Abstract
Although there have been many definitions for high-risk (HR) myeloma, most recent consensus for classifying risk in patients with newly diagnosed multiple myeloma (NMM) comes from the International Myeloma Working Group. This recently published revised International Staging System includes del(17p) or t(4;14) by fluorescence in situ hybridization, β-2 microglobulin, albumin, and lactate dehydrogenase. These elements should be captured in all NMM patients. The optimal treatments for HR myeloma have not been fully worked out; therefore, these patients should be considered for clinical trials. Outside of the trial setting for those patients who are not eligible for autologous stem cell transplantation (ASCT), a regimen with bortezomib, but not thalidomide, should be considered, with a duration of therapy of at least 1 year. The regimen with the best results to date is bortezomib, melphalan, and predisone. A nonthalidomide maintenance could also be considered. In patients who are eligible for ASCT, an induction regimen with bortezomib and an immunomodulatory drug should be administered for 3 to 6 months followed by 2 ASCTs. Finally, a consolidation/maintenance regimen containing at least 1 year of bortezomib should be administered followed by maintenance thereafter. For patient convenience, an oral agent that is not thalidomide could be prescribed as maintenance. Finally, in patients with HR myeloma, allogeneic SCT may be associated with reasonable outcomes, but this too will require further research.
Learning Objectives
Identify HR NMM patients
Be familiar with best practices for patients with HR NMM
Introduction
The advances in the treatment of multiple myeloma (MM) in the past 2 decades have been unprecedented, with survival rates nearly tripling during this time period. For those patients with standard risk fluorescence in situ hybridization (SR-FISH) who undergo early autologous stem cell transplantation (ASCT), median overall survival (OS) approaches 10 years.1 For those with high-risk FISH (HR-FISH), outcomes have also improved, but to a lesser degree. Herein, we will discuss the evolving definitions of HR newly diagnosed MM (NMM), outcomes for these subsets of patients with HR disease, and finally a recommended strategy to treat these patients.
Defining HR
Two challenges for understanding best management strategies for HR NMM is that the term “high-risk or HR” can refer to many different characteristics or combinations thereof (Table 1), and the magnitude of risk can be influenced by different treatments. Of late, more attention has been applied to host-dependent risk factors, most notably advanced age, but also performance status, other comorbidities, and frailty scores.2 The International Staging System (ISS), which was published in 2005, captured some of these host-dependent factors by incorporating low albumin and high β-2 microglobulin (B2M), the latter of which reflects renal dysfunction as well as tumor burden.3 At the end of the 20th century and the early 21st century, HR disease has most commonly referred to a complement of genetic risk factors.4,5 However, there are other tumor/tumor microenvironment factors, which include proliferation indices, lactate dehydrogenase, extramedullary disease, and numbers of circulating plasma cells with or without primary plasma cell leukemia. Finally, the last broad category of HR relates to characteristics not identified at diagnosis, but after institution of therapy: the lack of response to therapy and early relapse. Deeper responses more often than not portend better long-term outcomes,6 but in today’s parlance the lack of complete response (CR) is not considered “HR disease.” In contrast, relapse within 1 year after unmaintained ASCT would be considered HR.7 With increased use of maintenance, the best cut-point for early relapse in a maintained setting is less well defined, but 3 years may be the correct time point based on data from the University of Arkansas, where the approach of intensive therapy with prolonged maintenance has been the rule.8 These authors found that the lack of a sustained CR at 3 years portended a worse outcome than never having achieved a CR. Further confirmation of these findings will be required.
Risk factors in NMM
| . | Host . | Genetics . | Other . |
|---|---|---|---|
| Risk factors | Advanced age | Hypodiploidy | High S phase |
| Higher PS | Deletion (17p) | Circulating PCs | |
| Increased comorbidities | t(4;14), t(14;16), and t(14;20) | Reduced polyclonal BMPCs | |
| Deletion (1p) | High serum FLC | ||
| Addition (1q) Deletion (13q) by metaphase cytogenetics HR GEP signatures | Other extramedullary disease Early relapse |
| . | Host . | Genetics . | Other . |
|---|---|---|---|
| Risk factors | Advanced age | Hypodiploidy | High S phase |
| Higher PS | Deletion (17p) | Circulating PCs | |
| Increased comorbidities | t(4;14), t(14;16), and t(14;20) | Reduced polyclonal BMPCs | |
| Deletion (1p) | High serum FLC | ||
| Addition (1q) Deletion (13q) by metaphase cytogenetics HR GEP signatures | Other extramedullary disease Early relapse |
BMPCs, bone marrow plasma cells; FLC, free light chain; GEP, gene expression signature; PCs, plasma cells; PS, performance status.
Systems for defining genetic HR myeloma
Historically, cytogenetic risk had been defined by metaphase cytogenetics. Abnormal metaphase cytogenetics was associated with poor OS, but only 20% to 30% of patients with NMM have abnormal metaphase cytogenetics. In contrast, interphase FISH has the potential to reveal abnormalities in any patient’s myeloma cells, regardless of their proliferative rate. Because FISH is dependent on the probes used to interrogate the plasma cells, FISH panels have expanded over the years from a bare panel of del(13/13q) (RB1/LAMP1), del(17p)13 (TP53), break apart probes for chromosome 14q32 (immunoglobulin H [IgH]) with probes looking for the partners 11q13 (CCND1), 4p16 (FGFR3), and 16q32 (MAF). Over time, probes have been added to identify trisomies, most commonly +3, +7, +9, and +15, additions of 1q (CKS1B), deletions of 1p, IgH translocation partners 6p21 (CCND3) and 20q12 (MAFB), and 8q24.1 (MYC) rearrangements. There is considerable variability in how FISH is performed and interpreted. It is imperative that FISH be done on either CD138-sorted cells or with cytoplasmic Ig staining.
