM patients with excellent tolerability and was higher in patients who received 10 mg/kg ELO (92%) than in those who received 20 mg/kg (76%). Steroids were given to patients in the higher dose cohort to prevent infusion reactions, and whether this decrease in ORR with the higher dose of ELO was due to abrogation of host immune effector cell response by DEX is unknown.25 Subsequently, 646 patients with RRMM with 1 to 3 lines of prior therapy were randomized to receive LEN/DEX alone or with ELO at the 10 mg/kg dose in a randomized phase 3 trial (Eloquent-2).3 Previously noted activity was confirmed with a 79% ORR for the ELO combination compared with 66% for patients who received LEN/DEX (P < .001). One-year, 2-year, and median PFS were superior in patients who received the mAb combination compared with those treated with LEN/DEX (PFS 19.4 vs 14.9 months, 1-year PFS 68% vs 57%, 2-year PFS 41% vs 27%; hazard ratio [HR] 0.7; P < .001).5 Patients with a diagnosis of MM ≥3.5 years prior to study entry experienced the longest benefit in median PFS (26 months vs 17.3 months; P < .001); this improvement may suggest that these patients had a longer remission after induction therapy and only 1 prior line of therapy, but this remains unclear from the current data as presented. Improvements in PFS were noted even among patients traditionally considered to have higher risk disease, including those ≥65 years of age, resistant to their most recent therapy, and those with either International Staging System stage III disease, impaired renal function (creatinine clearance <60 mL/min), and/or deletion of chromosome 17p and t[4;14]. Based on the results of this trial, ELO in combination with LEN and DEX was granted regulatory approval in 2015 for MM patients who have received 1 to 3 lines of prior therapy.
The use of ELO/LEN/DEX has also been evaluated in a limited number of patients with renal impairment (n = 26), and the 10 mg/kg dose of ELO was considered safe for use in this population.26 Patients with varying degrees of renal function from normal to those requiring dialysis were evaluated and found to have similar pharmacokinetic and area under the curve data. Grade 3-4 adverse events (AEs) were similar for all groups, but grade 3-4 serious AEs had a trend toward being higher in patients requiring dialysis; a trend toward higher ORR in patients with better renal function appear similar to what was previously reported for LEN/DEX. ELO-based combinations are also actively being explored in the frontline and maintenance settings, as well as special subsets of MM patients including newly diagnosed high-risk MM (#NCT01668719) and high-risk smoldering MM (#NCT02279394).
DARA (anti-CD38)
CD38, a 45 kDa transmembrane glycoprotein, has receptor-like function in modulating adhesion and migration between circulating lymphocytes and endothelial cells through interactions with its ligand CD31. Moreover, its ecto-enzyme function has been well established through its cyclase and hydrolase activity that mobilizes intracellular calcium stores important for downstream signal transduction pathways. The functional importance of CD38 and its increased expression, particularly on malignant plasma cells, identified it as an attractive target for antibody-based therapeutic approaches in MM. Notably, it is also expressed on NK cells and at low levels on lymphoid, myeloid, and erythroid cells.27
DARA, an IgG1-κ anti-CD38 mAb, was the first mAb to gain regulatory approval for the treatment of MM in 2015. After screening a panel of 42 novel anti-CD38 mAbs, DARA was established as a lead candidate for further studies due to its high affinity for CD38 and its ability to induce CDC in vitro.28 Further preclinical studies confirmed other mechanisms of action including ADCC,28 ADCP,29 and apoptosis through FcγR-mediated crosslinking of bound antibody on the cell surface and CD38 ecto-enzyme inhibition,27 resulting in potent MM antitumor activity both in vitro and in vivo.
Based on these encouraging preclinical studies, a first-in-human phase 1/2 clinical study was conducted in RRMM patients with single-agent DARA.4 The maximum tolerated dose was not reached in the dose-escalation phase, and several dosing schedules of either 8 mg/kg or 16 mg/kg were evaluated in the phase 2 portion of the study. In patients receiving the 16 mg/kg dose, ORR was 36%, which was comparable to the 33% response rate observed in the subset of 27 (64%) patients that were dual refractory to both BOR and LEN. Estimated median PFS was 5.6 months, and 1-year survival was 77%. Although, ORR was higher in patients who had previously received ≤3 lines of therapy ORR 56%), 23% of patients with ≥4 prior therapies responded to DARA.
