Abstract
The majority of myelodysplastic syndrome (MDS) patients belong to the International Prognostic Scoring System (IPSS) and IPSS-revised (IPSS-R) lower-risk categories. Their precise diagnostics and prognostic stratification is often a challenge, but may ensure the optimization of therapy. The availability of diverse treatment options has significantly improved the quality of life and survival of this group of patients. Anemia is the most relevant cytopenia in terms of frequency and symptoms in lower-risk MDS, and may be treated successfully with erythropoietic stimulating agents, provided a careful selection is performed on the basis of IPSS-R, endogenous erythropoietin levels, and transfusion independence. Doses and duration of therapy of erythropoietic-stimulating agents (ESAs) are critical to determine efficacy. In case a patient fails ESA treatment, the available options may include lenalidomide (approved for del5q positive cases), hypomethylating agents, and a rather large number of experimental agents, whose clinical trials should be offered to a larger number of MDS patients. The choice for second-line treatment must take into account biologic, cytogenetic, and molecular-identified characteristics of individual patients, as well as frailty and comorbidities. Other cytopenias are less frequently presenting as isolated. Specific therapy for thrombocytopenia has been proposed in experimental clinical trials with thrombomimetic agents that have shown good efficacy, but raised some safety concern. Although neutropenia is targeted symptomatically with growth factor supportive care, the immunosuppressive treatments are indicated mainly for pancytopenic, hypoplastic lower-risk MDS; they are not widely used because of their toxicity, despite the fact that they may induce responses. Finally, hematopoietic stem cell transplant is the curative option also for lower-risk MDS and timing should be carefully evaluated, balancing toxicity and the possibility of survival advantage. Finally, even when considered suitable for lower-risk MDS, transplant application is limited to the rarer fit and younger MDS patient.
Learning Objectives
To appreciate the importance of correct diagnosis and risk stratification in MDS for therapeutic decision-making
To optimize standard therapies for lower-risk MDS in terms of choice, schedule and timing of drugs, and evaluation of response
To get to know the availability and characteristics of experimental drugs for patients who failed standard treatment
From nearly 2 decades ago, hematologists have considered myelodysplastic syndromes (MDSs) on the basis of their prognostic risk category, calculated according to the International Prognostic Scoring System (IPSS)1 and more recently, according to the revised form of it, the IPSS-revised (IPSS-R).2 Classically, this stratification of risk allows to distinguish 2 broad categories of MDS: lower risk and higher risk, and therapeutic options are based first on these, then adapted according to individual characteristics like comorbidities, age, and eligibility for transplant. In practice, both peer discussions and the informative conversation with patients and caregivers on goals of therapy stem from such risk evaluation, which must at present remain the essential step before treatment decision-making. Nevertheless, as times goes by and MDS outcome measures are more refined, it is clear that there are some “shadow zones,” and that more frequently than predicted, clinical behaviors differ from what is expected on the basis of calculated prognostic indexes. These discrepancies are often due to the intrinsic difficulty in formulating a precise diagnosis in this group of diseases.3 Diagnosis of MDS is a demanding exercise: the presence of dysplastic features in the marrow accompanied by peripheral cytopenias are not necessarily indicative of MDS, and even in the presence of an incontestable MDS form, a superficial evaluation can lead to misleading conclusions in terms of risk,3-5 especially for lower-risk ones. The presence of somatic mutations cannot be a secure attribution of MDS, because clonal hematopoiesis can be detected in idiopathic cytopenias of undetermined significance and in clonal cytopenias of undetermined significance.6 Moreover, for prognosis, factors not included in scoring systems may influence MDS outcome beyond the IPSS-calculated risk. These variables are not all yet ready to be evaluated routinely, but their roles and weight in determining progression or stability of the disease as well as response to therapies are the subject of active current investigation. Such variables include:
When the physiopathological and prognostic importance of the above-mentioned variables will be completely clarified, they could be used as an additional tool to diversify treatment, especially in lower-risk MDS.