Del(17p)13 and t(4:14) have most consistently been considered HR abnormalities, but even with these two abnormalities, there is heterogeneity. Patients with t(4;14) and hemoglobin >10 g/dL and a B2M <4 mg/L do much better than expected.9 In addition, the coexistence of trisomies has been deemed to attenuate the risk associated with these high-risk abnormalities in 1 study,10 but not another.11 Recent data have questioned whether t(11;14) is associated with more risk than previously believed.1 Del(13q) by FISH was also initially thought to be associated with increased risk of death, but further work has demonstrated that in isolation, it only marginally increases risk. The higher the proportion of myeloma cells that harbor del(17p), the worse the outcome. The French are proponents for a 60% cutoff,12 but the Spanish myeloma group have demonstrated that 20% of myeloma cells with del(17p) is also associated with adverse outcome. The Medical Research Council (MRC) IX myeloma trial has also demonstrated that the more HR-FISH features a patient has, the worse the prognosis.13
FISH incorporated into prognostic systems.
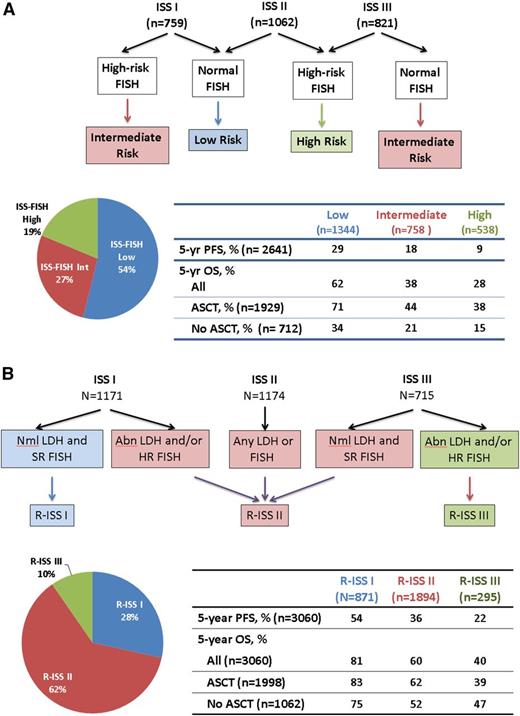
Myeloma FISH are increasingly available in laboratories throughout the world, and they are an important part of the initial work-up of a patient with NMM. A multinational effort at combining ISS and HR-FISH was reported by the International Myeloma Working Group (IMWG) (Figure 1A).3,12,14 In short, 2642 NMM patients who were treated in the era before lenalidomide and bortezomib available for induction were divided by ISS and by HR-FISH, generating low, intermediate, and HR populations with desperate progression-free survival (PFS) and OS rates. The respective 4-year PFS rates were 39%, 20%, and 11%, and the respective 4-year OS rates were 71%, 45%, and 33%.
Novel risk assignment schemes. (A) Risk assignment based on FISH and ISS. ISS 1 includes patients with albumin ≥3.5 g/dL and B2M <3.5 mg/L; ISS III includes patients with B2M >5.5 mg/L; and ISS II includes any patient who is not ISS I or III. HR-FISH was defined as del(17p) and/or t(4;14). Data are derived from an analysis of 2642 patients with FISH from the original cohort of 10 750 patients.3,12 No patients received lenalidomide or bortezomib as induction. (B) The R-ISS. HR-FISH was del(17p) and/or t(4;14). Data are generated from 3060 patients from 11 international trials conducted from 2005 to 2012 that were pooled and analyzed by the IMWG.14 Abl, abnormal; Int, intermediate; Nml, normal.
Novel risk assignment schemes. (A) Risk assignment based on FISH and ISS. ISS 1 includes patients with albumin ≥3.5 g/dL and B2M <3.5 mg/L; ISS III includes patients with B2M >5.5 mg/L; and ISS II includes any patient who is not ISS I or III. HR-FISH was defined as del(17p) and/or t(4;14). Data are derived from an analysis of 2642 patients with FISH from the original cohort of 10 750 patients.3,12 No patients received lenalidomide or bortezomib as induction. (B) The R-ISS. HR-FISH was del(17p) and/or t(4;14). Data are generated from 3060 patients from 11 international trials conducted from 2005 to 2012 that were pooled and analyzed by the IMWG.14 Abl, abnormal; Int, intermediate; Nml, normal.
The IMWG has done further analysis in a more modern cohort of NMM patients (3060 patients from 11 international trials conducted from 2005 through 2012) and has proffered a “revised ISS” (R-ISS), which incorporates the original ISS (B2M and albumin), myeloma FISH (t[4;14], t[14;16], or del[17p]), and lactate dehydrogenase (Figure 1B).14 Using this system, nearly two-thirds of patients are R-ISS II, 28% are R-ISS I, and only 10% are R-ISS III. Five-year OS rates are 81%, 60%, and 40% for the R-ISS I, II, and III, respectively.
Gene expression profiling.
There are 7 gene expression-based prognostic signatures, which are powerful, but not completely overlapping, and often not as accessible in routine practice.4
Therapy for patients with HR-FISH
In the days when the only treatments included alkylators, anthracyclines, and corticosteroids, patients with hypodiploid disease, t(4;14), and/or del(17p) had PFS and 5-year OS that were approximately half that of their SR counterparts. A summary of outcomes normalized to 3-year outcomes are shown in Tables 2 and 3.6,15-32 Response rates are not shown because these end points are less interesting in HR-FISH defined patients, who often achieve comparable response rates, although perhaps lower rates of CR and stringent CR but do not sustain their remissions.