Given the promising results from this initial study, DARA was granted breakthrough designation by the FDA for patients receiving at least 3 lines of therapy including a PI and IMiD or who were dual refractory to both classes of drugs. The efficacy of single-agent DARA was confirmed in the phase 2 SIRIUS study where ORR was 29% and median PFS was 3.7 months, and median overall survival of 17.5 months for 106 patients who received a 16 mg/kg dosing of DARA.5 Among 87 (82%) patients refractory to both LEN and BOR, ORR was 26%, and a comparable ORR (20%) was seen in patients with high-risk cytogenetics. Based on these positive data, DARA was granted accelerated FDA approval in 2015 for patients indicated in its breakthrough designation.
The use of DARA is also being explored in combination with other approved MM agents including DEX with either LEN, pomalidomide (POM), or BOR, and such combinations have shown safety, tolerability, and encouraging efficacy in early phase clinical trials, prompting the launch of several larger registration-enabling studies. Interim results of a phase 3 randomized trial (CASTOR) of DARA/BOR/DEX vs BOR/DEX were recently reported in 498 RRMM patients with ≥1 line of prior therapy.14 With a median follow-up of 7.2 months, the addition of DARA significantly prolonged PFS at 1 year (60.7% vs 26.9%; HR, 0.39; P < .0001). ORR also favored the DARA/BOR/DEX arm (83% vs 63%; P < .0001) and was consistent across response categories (≥ very good partial response [VGPR] 59% vs 29%, ≥ complete response (CR) 19% vs 9%). Overall, DARA/BOR/DEX was well tolerated with no increased risk of cumulative toxicity compared with the BOR/DEX arm. Shortly thereafter, interim results from a phase 3 randomized study (POLLUX) of DARA/LEN/DEX vs LEN/DEX were reported in 569 RRMM patients receiving ≥1 line of therapy.30 After a median follow-up of 13.5 months, median PFS was superior in the DARA/LEN/DEX group (not reached vs 18.4 months; HR, 0.24; P < .0001). Moreover, response rates across all categories were significantly higher in the DARA/LEN/DEX arm than in the control arm (ORR, 93% vs 76%; ≥ VGPR, 76% vs 44%; CR, 43% vs 19%, respectively), perhaps in part due to the augmentation of DARA’s ADCC activity in the presence of LEN that has been observed in preclinical studies.31 In an on-going trial with multiple arms combining standard doses of DARA with various standard-of-care regimens, 77 RRMM patients with ≥2 lines of therapy, including at least 2 cycles of BOR and LEN, received 28-day cycles of DARA/POM/DEX at standard doses.15 Approximately two-thirds of patients had disease resistant to BOR, one-third to carfilzomib, and 88% to LEN; additionally, nearly two-thirds had disease refractory to a PI and an IMiD. With a short median follow-up of 72 days, 36% of patients had discontinued therapy, usually due to disease progression (20%) or AEs (8%). Among 53 patients evaluable for response, the ORR was 59% with 8% ≥ CR; similarly, ORR among 40 dual refractory patients was 58% (4% ≥ CR). Five patients have died, 4 from AEs and 1 from progressive disease.