Is IPSS lower-risk MDS always really low risk?
A precise diagnosis and prognostication of MDS is in fact the first step toward a successful treatment. It is of extreme importance to identify those lower-risk MDS patients who would benefit from an earlier treatment.
IPSS-R scoring was recently compared with the MD Anderson Lower-risk Prognostic System,11 and was shown to have lower discriminatory power in determining prediction of overall survival (OS).11,12 A substantial number of patients defined as low- or intermediate 1 (INT-1)-risk according to IPSS, could be reclassified as IPSS-R intermediate or more, and Lower-risk Prognostic System category 3, and therefore opt for a more aggressive therapeutic approach.12 Thus, in perspective, more efforts are required to integrate weighted clinical variables (eg, the extremely important degree of cytopenias) to which biological characteristics, such as somatic mutations should be added.
Lower-risk patients harboring mutations of TP53, EZH2, ASXL1, CBL, and U2AF1 will fare worse than predicted by disease risk.7,13 On the contrary, SF3B1 mutations that are frequent in ring sideroblasts (RARS) and RARS associated with marked thrombocytosis, and myelodysplastic/myeloproliferative neoplasms subtypes are associated with longer OS than that calculated by the IPSS-R.13,14 It is clear that although sharing the same IPSS-R score, the patients carrying different mutations would require a different treatment approach.
The majority of patients with MDS have an IPSS lower-risk disease: in the Italian National MDS registry (FISMonlus),15 among a total of 4300 MDS cases, 20% have IPSS-R very low, 41% low, and 19% intermediate. Treatment of these diseases, which was once mainly based on resolution of symptoms due to cytopenias, can now have more ambitious goals.
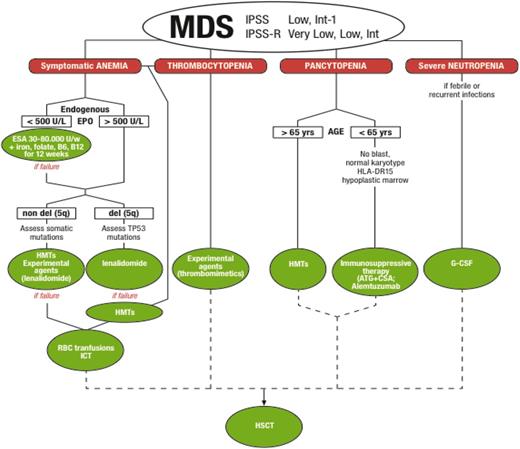
In terms of treatment options, the therapeutic algorithm for lower-risk MDS is more articulated than that for higher-risk ones, with an array of drugs that require selection of patients and a specific sequence in the choice of agents (Figure 1). The most recent guidelines indicate quite clearly how to proceed (National Comprehensive Cancer Network).16
Treatment algorithm for IPSS lower-risk MDS. ATG, antithymocyte globulins; CSA, cyclosporine; ESAs, erythropoietic-stimulating agents; G-CSF, granulocyte colony-stimulating factor; HMTs, hypomethylating agents; HSCT, hematopoietic stem cell transplant; ICT, iron chelation therapy.
Treatment algorithm for IPSS lower-risk MDS. ATG, antithymocyte globulins; CSA, cyclosporine; ESAs, erythropoietic-stimulating agents; G-CSF, granulocyte colony-stimulating factor; HMTs, hypomethylating agents; HSCT, hematopoietic stem cell transplant; ICT, iron chelation therapy.
For simplicity, treatment of single cytopenias will be addressed.