Outcomes based on FISH risk among patients not eligible for ASCT
| Reference (trial) . | FISH . | Regimen . | HR-FISH/ SR-FISH (N) . | 3-y PFS, % . | 3-y OS, % . | ||
|---|---|---|---|---|---|---|---|
| HR-FISH . | SR-FISH . | HR-FISH . | SR-FISH . | ||||
| 20 (E9486) | Del(17p) | VBMCP | 37/308 | 17* | 30* | 32 | 68 |
| t(4;14) | VBMCP | 42/290 | 17* | 31* | 24 | 64 | |
| 18 (MRC IX non-intensive) | HR† | Melphalan/prednisone | 90/125 | N/A | 10 | 26 | 48 |
| CTDa | 96/129 | N/A | 20 | 58 | 78 | ||
| 15 (MRC IX maintenance) | HR† | Placebo maintenance (after all 4 inductions) | 98/129 | 18 | 30 | 69 | 72 |
| Thalidomide maintenance (after all 4 inductions) | 99/125 | 11 | 47 | 45 | 76 | ||
| 21 (FIRST trial) | HR‡ | Rd continuous | 43/205 | 3 | 45 | 41 | 77 |
| Rd 18-mo | 52/209 | 10 | 20 | 40 | 71 | ||
| MPT | 47/206 | 3 | 25 | 47 | 65 | ||
| 22 (Mayo Clinic) | HR§ | RD | 16/84 | 18 | 36 | 77 | 86 |
| 23 (E4A03) | HR§ | RD/Rd | 21/105 | 24 | 46 | 63 | 88 |
| 19 (VISTA) | HR‡ | VMP | 28/140 | N/A | N/A | 56 | 72 |
| 16,17 (GEM2005>65) | HR‡ | VMP→VT or VP | 44/187 | 18* | N/A | 51* | N/A |
| 24* | 33* | 55 | 77 | ||||
| HR‡ | VTP→VT or VP | 20* | N/A | 29* | N/A | ||
| Reference (trial) . | FISH . | Regimen . | HR-FISH/ SR-FISH (N) . | 3-y PFS, % . | 3-y OS, % . | ||
|---|---|---|---|---|---|---|---|
| HR-FISH . | SR-FISH . | HR-FISH . | SR-FISH . | ||||
| 20 (E9486) | Del(17p) | VBMCP | 37/308 | 17* | 30* | 32 | 68 |
| t(4;14) | VBMCP | 42/290 | 17* | 31* | 24 | 64 | |
| 18 (MRC IX non-intensive) | HR† | Melphalan/prednisone | 90/125 | N/A | 10 | 26 | 48 |
| CTDa | 96/129 | N/A | 20 | 58 | 78 | ||
| 15 (MRC IX maintenance) | HR† | Placebo maintenance (after all 4 inductions) | 98/129 | 18 | 30 | 69 | 72 |
| Thalidomide maintenance (after all 4 inductions) | 99/125 | 11 | 47 | 45 | 76 | ||
| 21 (FIRST trial) | HR‡ | Rd continuous | 43/205 | 3 | 45 | 41 | 77 |
| Rd 18-mo | 52/209 | 10 | 20 | 40 | 71 | ||
| MPT | 47/206 | 3 | 25 | 47 | 65 | ||
| 22 (Mayo Clinic) | HR§ | RD | 16/84 | 18 | 36 | 77 | 86 |
| 23 (E4A03) | HR§ | RD/Rd | 21/105 | 24 | 46 | 63 | 88 |
| 19 (VISTA) | HR‡ | VMP | 28/140 | N/A | N/A | 56 | 72 |
| 16,17 (GEM2005>65) | HR‡ | VMP→VT or VP | 44/187 | 18* | N/A | 51* | N/A |
| 24* | 33* | 55 | 77 | ||||
| HR‡ | VTP→VT or VP | 20* | N/A | 29* | N/A | ||
OS and PFS are estimated from Kaplan-Meier survival curves when necessary in an effort to normalize results to 3-year outcomes.
CTDa, attenuated cyclophosphamide, thalidomide, and dexamethasone regimen; MPT, melphalan, prednisone, and thaldiomide; N/A, not applicable; RD, lenalisomide plus dexamethasone; VBMCP, vincristine-carmustine (BCNU)-melphalan-cyclophosphamide-prednisone; VMP, bortezomib, melphalan, and prednisone; VP, bortezomib and prednisone; VT, bortezomib and thalidomide; VTP, bortezomib, thalidomide, and prednisone.
Median PFS, in months.
HR: Any of the following: gain(1q), t(4;14), t(14;20), t(14;16), and del(17p13).
HR: Any of the following: t(4;14), t(14;16), or del(17p13).
HR: Any of the following: hypodiploidy, monoallelic loss of chromosome 13 or its long-arm by metaphase cytogenetics only, deletion 17p13, t(4;14), or t(14;16), or plasma cell labeling index ≥3%.
Outcomes based on FISH risk among patients eligible for ASCT
| Reference (trial) . | FISH . | Regimen . | HR-FISH/ SR-FISH (N) . | 3-y PFS, % . | 3-y OS, % . | ||
|---|---|---|---|---|---|---|---|
| HR-FISH . | SR-FISH . | HR-FISH . | SR-FISH . | ||||
| 24 (GEM05MENOS65) | HR* | VBMCP/VBAD → Bz × 2 → ASCT × 1 → 3 y maintenance | 18/111 | 39 | 50 | 48 | 84 |
| TD → ASCT × 1 + 3 y maintenance† | 17/110 | 24 | 43 | 56 | 85 | ||
| VTD →ASCT × 1+ 3 y maintenance† | 18/112 | 47 | 54 | 60 | 88 | ||
| 25 (MRC IX intensive) | HR‡ | CVAD → ASCT × 1 | 141/166 | 18§ | 32§ | 58 | 81 |
| CTD → ASCT × 1 | 152/167 | 20§ | 34§ | 59 | 82 | ||
| 29 (RV-MM-EMN-441) | HR* | Rd → CRD → Len ± prednisone | 30/57 | 17 | 51 | 67 | 92 |
| Rd → ASCT × 2 → Len ± prednisone | 23/70 | 43 | 59 | 78 | 88 | ||
| 30 (IFM-99) | t(4;14) | VAD → ASCT × 2 | 100/616 | 23 | 50 | 58 | 80 |