Safety of ELO and DARA in myeloma
Infusion-related reactions (IRR) represent the most common AEs noted with mAbs. In the trial of ELO given as monotherapy, IRR occurred in 13 of the first 25 patients (chills [32.4%], pyrexia [17.6%], flushing [11.8%], and chest discomfort, headache, and tachycardia [8.8% each]), and nearly all were grade 1 and 2.12 Subsequently, premedication with antihistamines and acetaminophen was required; additionally, if not contraindicated, all patients in the 20 mg/kg cohort were given DEX 20 mg IV prior to the initial dose of ELO. Approximately 59% of patients (n = 20) experienced an IRR to the first infusion, and 10 patients had IRR during subsequent infusions. However, after implementation of antihistamine/acetaminophen/steroid premedication, no further grade 3 to 4 IRR occurred, and all other IRRs subsided within 24 hours. Subsequent combination therapy of ELO with either BOR/DEX or LEN/DEX was performed with premedication for the majority of patients, and the rate of IRR occurred in only 5% and 11% of patients, respectively, and were generally grade 1 to 2.13,25 Similarly, during the phase 2 dose-expansion trial of DARA with the clinically relevant DARA dose of 16 mg/kg, IRR occurred in 71% of patients, despite premedication with acetaminophen, antihistamine, and steroids.4 All but one IRR was grade 1 to 2, and the majority of reactions occurred during the first infusion, with only 7% experiencing IRR with subsequent infusions. The SIRIUS trial showed similar results at the same dose.5 IRR occurred in 40% and were usually grade 1 to 2, although 5% experienced grade 3 reactions; nasal congestion, throat irritation, cough, dyspnea, and chills were among the reactions most commonly observed. Only 6% of patients experienced IRR beyond cycle 1.
Unique to the combination of ELO with LEN/DEX was a 77% grade 3 to 4 lymphopenia, which was significantly higher than the control arm at 49%; the authors hypothesized that this may have been due to the immunologic effects of ELO on lymphocytes and NK cells.3 Rates of grade 3 to 4 infections were similar when drug exposure was considered and equalized, with the exception of varicella zoster, which the authors reasonably proposed may be related to opportunistic susceptibility attributable to lymphopenia, although other opportunistic infections were not reported.3
Special laboratory considerations for currently approved mAb therapy
ELO and DARA are IgG-κ mAbs, and subsequently may impact or interfere with laboratory testing that relies on detection or measurement of an antibody. This becomes particularly important for management of MM patients who often have significant anemia that necessitates an indirect Coombs test for antibody testing for transfusions, measurement of a mAb for response determination by electrophoresis and/or immunofixation (IFE) studies, and flow cytometry for detection of plasma cells by CD38 expression.32,33
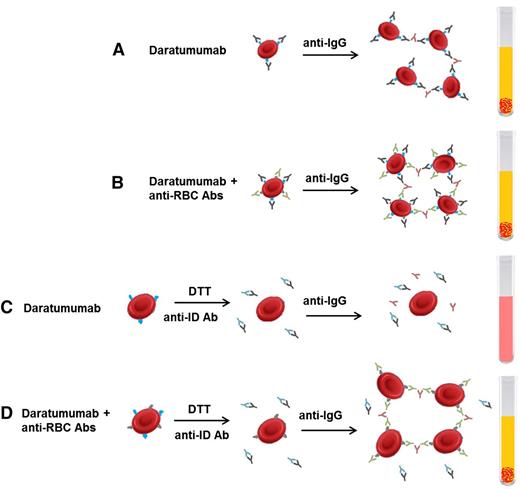
The challenges with blood typing are most pronounced in patients receiving anti-CD38 mAbs because CD38 is weakly expressed on erythrocytes.33 Although ABO/RhD blood typing is not typically affected, DARA interacts with routine testing for other antibodies because of binding to reagent erythrocytes with continued interference for up to 6 months after cessation of therapy (Figure 2).32,33 Both the indirect Coombs test and antibody screens may be falsely positive. Several strategies have since been developed to overcome the interference of blood compatibility testing in these patients. These have included adding a reducing agent dithiothreitol (DTT) to denature surface CD38 of reagent red blood cells (RBCs) and prevent their reactivity with DARA. However, DTT can also denature a limited number of other surface RBC antigens, among which the most clinically relevant and frequent is the Kell antigen, which may lead to the failed identification of anti-Kell alloantibodies. This can be addressed by providing Kell-negative blood units to these patients. Other techniques have involved adding an anti-idiotype antibody or adding excess recombinant soluble CD38 to neutralize DARA and prevent its binding to CD38 on reagent RBCs, although these assays are not widely available.32 Hence, current recommendations are to perform RBC phenotyping on patients receiving CD38-directed therapy prior to their first dose and provide phenotypically matched RBCs thereafter. For urgent situations, appropriate ABO/RhD compatible units (phenotypically compatible to the best ability) can be considered for transfusion or O RhD negative blood in emergent circumstances.