Treatment of anemia
Anemia is the most common cytopenia in MDS, present in 89% of the cases15 and is the major determinant of symptoms in low and INT-1 IPSS, and low and very low IPSS-R MDS. When symptomatic, anemia should be promptly treated, avoiding transfusions and improving quality of life (QoL).17-19 With a median age of 71 to 74 years for MDS patients in Western countries,15 it is evident that chronically low levels of hemoglobin (Hb) can severely impact already frail subjects, causing not only the worsening of cardiac function but also increasing falls and inducing cognitive impairment. Therefore, anemia of MDS constitutes an important social and economic burden. The first choice of treatment should be ESAs, and although used for decades, are still not yet managed optimally. At the time of writing this manuscript, no ESA has been approved for treatment of MDS by health authorities. Two separate randomized registrative trials comparing the safety and efficacy of erythropoietin α (EPO) and darbepoetin with placebo (PBO) have concluded and preliminary results confirm the well-known activity20 of both agents; erythroid response seems to be lower than the one present in literature but definitive results are not yet available.21,22
In practice, anemic lower-risk MDS patients should start treatment with fixed doses, rather than weight-adjusted doses of ESAs. The optimal treatment doses are 30 000 to 80 000 U of EPO23 and 150 to 300 μg of darbepoetin subcutaneous (SC) injection per week.24 Provided endogenous EPO is measured and is below 500 U/L (see section to follow), treatment with ESAs should start in a timely manner, before transfusion-dependence is established, because the probability to respond is higher for early treated, transfusion-independent patients.25 Response to ESAs is generally observed within 12 weeks17 and should not be evaluated before then to avoid missing some cases that show later increases of Hb. Together with optimal doses and periods of treatment, it is important to administer ESAs regularly and without interruptions in order to maintain stable levels of Hb, whose target values are 12 g/dL, traditionally derived from recommendations regarding renal-insufficient and solid tumor patients. When a positive response is achieved, ESA doses can be tapered to reach the lowest effective dose to maintain Hb. As such, a high percentage of lower-risk MDS patients will respond to ESA treatment, with the highest rates of response (as much as 70%) being obtained, provided selection criteria are followed.17 Endogenous EPO levels should be <500 U/L as per the Nordic score26 (better if <200 U/L), transfusion requirement should be absent or limited (<2 U/month), cytogenetics normal, marrow blasts absent, and of course IPSS should be low or INT-1.17,20 Recent evidence indicated that in IPSS lower-risk MDS patients selected with the above-mentioned criteria, application of IPSS-R risk score can refine prediction of response: very low-risk patients responded to ESA therapy in 85% of the cases, compared with 68%, 48%, and 31% of low-, intermediate-, and high-risk scores, respectively.27 Other determinant of response was low serum ferritin.
Recurrent individual somatic mutations (SF3B1, TET2, ASXL1, and DNMT3A) evaluated in a selected group of anemic lower-risk MDS had no impact on response to ESAs, nor had the size of the mutated clones,28 whereas the presence of >2 mutations was confirmed to be predictive of shorter survival.13,28
During treatment with ESAs, MDS lower-risk patients did not experience an increase of thrombotic events compared with nontreated patients,29 quite different to what was observed for other hematologic neoplasias. It is therefore not necessary to establish any antithrombotic therapy, unless in the presence of thrombophilia. Response duration to ESAs ranges from 20 to 24 months,30 and response is present but shorter for lower-risk MDS cases with del(5q).31 The addition of G-CSF to ESAs may contribute to increased response, especially in RARS,32 but randomized prospective studies to sustain this synergy are scarce.
At failure/relapse to ESAs, bone marrow aspiration must be performed to exclude disease progression, as well as evaluation of iron balance, and of vitamin B12 and folate levels. In fact, quite often, a state of functional iron deficiency (both for low-serum iron with/without low-serum ferritin) and acquired vitamin deficiency is the direct cause of loss of response to ESAs. For MDS cases with no sign of progression, the subsequent therapeutic choice is driven by the specific karyotype abnormality.