| 27 (IFM 2005-01) | t(4;14) | VAD → ASCT × 1 or 2 ± Len maintenance | 98/414 | 17 | N/A | 32‖ | 72‖ |
| VD → ASCT × 1 or 2 ± Len maintenance | 106/401 | 36 | N/A | 63 | 85 | ||
| 26 (GIMEMA MM-BO2005) | t(4;14) | TD → ASCT × 2 → TD → Dex | 57/181 | 37 | 63 | N/A | N/A |
| VTD → ASCT × 2 → VTD → Dex | 53/183 | 69 | 74 | N/A | N/A | ||
| 28 (HOVON65/GMMG-HD4) | t(4;14) | VAD → ASCT × 1 or 2 → Thal maintenance | 35/227 | 20 | 40 | 40 | 73 |
| PAD → ASCT × 1 or 2 → Bz maintenance | 35/215 | 28 | 48 | 60 | 79 | ||
| 31, 32 (IFM-2005-02) | t(4;14) | No Len maintenance post-ASCT | N/A | 15‖ | N/A | N/A | N/A |
| Len maintenance post-ASCT | 26/229 | 27¶ | 42¶ | N/A | N/A | ||
| 27 (IFM 2005-01) | Del(17p) | VAD → ASCT × 1 or 2 ± Len maintenance | 119/393 | 21 | N/A | 41 | N/A |
| VD→ ASCT × 1 or 2 ± Len maintenance | 54/427 | 21 | N/A | 68 | 82 | ||
| 30 (IFM-99) | Del(17p) | VAD → ASCT × 2 | 58/474 | 25 | 49 | 50 | 78 |
| 28 (HOVON65/GMMG-HD4) | Del(17p) | VAD → ASCT × 1 or 2 → Thal maintenance | 40/273 | 14 | 41 | 17 | 80 |
| PAD → ASCT × 1 or 2 → Bz maintenance | 25/264 | 32 | 48 | 66 | 80 | ||
| 31, 32 (IFM-2005-02) | Del(17p) | Len maintenance post-ASCT | 30/235 | 29¶ | 42¶ | N/A | N/A |
| No Len maintenance post-ASCT | N/A | 14¶ | N/A | N/A | N/A | ||
| Reference (trial) . | FISH . | Regimen . | HR-FISH/ SR-FISH (N) . | 3-y PFS, % . | 3-y OS, % . | ||
|---|---|---|---|---|---|---|---|
| HR-FISH . | SR-FISH . | HR-FISH . | SR-FISH . | ||||
| 24 (GEM05MENOS65) | HR* | VBMCP/VBAD → Bz × 2 → ASCT × 1 → 3 y maintenance | 18/111 | 39 | 50 | 48 | 84 |
| TD → ASCT × 1 + 3 y maintenance† | 17/110 | 24 | 43 | 56 | 85 | ||
| VTD →ASCT × 1+ 3 y maintenance† | 18/112 | 47 | 54 | 60 | 88 | ||
| 25 (MRC IX intensive) | HR‡ | CVAD → ASCT × 1 | 141/166 | 18§ | 32§ | 58 | 81 |
| CTD → ASCT × 1 | 152/167 | 20§ | 34§ | 59 | 82 | ||
| 29 (RV-MM-EMN-441) | HR* | Rd → CRD → Len ± prednisone | 30/57 | 17 | 51 | 67 | 92 |
| Rd → ASCT × 2 → Len ± prednisone | 23/70 | 43 | 59 | 78 | 88 | ||
| 30 (IFM-99) | t(4;14) | VAD → ASCT × 2 | 100/616 | 23 | 50 | 58 | 80 |
| 27 (IFM 2005-01) | t(4;14) | VAD → ASCT × 1 or 2 ± Len maintenance | 98/414 | 17 | N/A | 32‖ | 72‖ |
| VD → ASCT × 1 or 2 ± Len maintenance | 106/401 | 36 | N/A | 63 | 85 | ||
| 26 (GIMEMA MM-BO2005) | t(4;14) | TD → ASCT × 2 → TD → Dex | 57/181 | 37 | 63 | N/A | N/A |
| VTD → ASCT × 2 → VTD → Dex | 53/183 | 69 | 74 | N/A | N/A | ||
| 28 (HOVON65/GMMG-HD4) | t(4;14) | VAD → ASCT × 1 or 2 → Thal maintenance | 35/227 | 20 | 40 | 40 | 73 |
| PAD → ASCT × 1 or 2 → Bz maintenance | 35/215 | 28 | 48 | 60 | 79 | ||
| 31, 32 (IFM-2005-02) | t(4;14) | No Len maintenance post-ASCT | N/A | 15‖ | N/A | N/A | N/A |
| Len maintenance post-ASCT | 26/229 | 27¶ | 42¶ | N/A | N/A | ||
| 27 (IFM 2005-01) | Del(17p) | VAD → ASCT × 1 or 2 ± Len maintenance | 119/393 | 21 | N/A | 41 | N/A |
| VD→ ASCT × 1 or 2 ± Len maintenance | 54/427 | 21 | N/A | 68 | 82 | ||
| 30 (IFM-99) | Del(17p) | VAD → ASCT × 2 | 58/474 | 25 | 49 | 50 | 78 |
| 28 (HOVON65/GMMG-HD4) | Del(17p) | VAD → ASCT × 1 or 2 → Thal maintenance | 40/273 | 14 | 41 | 17 | 80 |
| PAD → ASCT × 1 or 2 → Bz maintenance | 25/264 | 32 | 48 | 66 | 80 | ||
| 31, 32 (IFM-2005-02) | Del(17p) | Len maintenance post-ASCT | 30/235 | 29¶ | 42¶ | N/A | N/A |
| No Len maintenance post-ASCT | N/A | 14¶ | N/A | N/A | N/A | ||
OS and PFS are estimated when necessary from Kaplan-Meier survival curves in an effort to normalize results to 3-y outcomes.
Bz, bortezomib; CRD, carfilzomib-revlimid-dexamethasone; Dex, dexamethasone; Len, lenalidomide; PAD, bortezomib-doxorubicin-dexamethasone; TD, thalidomide-dexamethasone; Thal, thalidomide; VAD, vincristine-adriamycin-dexamethasone; VBAD, vincristine-BCNU-doxorubicin-dexamethasone; VD, bortezomib-dexamethasone; VTD, velcade-thalidomide-dexamethasone.
HR: Any of the following: t(4;14), t(14;16), or del(17p13).
Secondary randomization to 1 of 3 maintenance regimens: interferon, thalidomide, or bortezomib + thalidomide.
HR: Any of the following: gain(1q), t(4;14), t(14;20), t(14;16), del(17p13), and del (1p32).
Median PFS, in months.