Interference with blood compatibility testing with DARA. (A) DARA binds to CD38 expressed on RBCs, leading to agglutination on indirect Coombs testing even in the absence of allo- or autoantibodies against RBC antigens. (B) In the presence of DARA and RBC antibodies, agglutination occurs. (C) Incubating reagent RBCs with DTT before adding patient serum will denature CD38 on RBC cells and prevent agglutination. Alternatively, the patient’s serum can be incubated with an anti-ID that neutralizes DARA and prevents its interaction with CD38. Either technique allows for the discrimination of clinically relevant RBC antibodies during pretransfusion antibody screening (D). Abs, antibodies; anti-ID, anti-idiotype Ab. Adapted from van de Donk et al32 with permission.
Interference with blood compatibility testing with DARA. (A) DARA binds to CD38 expressed on RBCs, leading to agglutination on indirect Coombs testing even in the absence of allo- or autoantibodies against RBC antigens. (B) In the presence of DARA and RBC antibodies, agglutination occurs. (C) Incubating reagent RBCs with DTT before adding patient serum will denature CD38 on RBC cells and prevent agglutination. Alternatively, the patient’s serum can be incubated with an anti-ID that neutralizes DARA and prevents its interaction with CD38. Either technique allows for the discrimination of clinically relevant RBC antibodies during pretransfusion antibody screening (D). Abs, antibodies; anti-ID, anti-idiotype Ab. Adapted from van de Donk et al32 with permission.
The use of mAbs in MM may interfere with response assessments using serum protein electrophoresis (SPEP) and IFE due to co-migration of the therapeutic antibody with the patient’s endogenous M protein, particularly for patients with a known IgG-κ M protein, because mAbs are most frequently IgG-κ proteins.32,34 Because clearance of the M protein on IFE is necessary to document a CR or stringent CR, accurate response assessments by conventional methods may not be feasible. For example, DARA, an IgG1-κ mAb, can reach peak plasma concentrations of 0.1 g/dL, and thus discriminating between DARA and a patient’s endogenous paraprotein may not be possible at levels <0.2 g/dL.32,34 Moreover, the appearance of a new low-level IgG-κ M protein in MM patients with non–IgG-κ isotypes receiving DARA should be interpreted cautiously. To address this issue, the DARA IFE reflex assay was developed in which patient serum samples are pre-incubated with an anti-idiotype antibody against DARA prior to electrophoresis and IFE, thus shifting the migration of DARA on the electrophoresis gel, allowing for discrimination between circulating serum DARA and disease-related M protein.34 Similar assays are in development for ELO, ISA, and MOR202.32 Additionally, DARA (and other anti–CD38-directed therapy) may interfere with the detection of cells on flow cytometry that is sorted by CD38 expression; therefore, other markers nearly ubiquitous on malignant plasma cells should be considered for samples from patients receiving anti-CD38 mAbs.32
Other mAbs demonstrating clinical efficacy in myeloma
ISA
ISA (SAR650984), a humanized IgG1-κ antibody against CD38, also demonstrated potent in vitro and in vivo activity in MM preclinical models through Fc-mediated CDC, ADCC, and ADCP, and enhanced NK cell-mediated ADCC in combination with LEN and POM. However, unlike DARA, it also possesses direct cytotoxic activity in the absence of crosslinking agents or immune effector cells, in part through homotypic aggregation-associated cell death through caspase- and lysosomal-dependent apoptotic pathways.35 Moreover, ISA induces more potent inhibition of CD38 enzymatic activity relative to other clinically relevant CD38 antibodies, which may play a role in its distinct mechanistic properties.36
In a phase 2 dose-finding trial of ISA in RRMM with ≥3 lines of therapy, ORR was 24% in 74 patients receiving dose levels ≥10 mg/kg and median duration of response at the time of data cutoff was 6.6 months.7 IRR were seen in 49% of patients, although were predominately grade 1 to 2 (grade ≥3, 6%). The combination of ISA/LEN/DEX has also been evaluated in a phase 1b dose-escalation trial in RRMM with ≥2 lines of therapy. Overall, the combination was well tolerated and demonstrated an ORR of 57% (33% VGPR) with a median duration of response of 7.6 months.16 In ongoing trials, ISA is also being tested in combination with carfilzomib (#NCT02332850) or POM and DEX (#NCT02283775) in the RR setting, and in combination with cyclophosphamide, BOR, and DEX (CyBorD) in newly diagnosed transplant-ineligible patients (NCT02513186).