MDS lower risk with del(5q)
Lenalidomide is approved for the treatment of MDS patients with transfusion-dependent anemia due to low-/INT-1–risk MDS del(5q) with or without additional cytogenetic abnormalities, but in Europe its use is restricted to IPSS low-/INT-1 MDS with an isolated del(5q) when other therapeutic options (eg, EPO and G-CSF) are insufficient or inadequate. Actually, there is evidence that the drug is active in MDS del(5q) patients before transfusion dependence33 and the Spanish SINTRA-REV study (#NCT01243476) will prospectively assess whether lenalidomide delays the onset of transfusion dependence in MDS del(5q) patients. For now, based on the evidence of efficacy and safety obtained in several studies,34,35 lenalidomide is used at the starting dose of 10 mg per day for 21 days on a 28-day cycle. The 10-mg dose is more effective than the 5-mg dose in inducing transfusion independence (61% vs 49%),34 and cytogenetic response,36 with a similar safety profile. Achievement of durable transfusion independence with lenalidomide was associated with a significantly reduced risk of acute myeloid leukemia (AML) progression (45%; P = .022) and death (51%; P = .008).34 Very few lower-risk MDS del(5q) patients are not eligible for lenalidomide treatment, ie, those with severe renal insufficiency and those who have severe cytopenias in addition to anemia. In any case, dose adjustment and use of G-CSF may allow therapy,37 during which renal and thyroid function control is mandatory. A clinical trial of the combination of lenalidomide and eltrombopag in thrombocytopenic del(5q) patients is ongoing (www.clinicaltrials.gov; #NCT01772420). Onset of response to lenalidomide occurs for half of the patients at the first cycle.34 Karyotype complexity has a negative impact both on OS and AML evolution of lenalidomide-treated patients.35 A negative impact on OS is also due to TP53 mutations, which are more frequent in isolated del5q (19%) and complex karyotype with −5/5q- (72%).38 Despite these observations, TP53 mutations are not at present sufficient to exclude lower-risk MDS del(5q) patients from treatment with lenalidomide after loss of response to ESAs. In fact, their probability to respond to lenalidomide is not negligible, and TP53 mutations were associated with poor cytogenetic response but had no effect on achievement of transfusion independence after lenalidomide.39 Keep in mind nonetheless that a more aggressive therapy will be needed for TP53-mutated cases, although, even after transplant, TP53 mutations are independently associated with shorter OS.40 The assessment of TP53 mutations should therefore be implemented in clinical practice to plan ahead treatment strategies. Generally, lenalidomide treatment is maintained until loss of response, but indeed it would be interesting to investigate the possibility of interruption, although at present there are very few reports.41 In case of loss of response/failure to lenalidomide, available treatment options for del(5q) lower-risk MDS are very limited and experimental, with the exclusion of azacitidine, which is accessible to lower-risk MDS patients outside Europe. Studies are ongoing to clarify the mechanisms of action and of resistance to lenalidomide in lower-risk MDS with del(5q) and will possibly indicate the use of specific alternative drugs.42 A post-authorization study is ongoing worldwide to clarify whether therapy with lenalidomide of lower-risk MDS del(5q) patients is associated with an increase of AML progression or secondary tumors.
MDS lower risk without del5q
Although frequent and rather long lasting, as mentioned earlier, responses to ESAs are bound to be lost over time. Lower-risk MDS patients without del5q, who fail or relapse after ESAs have scarce therapeutic options, often requiring chronic transfusions, and have quite a poor QoL.43 In fact, HMTs are approved in the United States and other countries in this setting, but there are no approved treatments in the European Union. There is thus a substantial number of lower-risk MDS patients who could be candidates for supportive therapy or experimental drugs.