4-year OS from maintenance randomization, %.
Median PFS from maintenance randomization, month.
There are 2 ways the question of differential impact of therapy in patients with HR disease can be posed. The first is whether a given therapy can make the HR patients do as well as the SR patients. The second is whether there is a “less than expected” difference in outcomes among HR patients than their SR counterparts with a given therapy. So far, no therapy has definitively been proved to remove all of the risk associated with HR-FISH.
How to treat SR disease
Although this is not the subject of this review, most experts would agree that for the elderly and/or those who are not candidates for ASCT who have SR NMM, the 2 leading regimens are: lenalidomide and dexamethasone; and bortezomib, melphalan (or cyclophosphamide), and dexamethasone (Figure 2).33 The lenalidomide and dexamethasone is typically given until toxicity or progression. The alkylator-containing regimen is typically given for 1 year; the role maintenance plays in this population is less clear given the need to balance toxicity, age, and frailty. The doublet lenalidomide and dexamethasone is also a reasonable option in the very elderly.34
Treatment algorithm for NMM. Modified from mSMART algorithm.33 Bor, bortezomib; Car, carfilzomib; KRd, carfilzomib, lenalidomide, and dexamethasone; Rd, lenalidomide and dexamethasone; VRd, bortezomib, lenalidomide, and dexamethasone.
Treatment algorithm for NMM. Modified from mSMART algorithm.33 Bor, bortezomib; Car, carfilzomib; KRd, carfilzomib, lenalidomide, and dexamethasone; Rd, lenalidomide and dexamethasone; VRd, bortezomib, lenalidomide, and dexamethasone.
For patients who are candidates for ASCT and who have SR NMM, induction should include a triplet, most commonly a proteasome inhibitor (PI), an IMiD (although substitution with an alkylator can be considered), and a corticosteroid. After 3 to 6 months of induction, stem cells should be collected for at least 2 ASCTs, and patients should move to 1 or 2 ASCTs. Those patients who do not achieve a very good partial response (VGPR) or better after induction and their first ASCT could be considered for either a second ASCT or for consolidation and/or maintenance (see McCarthy et al, in this book35 ). For those in VGPR or better after 1 ASCT, the data are less clear, although many experts would recommend maintenance in this setting as well.
Treating patients with HR-FISH NMM who are not candidates for ASCT
Studies have not demonstrated normalization of outcomes with regimens incorporating thalidomide (Table 2).15-18 In the MRC IX trial, patients with HR-FISH randomized to thalidomide maintenance had worse PFS and OS than those without maintenance.15 In the GIEMEMA-MM-03-05 trial, which randomized patients to VMP vs bortezomib, melphalan, prednisone, and thalidomide followed by VT maintenance, there was no difference in PFS between the treatment arms among patients with HR-FISH in contrast to the improved outcome observed in SR-FISH patients treated with bortezomib, melphalan, prednisone, and thalidomide-VT.36 Therefore, thalidomide is not typically recommended in this nontransplant, HR-FISH NMM population.
In contrast, bortezomib appeared to normalize outcomes among patients with t(4;14) in the VISTA trial, which included 1 year of bortezomib, melphalan, and prednisone.19 In contrast, in the VMP vs bortezomib, thalidomide, prednisone trial (GEM2005 >65 trial), which randomized patients to 36 weeks of induction with standard-intensity bortezomib followed by up to 3 years of maintenance using 1 standard cycle of bortezomib (days 1, 4, 8, and 11) every 3 months in association with either daily thalidomide or alternate day prednisone, patients with deletion 17p or t(4;14) had worse outcomes than the SR-FISH counterparts. It is notable, however, that the 44 patients with HR-FISH had a 3-year OS of 55%,17 which is better than what has been seen in alkylator-only studies in the past.20 The authors speculated that the difference in PFS seen in this study as compared with the VISTA study results may be due to the limited duration of dose-intensive bortezomib (36 weeks rather than 54 weeks). Alternatively, the lack of risk reduction might relate to the fact that thalidomide was part of the maintenance in half of the patients.
Lenalidomide induction as a doublet does not normalize the risk imposed by HR-FISH.21-23 It is noteworthy that although patients treated with either RD or Rd in a Mayo Clinic trial and E4A03 trial who had HR-FISH did not do as well as their SR-FISH counterparts, they still had 3-year OS rates of 77% and 63%.22,23 These data cannot be generalized to the nontransplant population because a fraction of these patients received ASCT. Finally, preliminary data on the FISH characteristics of patients treated on the FIRST trial do not suggest reduction of risk of death or progression among patients treated with continuous lenalidomide21 ; moreover, the HR-FISH group is the only subpopulation in which Rd does not outperform 1 year of melphalan, prednisone, and thalidomide induction. Further study will be required to determine whether there may be a negative interaction between long-term use of the IMiD class of drugs and HR-FISH. More information will be forthcoming from the S0777 (VRd vs Rd; #NCT01530594) and Clarion (VMP vs CarMP; #NCT01818752) trials.
To date, there is little data on the role of maintenance lenalidomide in the nontransplant HR-FISH NMM population. In a pooled analysis of 827 patients treated in 3 randomized trials evaluating the role of maintenance therapy,37 586 had FISH data available; of these 23% were HR-FISH. Two of the trials were for nontransplant candidates; the other included one arm assigned to tandem ASCT. A little over half of the patients receiving maintenance were scheduled to receive lenalidomide until progression, whereas the other half randomized to maintenance were scheduled to receive VT for 24 months. The effect of “continuous therapy” in this HR-FISH population was a hazard ratio of 0.56 (95% confidence interval [CI], 0.38-0.84) for PFS and a hazard ratio of 0.73 (95% CI, 0.55-0.96) for OS. Extrapolating this data to non-ASCT NMM patients with HR-FISH is difficult because there were as many as 20% to 30% of patients who received tandem ASCT, and separating the lenalidomide and bortezomib effects is also not possible. More information will be forthcoming from the ARUMM study (VMP followed by lenalidomide vs placebo; #NCT02112175).