MOR202
Early results of the safety and preliminary efficacy of MOR202, a humanized IgG1-λ mAb against CD38, was reported in a phase 1/2a study in RRMM; ORR was 27% (4/15 patients) with DEX, and 50% (5/10 patients) when used in combination with LEN/DEX or POM/DEX.6 Unlike DARA and ISA, IRR were rare (14%) when DEX was used as a premedication, which may be due to the absence of CDC with MOR202, which is suspected to be a major contributor of IRR.37
PD-1 targeted therapy
Checkpoint inhibitors to augment the host immune response against cancer cells are also being evaluated in MM with anti-PD1 mAbs furthest along in development. Increased expression of PD-L1 in malignant plasma cells and upregulation of PD-1 on effector T and NK cells of MM patients38 provided a rationale that targeting PD-1 may be an effective therapeutic approach in MM. Moreover, blockade of the PD-1/PD-L1 axis led to increased CD8+ cytotoxic T-lymphocyte activity and NK-mediated cell lysis in MM preclinical models.39
As a single agent, the anti-PD1 mAb nivolumab demonstrated no objective responses in RRMM.17 The observation that IMiDs increase effector immune cell activity provided rationale for a phase 1 study of the anti-PD1 antibody pembrolizumab (PEMBRO), in combination with LEN/DEX in RRMM with ≥2 lines of therapy; ORR was 50% in the response-evaluable cohort, including responses seen in IMiD- and dual-refractory MM, and median duration of response was 11.3 months.18 Similarly, a 50% ORR was noted in a phase 1 trial with PEMBRO/POM/DEX; 72% had (+1q) and 40% had del 17p, t(4:14), and/or t(14:16); and IRR occurred in 5 (21%) patients.19 Phase 3 trials of POM/DEX with or without PEMBRO in RRMM (#NCT02576977) and LEN/DEX with or without PEMBRO in newly diagnosed patients are ongoing (#NCT02579863). A phase 3 study evaluating ELO/LEN/DEX with or without nivolumab in RRMM is also underway (#NCT02726581).
Summary.
Currently approved mAbs for the treatment of MM are DARA and ELO. Practically, ELO is not effective alone, but demonstrates its highest activity in combination with IMiDs and DEX. It is approved for use in patients with 1 to 3 prior lines of therapy for MM. Data suggests ELO may be most effective in patients with fewer lines of prior therapy and in those with a diagnosis of myeloma ≥3.5 years. Subsequently, treatment with ELO/LEN/DEX may be of greatest benefit in patients relapsing after long remissions following induction therapy (with or without consolidation with myeloablative therapy with autologous stem cell transplant) and maintenance therapy. Although DARA is effective as monotherapy, and is approved in either patients with double-refractory disease to a PI and IMiD, or in patients who have had at least 3 lines of prior therapy (including a PI and IMiD), responses only occurred in ∼30% of patients treated with monotherapy. However, ORR substantially improves when DARA is combined with LEN/DEX or BOR/DEX. It therefore seems reasonable to consider DARA monotherapy for either patients with slowly relapsing/progressive disease or for those with poor tolerance to therapy with multiple agents, and to consider combination therapy for patients with either advanced/aggressive disease, those with high-risk disease determined by cytogenetics, or for patients progressing after monotherapy. To prevent IRR for currently approved mAbs, pretreatment with an antihistamine, acetaminophen, and a steroid is advisable. Varicella zoster prophylaxis should be initiated with both ELO and DARA. Prior to therapy with DARA, RBC allotyping and phenotyping should be performed, and the blood bank should be notified that the patient would be starting mAb therapy to help facilitate prompt availability of blood products after beginning therapy. The patient should be notified to let all providers know of mAb treatment prior to subsequent typing/transfusion. Similarly, the laboratory should be notified of treatment with mAbs for appropriate use of assays to counteract therapeutic mAb interference with paraprotein evaluation by electrophoresis and/or IFE, and clonal plasma cell evaluation by flow cytometry (Table 2).