HMTs, azacitidine, and decitabine have shown efficacy in lower-risk MDS patients both as first-line and second-line therapy.44,45 Both drugs are active in inducing transfusion independence and hematologic improvement in up to 60% of treated patients. There are no final data on their impact on OS in lower-risk MDS, nor on the duration of response and treatment. Published studies have employed standard as well as “adapted” doses and schedules of HMTs with apparently similar results in terms of hematologic improvement.44-46 Employing HMTs after ESAs is however recommended, and possibly in the presence of thrombocytopenia, to balance safety and efficacy.16 Azacitidine 75 mg/m2 SC for 7 days every 28 days and decitabine 20 mg/m2 IV for 5 days every 28 days have been replaced by “low-dose” HMTs in a recent study by the MDS Clinical Research Consortium. IPSS low- or INT-1–risk MDS, chronic myelomonocytic leukemia, or MDS/myeloproliferative neoplasms were treated with standard daily doses of azacitidine or decitabine for 3 days every 28 days for a median of 9 cycles. With a median follow-up of 13 months, median OS has not been reached, and the response rate was 61%.46 Despite these interesting results, other studies indicate a limited efficacy of HMTs in lower-risk MDS,47 but apparently activity is more pronounced for SF3B1-mutated cases.48 An international randomized phase 3 clinical trial with oral azacitidine (cc-486) is ongoing for lower-risk MDS patients who are red blood cell (RBC) transfusion dependent, and concomitant thrombocytopenia (www.clinicaltrials.gov; #NCT01566695).
Lenalidomide has been used in non-del5q lower-risk MDS patients ineligible for or refractory to ESAs and its efficacy at 10 mg per day vs PBO was recently evaluated in transfusion-dependent lower-risk MDS patients.49 Transfusion independence was obtained in 26.9% of the cases. A subgroup of patients with endogenous EPO levels <100 U/L who had received previous ESA treatment, reached transfusion independence in 42.5% of the cases.49 These 2 simple variables, ie, previous therapies and low endogenous EPO, could be used to select patients more prone to take advantage of lenalidomide after ESA failure, whereas investigation on molecular characteristics to better identify responsive subjects are ongoing. At present, no somatic mutation predicts for response.49 Quite interestingly, the combination of lenalidomide and ESAs has also been recently shown to have additive effects in restoring erythropoiesis when ESAs fail,50 and the results from 2 other just completed trials are awaited (www.clinicaltrials.gov: #NCT00843882; and EudraCT: #2008-002195-10). Consistent with results obtained with the single drug lenalidomide, patients with EPO levels <100 U/L experienced higher erythroid responses; CRBNrs1672753 polymorphism was also predictive of erythroid improvement.50
Transforming growth factor β activation in MDS contributes to impairment of erythropoiesis: the promising strategy of blocking this pathway was evaluated more recently with luspatercept and galunisertib.51,52 The modified activin receptor IIB containing molecule ACE-536 (luspatercept), which inhibits SMAD2/3 signaling by blocking GDF11 when administered in ESA-resistant lower-risk MDS patients, induced transfusion independence in >40% of cases and hematologic improvement in >70%, the majority response being the RARS subtype of MDS.51 A randomized trial of luspatercept vs PBO is ongoing for transfusion-dependent RARS patients who have lost response to ESAs (irrespective of SF3B1 mutation) (www.clinicaltrials.gov; #NCT02631070).
The small activin receptor-like kinase 5 inhibitor, LY-2157299 (galunisertib), induced erythroid response in 26% of ESA resistant/relapsed IPSS lower-risk MDS patients50 in a phase 2 study; no selectively sensitive subpopulation was identified and further trials are not expected.
For SF3B1-mutated low-risk MDS cases, as well as for SRSF2-, U2AF1-, and/or ZRSR2-mutated ones, a phase 1 trial with the oral splicing modulator H3B-8800 was just initiated (www.clinicaltrials.gov; #NCT02841540).
The many therapies attempted with experimental agents blocking different signal transduction pathways activated in MDS have yielded quite disappointing results.
In an ongoing phase 2/3, multicenter study (www.clinicaltrials.gov; #NCT02598661), the activity of the telomerase inhibitor imetelstat is evaluated in transfusion-dependent IPSS lower-risk MDS, relapsed/refractory to ESA treatment.