In patients who are not eligible for ASCT, there are limited data to direct best therapy for patients with HR-FISH. A regimen with bortezomib should be considered, with a duration of therapy of at least a year. The regimen with the best results so far is VMP. Although data are still lacking, the combination of lenalidomide, dexamethasone, and bortezomib (or carfilzomib or ixazomib) is appealing given unprecedented rates of response, PFS, and OS for all comers with NMM.
Treating patients with HR-FISH NMM who are candidates for ASCT
Isolating the effect of induction, ASCT, and consolidation/maintenance in patients who undergo early ASCT is a challenge, based on how trials are reported and given the difficulty of breaking an already small subset of patients (HR-FISH) into further subgroups, but the following section is an attempt to highlight these distinctions where possible (Table 3).
Induction pre-ASCT.
There is no data suggesting that thalidomide induction normalizes outcomes among patients with HR-FISH.24-26 Although bortezomib does not completely abrogate the risk of HR-FISH, bortezomib induction is associated with an increased odds ratio (2.44 [95% CI, 1.72-3.46]) of achieving CR,38 and some of the best PFS and OS rates have been shown among HR-FISH patients who received bortezomib-containing regimens as induction.24,26,27,28 With bortezomib in induction prior to ASCT, patients with HR-FISH have 3-year PFS and 3-year OS of 21% to 47% and 48% to 68%, respectively; without bortezomib in induction, 3-year PFS and 3-year OS ranged from 14% to 37% and 17% to 58%, respectively.
The IFM 2005-1 trial randomized 482 patients to either 4 cycles of VAD or VD39 followed by dexamethasone-cyclophosphamide-etoposide-cisplatin for 2 cycles vs no dexamethasone-cyclophosphamide-etoposide-cisplatin. Patients were to have a single ASCT, and those without a VGPR or better were to receive a second ASCT. An undefined number of patients received maintenance lenalidomide as part of a different trial of lenalidomide vs placebo (IFM 2005-2 trial). Patients treated with VD who had t(4;14), had 3-year PFS and 4-year OS rates that were nearly twice as good as their VAD counterparts. The difference in 4-year OS between patients harboring t(4;14) vs those without t(4;14) was less striking among the VD-treated patients (63% vs 85%) than among the VAD-treated patients (32% vs 72%). For patients with del(17p), the 3-year PFS was not different regardless of treatment arm (median, 17 months), but there was a trend for better 3-year OS for those receiving VD induction (68% vs 41%).
The Spanish Myeloma Group conducted a trial to compare VTD vs TD vs vincristine-BCNU-melphalan-cyclophosphamide-prednisone alternating with VBMCP/VBAD/B in patients with NMM aged 65 years or younger who were destined for ASCT.24 After ASCT, patients had a second randomization to 1 of 3 maintenance arms: interferon-α, thalidomide, or bortezomib and thalidomide. A total of 386 patients were allocated. The median PFS by induction arm among patients with HR-FISH were 23.5 months for VTD, 8.9 months for TD, and 18 months for VBMCP/VBAD/B. OS was significantly worse regardless of arm among patients with HR-FISH. No information is provided about outcomes by FISH risk and maintenance arm.
In the GMMG-MM-5 trial, patients were randomized to either PAD induction or bortezomib, cyclophosphamide, and dexamethasone induction.40 Patients were then submitted to either 1 or 2 ASCTs and 2 cycles of lenalidomide. There was a secondary randomization to either lenalidomide continuously or until CR. Little information based on FISH risk has been provided, but it was shown that rates of progression were lower in the bortezomib, cyclophosphamide, and dexamethasone arm than in the PAD arm.
Autologous SCT in patients with HR-FISH.
There is a strong suggestion that patients with HR-FISH fare better with ASCT than without,12,29 and that in certain instances, tandem ASCT may be preferred to single ASCT in patients with HR-FISH.28 The argument for ASCT in HR-FISH patients is complex, because in the era before lenalidomide and bortezomib-based induction and before incorporation of maintenance in HR-FISH patients, PFS duration post a single ASCT was ∼9 months and approximately double that for a tandem ASCT30 ; however, despite these short PFS statistics, there was already a trend in better OS in HR-FISH patients with the more routine use of ASCT.12 If one considers instances with novel induction and maintenance, the PFS and OS are still not typically comparable to what is seen in SR-FISH patients, but they are better than what is seen without ASCT.
An example is the compilation of the 2642 patients reported by Avet-Loiseau et al on behalf of the IMWG12 ; those NMM patients with HR-FISH who received ASCT had 5-year OS that was more than twice that of their no-ASCT counterparts (Figure 1A). This was not corrected for age and comorbidity, but it was corrected for ISS, that is B2M and albumin; patients receiving 1 or tandem ASCT were included. In contrast, using the R-ISS (Figure 1B), outcomes of R-ISS III NMM patients who received ASCT were not superior to those who did not, although no direct comparisons were made in the manuscript.14 This may be in part due to the fact that many of the HR-FISH patients are included in R-ISS II.
Another example supporting ASCT in HR-FISH patients is that of Gay et al. Patients with NMM were all given lenalidomide and dexamethasone induction, and then in a 1:1:1:1 strategy randomized to 1 of 2 consolidations and 1 of 2 maintenance strategies.29 Consolidation was with cyclophosphamide, lenalidomide, and dexamethasone for 6 months or ASCT (1 or 2, and physician’s choice if VGPR after first ASCT). Maintenance was with either lenalidomide or lenalidomide and prednisone. Most of the data pertaining to FISH risk is in their supplemental material, and many of the formal statistical comparisons are lacking, but the data are still quite suggestive. With a median follow-up of 52 months, the HR-FISH group randomized to ASCT enjoyed better overall PFS and OS than did the patients randomized to cyclophosphamide, lenalidomide, and dexamethasone. Moreover, the relative risk for progression (and death) between HR-FISH and SR-FISH patients was lower in the ASCT arm than the no-ASCT arm (Table 3). The differences in the SR-FISH group were less striking.
Consider tandem transplant for patients with del(17p) or t(4;14).
Some of the best PFS and OS statistics for patients carrying del(17p) have come from studies that include tandem transplant.26,30,39 This observation could be artifactual because it includes pooling information from relatively small numbers of patients from multiple trials across time and centers using different methods and cutoffs for calling deletion 17p, but the data are quite suggestive.