Practical clinical guidelines when administering DARA and ELO
| DARA Pretreatment Infectious disease prophylaxis Pulmonary function testing RBC compatibility testing Premedications | Begin VZV prophylaxis 1 week prior to DARA administration and for 3 months posttreatment |
| Perform pulmonary function testing in patients with history of severe COPD or moderate-to-severe reactive airway disease. If FEV1 <50%, strong caution advised in administering DARA because clinical studies with DARA excluded such patients | |
| Notify local blood bank that patient will be receiving anti–CD38-directed therapy | |
| Perform RBC phenotyping (or genotyping prior if patient has received RBC transfusion within 3 months) prior to 1st dose of DARA | |
| Provide wallet card for patient to inform blood banks and physicians of potential interference with RBC compatibility testing due to anti–CD38-directed therapy | |
| IV corticosteroids (methylprednisolone 100 mg prior to doses 1 and 2, 60 mg for subsequent doses or equivalent) | |
| Oral antipyretic (acetaminophen 650 mg to 1000 mg or equivalent) | |
| Oral or IV H1 receptor antagonist (diphenhydramine 25 to 50 mg or equivalent) | |
| Strongly consider: | |
| Oral or IV H2 receptor antagonist (famotidine 20 mg or equivalent) | |
| Oral leukotriene receptor antagonist (montelukast 10 mg or equivalent) | |
| If FEV1 ≤80%, administer β2-adrenergic agonist inhaler | |
| For at-risk patients for IRR, consider admitting to hospital for first dose of DARA for careful monitoring | |
| Treatment IRR | Hold infusion of DARA until symptoms resolve; administer corticosteroids, antihistamines and bronchodilators as needed. Restart infusion at 1/2 rate of when IRR began and can increase as patient tolerates to maximum rate of 200 mL/min |
| Post-treatment Posttreatment prophylaxis Response assessments with SPEP and IFE Plasma cell quantification with flow cytometry ELO Pretreatment Infectious disease prophylaxis Premedications Treatment IRR Posttreatment Response assessments with SPEP and IFE | Oral corticosteroids for 2 days after each DARA infusion (DEX 4 mg or equivalent) |
| If FEV1 ≤80%, continue β2-adrenergic agonist inhaler | |
| If M protein <0.2 g/dL, use DARA IFE reflex assay to discriminate between disease-related M protein and drug-related M protein to assess for CR Use alternative cell surface marker other than CD38 as a marker for plasma cell identification or use CD38 antibody that binds to different epitope than DARA for up to 6 months after treatment is completed Begin VZV prophylaxis 1 week prior to ELO administration and for 3 months posttreatment Oral DEX 28 mg 3 to 24 hours prior to each ELO dose IV DEX 8 mg 45 to 90 minutes prior to each ELO dose Oral antipyretic (acetaminophen 650 mg to 1000 mg or equivalent) Oral or IV H1 receptor antagonist (diphenhydramine 25 to 50 mg or equivalent) Oral or IV H2 receptor antagonist (ranitidine 150 mg PO or 50 mg IV or equivalent) Hold infusion of ELO until symptoms resolve to grade 1 or lower; administer corticosteroids, antihistamines, and bronchodilators as needed. Restart infusion at 0.5 mL/min and gradually increase by 0.5 mL/min every 30 minutes until target rate of 2 mL/min Assessment of CR with SPEP and IFE can be impaired due to co-migration of ELO band with disease-related M protein. Commercial anti-ELO antibodies for SPEP and IFE assays are in development to discriminate between ELO and endogenous M protein | |
| DARA Pretreatment Infectious disease prophylaxis Pulmonary function testing RBC compatibility testing Premedications | Begin VZV prophylaxis 1 week prior to DARA administration and for 3 months posttreatment |
| Perform pulmonary function testing in patients with history of severe COPD or moderate-to-severe reactive airway disease. If FEV1 <50%, strong caution advised in administering DARA because clinical studies with DARA excluded such patients | |
| Notify local blood bank that patient will be receiving anti–CD38-directed therapy | |