More frequently than wished, hematologists have no alternative therapies to offer, although experimental drugs, while numerous (Table 1) are not always immediately accessible.48-52 In the end, the majority of patients with lower-risk MDS would require RBC transfusions.17 Given their relatively long survival, this support therapy is chronic and as such may create long-term side effects, as well as a social and economic burden.53 Firstly, there are differences in the approach to transfusion therapy. In many countries transfusions are not performed to obtain normalization of the Hb level, and require a lower threshold of 8 g/dL or less (unless significantly symptomatic). The principal effect of the different transfusion strategies among countries and centers, is to create difficulties in the application of objective criteria of response in clinical trials.
Experimental treatments (phase 2/phase 3 studies) of IPSS lower-risk MDS patients refractory/relapsed after ESAs and severely anemic/RBC transfusion dependent
| Study . | Doses . | # Patients . | Inclusion criteria . | Results (HI-E per IWG criteria) % . |
|---|---|---|---|---|
| Azacitidine + ESAs phase 248 | 75 mg/m2 daily for 5-d/28-d cycle plus EPO β 60 000 U/wk | 49 | sEPO >500 U/L or ESA R/R | 34.7 |
| Oral azacitidine (cc-486) (www.clinicaltrials.gov; #NCT01566695) | 300 mg daily for 21-d/28-d cycle | Ongoing | RBC TD and PLT <75 × 109/L | Ongoing |
| Lenalidomide phase 349 | 10 mg os daily 28-d cycle | 239 (160) | sEPO >500 U/L or ESA R/R | 26.9 |
| Lenalidomide + ESAs phase 350 | 10 mg daily for 21-d/28-d cycle plus EPO β 60 000 U/wk | 131 (65) | sEPO >500 U/L or ESA R/R | 39.4 |
| Luspatercept phase 251 | 1 mg/kg* SC every 3 wk | 32 | sEPO >500 U/L or ESA R/R with/without RS | 69 |
| Galunisertib phase 252 | 150 mg os BID for 14-d/28-d cycle | 41 | sEPO >500 U/L or ESA R/R | 26 |
| Study . | Doses . | # Patients . | Inclusion criteria . | Results (HI-E per IWG criteria) % . |
|---|---|---|---|---|
| Azacitidine + ESAs phase 248 | 75 mg/m2 daily for 5-d/28-d cycle plus EPO β 60 000 U/wk | 49 | sEPO >500 U/L or ESA R/R | 34.7 |
| Oral azacitidine (cc-486) (www.clinicaltrials.gov; #NCT01566695) | 300 mg daily for 21-d/28-d cycle | Ongoing | RBC TD and PLT <75 × 109/L | Ongoing |
| Lenalidomide phase 349 | 10 mg os daily 28-d cycle | 239 (160) | sEPO >500 U/L or ESA R/R | 26.9 |
| Lenalidomide + ESAs phase 350 | 10 mg daily for 21-d/28-d cycle plus EPO β 60 000 U/wk | 131 (65) | sEPO >500 U/L or ESA R/R | 39.4 |
| Luspatercept phase 251 | 1 mg/kg* SC every 3 wk | 32 | sEPO >500 U/L or ESA R/R with/without RS | 69 |
| Galunisertib phase 252 | 150 mg os BID for 14-d/28-d cycle | 41 | sEPO >500 U/L or ESA R/R | 26 |
Of the numerous studies performed in this clinical setting, reported here are some of the most relevant phase 2/3 studies, as referenced and quoted in the text.
BID, twice daily; ESA R/R, erythropoietic stimulating agent relapsed/refractory; os, orally; RS, ring sideroblasts; sEPO, endogenous serum EPO; TD, transfusion dependent.
Starting dose, titration up to 1.75 mg/kg.