Cavo et al reported on a pooled analysis of 4 randomized controlled trials that incorporated bortezomib and transplant: IFM2005-01, HOVON 65/GMMG-HD4, GIMEMA MM-BO2005, and PETHEMA GEM05MENOS65.24,26,27,28,41 Only those patients randomized to a bortezomib-containing regimen were included, and only those patients who were prospectively assigned to receive either a single or double transplant were included, leaving 908 patients for the analysis. Twenty percent of patients had del(17p) and/or t(4;14). Those patients with del(17p) and/or t(4;14), who did not achieve CR after bortezomib-based induction, had significantly better PFS (median = 42 months vs 21 months) and OS (4-year OS = 76% vs 33%) if they had a preplanned tandem ASCT rather than a preplanned single ASCT.
Digging deeper into the studies comprising this pooled analysis, the HOVON-65/GMMG HD4 trial was a study in which practices differed between centers in regards to whether a single ASCT or tandem ASCT was standard practice. Among the patients with HR-FISH, the magnitude of the reduction in risk for death among patients receiving tandem ASCT was comparable to that of being on the experimental bortezomib arm.42 Along the same lines, in the GIMEMA MM-BO2005 trial, patients with t(4;14), those patients receiving VTD induction and consolidation along with planned tandem ASCT had comparable PFS as those without the abnormality.26 This was not the case for patients receiving TD induction and consolidation, and tandem ASCT.
Finally, the RV-MM-PI-209 trial, which was a 2 × 2 design that randomized patients to either melphalan, prednisone, and lenalidomide or tandem ASCT, and to maintenance with either lenalidomide or placebo all after 4 cycles of induction with lenalidomide and dexamethasone.43 Patients with HR-FISH randomized to the tandem ASCT had risk reduction for PFS (0.30 [0.16, 0.58]) and for OS (0.49 [0.20, 1.18]).
Consolidation/maintenance in patients with HR-FISH.
As mentioned in the section on patients not eligible for ASCT, thalidomide maintenance appears to be worse than no maintenance in patients with HR-FISH. This observation was most striking in the MRC-IX trial.15 Additional support comes from the HOVON65/GMMG-HD4 study, which randomized patients to VAD induction followed by ASCT followed by thalidomide maintenance (conventional arm) or PAD induction followed by ASCT followed by bortezomib 1.3 mg/m2 once every 2 weeks maintenance for 2 years (experimental arm).28 Among patients with del(17p), those treated on the conventional arm not only had PFS and OS that were inferior to the experimental arm, but their 3-year PFS of 14% and 3-year OS of 17% would appear to be inferior than comparable ASCT studies in which there was no thalidomide maintenance.27,30
In contrast, consolidation and/or maintenance with bortezomib in patients with HR-FISH abrogates risk. The strongest support also comes from the HOVON-65/GMMG-HD4 trial.28,44 Details from outcomes of the entire trial are shown in Table 3, which demonstrates that long-term bortezomib does not completely cancel out the risk introduced by the presence of HR-FISH, but it does abrogate it. In an update of the subset of 345 patients treated on this trial who received tandem ASCT,44 those receiving the bortezomib-containing regimen with and without t(4;14) had a 5-year PFS of 16% and 27%, and a 5-year OS of 52% and 75%; in contrast, the respective numbers for patients treated on the standard arm (VAD + ASCT + thalidomide maintenance) were 5-year PFS of 8% and 24%, and 5-year OS of 33% and 64%. In a similar vein, those patients receiving tandem ASCT who did and did not have del(17p) who were treated with bortezomib had a 5-year PFS of 22% and 27%, and a 5-year OS of 65% and 72%44 ; in contrast, patients with del(17p) treated on the standard arm had a 5-year OS of 18%. The risk of del(17p) was not completely abrogated, but aside from total therapy 3 (TT3),45 such excellent PFS has not been described in patients with del(17p). Clinical trial #NCT02181413, which randomizes patients post-ASCT to maintenance with ixazomib or placebo will also be very informative, and a potentially convenient oral option to parenteral bortezomib.
The GIMEMA MM-BO2005 trial also supports the value of bortezomib induction and consolidation among patients with t(4;14). These investigators randomly allocated 480 patients to either 3 21-day cycles of VTD or TD.26 After tandem ASCT, patients received 2 35-day cycles of their assigned drug regimen, VTD or TD, as consolidation therapy. All patients were subsequently maintained on dexamethasone 40 mg days 1 to 4 every 28 days. In the VTD group, 3-year PFS was comparable between those patients with and without t(4:14): 69% vs 74%; this was not true for the TD-treated patients who had respective 3-year PFS of 37% vs 63%.
Additional support comes from Straka et al, who have reported in abstract form their pooled results from 2 phase 3 studies of bortezomib consolidation vs observation post-ASCT.46 In the 278 patients who were assessed for FISH, 37% were HR; their definition included del(13q), t(4;14), and/or del(17p), and there was a trend toward better PFS among patients with HR-FISH if they received bortezomib consolidation (0.66 [0.41, 1.05]).
Yet another piece of supporting evidence that long-term use of bortezomib may help overcome the adverse impact of t(4;14) is a cross-trial comparison performed by the Arkansas group.45 Among patients with the MS molecular subgroup, which translates to FISH t(4;14), PFS increased significantly among patients treated on TT3 as compared with TT2, the former of which contained bortezomib. The 5-year PFS for the 2 arms of TT3 (TT3a included VTD in induction, consolidation, and maintenance, and TT3b contained VRd in induction, consolidation, and maintenance) were 64% and 52%, respectively. These figures contrast sharply with the 5-year PFS figures of TT2 (TT2 without thalidomide as part of induction, consolidation, and maintenance vs TT2 with thalidomide, which incorporated thalidomide throughout the regimen), which were 12% and 35%, respectively. Similar advances were made in this subgroup in terms of 5-year OS: TT3a and TT3b, 81% and 74%, respectively; and TT2 without thalidomide and TT2 with thalidomide, 40% and 54%, respectively. How the use of long-term thalidomide fits plays against other reports suggesting that long-term thalidomide may be detrimental in HR-FISH patients is unclear, especially given how different the TT approach is from many other treatment trials.