| Perform RBC phenotyping (or genotyping prior if patient has received RBC transfusion within 3 months) prior to 1st dose of DARA | |
| Provide wallet card for patient to inform blood banks and physicians of potential interference with RBC compatibility testing due to anti–CD38-directed therapy | |
| IV corticosteroids (methylprednisolone 100 mg prior to doses 1 and 2, 60 mg for subsequent doses or equivalent) | |
| Oral antipyretic (acetaminophen 650 mg to 1000 mg or equivalent) | |
| Oral or IV H1 receptor antagonist (diphenhydramine 25 to 50 mg or equivalent) | |
| Strongly consider: | |
| Oral or IV H2 receptor antagonist (famotidine 20 mg or equivalent) | |
| Oral leukotriene receptor antagonist (montelukast 10 mg or equivalent) | |
| If FEV1 ≤80%, administer β2-adrenergic agonist inhaler | |
| For at-risk patients for IRR, consider admitting to hospital for first dose of DARA for careful monitoring | |
| Treatment IRR | Hold infusion of DARA until symptoms resolve; administer corticosteroids, antihistamines and bronchodilators as needed. Restart infusion at 1/2 rate of when IRR began and can increase as patient tolerates to maximum rate of 200 mL/min |
| Post-treatment Posttreatment prophylaxis Response assessments with SPEP and IFE Plasma cell quantification with flow cytometry ELO Pretreatment Infectious disease prophylaxis Premedications Treatment IRR Posttreatment Response assessments with SPEP and IFE | Oral corticosteroids for 2 days after each DARA infusion (DEX 4 mg or equivalent) |
| If FEV1 ≤80%, continue β2-adrenergic agonist inhaler | |
| If M protein <0.2 g/dL, use DARA IFE reflex assay to discriminate between disease-related M protein and drug-related M protein to assess for CR Use alternative cell surface marker other than CD38 as a marker for plasma cell identification or use CD38 antibody that binds to different epitope than DARA for up to 6 months after treatment is completed Begin VZV prophylaxis 1 week prior to ELO administration and for 3 months posttreatment Oral DEX 28 mg 3 to 24 hours prior to each ELO dose IV DEX 8 mg 45 to 90 minutes prior to each ELO dose Oral antipyretic (acetaminophen 650 mg to 1000 mg or equivalent) Oral or IV H1 receptor antagonist (diphenhydramine 25 to 50 mg or equivalent) Oral or IV H2 receptor antagonist (ranitidine 150 mg PO or 50 mg IV or equivalent) Hold infusion of ELO until symptoms resolve to grade 1 or lower; administer corticosteroids, antihistamines, and bronchodilators as needed. Restart infusion at 0.5 mL/min and gradually increase by 0.5 mL/min every 30 minutes until target rate of 2 mL/min Assessment of CR with SPEP and IFE can be impaired due to co-migration of ELO band with disease-related M protein. Commercial anti-ELO antibodies for SPEP and IFE assays are in development to discriminate between ELO and endogenous M protein | |
COPD, chronic obstructive pulmonary disease; VZV, varicella-zoster virus.
Results from ongoing and future trials with mAbs will help further clarify their optimal use in rationale combinations with other novel agents, and their potential expanded role for frontline and maintenance therapy of symptomatic disease as well as for the treatment of smoldering MM. Future directions include identifying biomarkers that may predict response to therapy and exploring mechanisms of innate and acquired resistance, some of which may involve varying levels of expression of target antigens on tumor cells40 or the development of anti-drug antibodies over time with treatment. Moreover, new mAb targets beyond CD38 and SLAMF7 are being actively investigated in preclinical and clinical studies. The era of mAb therapy in MM is just beginning and represents one significant step in building upon the improvement in MM patient outcomes over the last decade.
Correspondence
Donna M. Weber, Department of Lymphoma/Myeloma, The University of Texas MD Anderson Cancer Center, 1515 Holcombe Blvd, Unit 429, Houston, TX 77030; e-mail: dmweber@mdanderson.org.
References
Competing Interests
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Author notes
Off-label drug use: None disclosed.