Also of note is a retrospective analysis of 19 patients with deletion 17p who were given continuous VRd after a single ASCT.47 The 3-year PFS duration was ∼45% and the 3-year OS was ∼94%. These patients were highly selected because in order to be included in this series, they had to make it through induction and ASCT.
Very little can be said about the role lenalidomide maintenance plays in ASCT patients with HR-FISH.31,32 Retrospective data would not suggest that there is harm, as there may be with thalidomide maintenance in this population.1,47 In the RV-MM-PI-209 trial, which was a 2 × 2 design that randomized patients to either melphalan, prednisone, and lenalidomide or tandem ASCT and to maintenance with either lenalidomide or placebo, the risk reduction introduced by maintenance lenalidomide for PFS (0.73 [0.37, 1.42]) and for OS (0.79 [0.31, 2.01]) was not dramatic or significant,43 although this could be in part attributable to the small sample size.
Some conclusions may also be gleaned by the retrospective analysis of 409 patients treated between 2003 and 2012 at the Mayo Clinic. Patients were treated with a risk-adapted approach. Maintenance or consolidation was used in 46% of patients with t(4:14) and in 68% of those with del(17p) and was most often lenalidomide or bortezomib based. What was notable was that there was no significant difference in PFS between the full cohort and either the t(4;14) or del(17p) group for patients with ISS I or II disease, although there was a difference in OS for those patients with ISS III disease and either t(4;14) or del(17p).1 How much these effects are due to lenalidomide vs bortezomib maintenance was not disclosed.
We await results from multiple trials (IFM 2008-01/DFCI 2009/#NCT01191060, BMT CTN 0702/#NCT01109004, and #NCT01208662) to better inform the role lenalidomide-based consolidation and/or maintenance will play in patients with HR-FISH.
In patients who are eligible for ASCT, an induction regimen with bortezomib and an IMiD should be administered for 3 to 6 months. This should be followed by a tandem ASCT. Finally, a regimen containing at least 1 year of bortezomib should be given as consolidation. Maintenance thereafter is worth serious consideration; for patient convenience, an oral agent that is not thalidomide could be prescribed. Regardless, these patients should be directed to clinical trials to unveil best strategies.
Other IMiDs, PIs, and antibodies in myeloma patients with HR-FISH
It is too soon to say what role other combinations, eg, those including antibodies such as elotuzumab or daratumumab and other IMiDs and PIs, may play. For example, the combination of carfilzomib (20-36 mg/m2), lenalidomide, and dexamethasone induction has been studied in 53 patients.48 The regimen is comprised of 8-standard cycles, followed by an additional 16 cycles with a modified carfilzomib schedule, followed by single-agent lenalidomide after cycle 24. Although the specific outcomes have not been described for patients with unfavorable cytogenetics, one-third of patients had unfavorable cytogenetics. The regimen is highly active, with CRs in 64%, stringent CR in 53%, and a 2-year PFS and OS of 93% and 98%, respectively. More information will arrive from the Eastern Cooperative Oncology Group-ACRIN (#NCT01863550) trial, which randomizes 756 patients with NMM to carfilzomib-lenalidomide-dexamethasone or bortezomib-lenalidomide-dexamethasone for 12 cycles. In addition, there are ongoing trials evaluating the addition of daratumumab to lenalidomide and dexamethasone (#NCT02541383) and to bortezomib, melphalan, and prednisone (#NCT02252172), and other trials evaluating the addition of elotuzumab to lenalidomide and dexamethasone (#NCT01335399 and #NCT01891643).
Allogeneic stem cell transplant (allo-SCT) in patients with HR-FISH
There are limited data on the role allo-SCT plays among patients with HR disease.49-51 Kröger et al studied 73 patients with NMM who received an ASCT followed by a planned allo-SCT.49 Sixteen patients had HR cytogenetics, defined by positive FISH for del(17p13) and/or t(4;14). After a median follow-up of 6 years, overall 5-year PFS was 29%, with no significant difference between del(17p)13/t(4;14)-harboring patients and others (24% vs 30%; P = .70). The 2 other studies addressing this question are too heterogeneous to draw any conclusions, although in a French cohort, there was no difference observed between the HR-FISH and SR-FISH patients in terms of 3-year PFS (30% vs 17%) or OS (45% vs 39%).50 In a German report, there was a trend toward inferior event-free survival and OS for patients with del(17p), but no difference in either for patients with t(4;14).51
How to manage HR myeloma: summary
An approach to dealing with patients with HR NMM is shown in Figure 2. Aside from clinical trials, our approach has been to use the most highly active regimens for patients with HR disease, followed by tandem ASCT as consolidation for those who are young and healthy enough, followed by consolidation/maintenance with a PI. The hypothesis is that these patients are those who are at most at risk for clonal evolution and lack of durable response to successive therapies. Not dissimilar from patients with other aggressive, highly proliferative hematologic malignancies, patients with genetically defined HR myeloma appear to need prolonged intensive therapy. Some may consider allo-SCT in younger patients with HR cytogenetics, understanding that there are not solid data to support this recommendation. Justification for this approach is that historically HR patients have done so poorly that the risk of treatment-related morbidity and mortality introduced by allo-SCT may be outweighed by the risk of death due to MM using standard approaches. The counter-argument, however, is that with newer classes of drugs including antibodies, histone deacetylase inhibitors, kinesin spindle protein inhibitors, and cyclin-dependent kinase inhibitors, the prognosis of these patients may be transformed.
Correspondence
Angela Dispenzieri, Division of Hematology, Department of Internal Medicine, Mayo Clinic, Mayo Medical School, 200 First St SW, Rochester, MN 55905; e-mail: dispenzieri.angela@mayo.edu.
References
Competing Interests
Conflict-of-interest disclosure: A.D. is on the Board of Directors or an advisory committee for GlaxoSmithKline, Takeda, and Prothena, and has received research funding from Takeda, Janssen, Pfizer, Alnylam, and Celgene.
Author notes
Off-label drug use: None disclosed.