A large proportion of lower-risk MDS patients experience a poor QoL, due to persistent anemia, but also fluctuations in Hb levels and frequent hospitalizations. Chronic RBC transfusions can lead to iron overload that should be treated in order to deliver optimized supportive care. Whether ICT is appropriate and fundamental in MDS management is the subject of a lively discussion.54 Nevertheless, there is evidence indicating its need before myeloablative HSCT, and guidelines recommend chelating patients with transfusion-dependent lower-risk MDS and expected survival of >1 year.16 Irrespective of the chelating agent employed (deferoxamine or deferasirox), retrospective studies have indicated a possible survival advantage,54 whereas some hematopoietic improvement is suggested in 10% to 20% of chelated patients.55 Both agents, for different reasons, are not extremely well tolerated and ICT requires careful management to prevent side effects. In conclusion, the decision for ICT in lower-risk MDS should rely first on burden of transfusions, then on expectancy of life, and followed by individual safety and tolerability of the chosen agent. In fact, the iron overload of MDS is not always secondary to transfusion, but may also be due to an imbalance in iron metabolism, intrinsic of the subtype of disease, as demonstrated by the finding of altered hepcidin levels in RARS56 but not in other World Health Organization (WHO) types of MDS.
Treatment of neutropenia
The updated WHO classification of MDS3 relies mainly on the degree of dysplasia for disease classification and specific cytopenias have only a minor impact on MDS classification, so the previous definitions of refractory neutropenia and refractory thrombocytopenia have been substituted by “MDS with single-lineage dysplasia.” In fact, neutropenia as single cytopenia is a rare finding that can be present at any rate at diagnosis of several WHO MDS subtypes.57 These infrequent cases have a rather good prognosis and have a low propensity to develop AML.57
The intrinsic functional defect of dysplastic neutrophils, together with iron overload and inefficient B, T, and natural killer cells, may indeed account for an unexpectedly higher susceptibility to infections in MDS. Although G-CSF is broadly used in febrile neutropenia, sporadically in severe neutropenia, or in combination with ESAs as well as during HMT therapy, there is no systematic study on its role in modifying disease history in terms of OS, progression to AML, or even in preventing infections.58 In any case, the majority of MDS patients who succumb because of infections belong to higher-risk categories.
Treatment of thrombocytopenia
Severe thrombocytopenia, whose frequency is <20% of the cases,15 is directly responsible for fatal events in patients with lower IPSS-risk MDS, where hemorrhage represents the third cause of MDS-related death (13%).59 The frequency and severity of bleeding events in MDS is increased also by the intrinsic functional defect of dysplastic thrombocytes. Severity of thrombocytopenia has prognostic significance,2,60 but platelet (PLT) transfusions are highly immunogenic and do not have long-lasting efficacy. Moreover, some of the treatments for MDS (ie, HMTs and lenalidomide) may initially worsen thrombocytopenia, and consequently augment the risk of bleeding. The availability of agents actively promoting megakaryocytopoiesis and PLT function could therefore represent an important possible therapeutic option for MDS (Table 2).61-66 Several agents (interleukin-11, recombinant human thrombopoietin [TPO], pegylated recombinant human megakaryocyte growth and development factor, and interleukin-6) have been investigated in MDS, but only 2 have demonstrated safe activity. AMG-531, now known as romiplostim, is a peptide TPO mimetic, synthesized as a dimer resulting from the fusion of a novel peptide and antibody or “peptibody” that can stimulate PLT production via the TPO receptor without competing with TPO.
Experimental treatments (phase 2/phase 3) of IPSS lower-risk MDS patients with severe thrombocytopenia
| Study . | Dose of drugs . | No. Patients . | Inclusion criteria . | Results (ORR) % . |
|---|---|---|---|---|
| ROM phase 3 randomized vs PBO61,62 | 750 μg/wk × 58 wk SC | 250 | PLT <50 × 109/L | 36.5 (IWG HI-P) |
| EL escalating dose phase 2 vs PBO 2:163 | 50-300 mg os daily | Ongoing | PLT <30 × 109/L | 32 (IWG HI-P) |
| ROM plus azacitidine64 | 500 and 750 μg/wk SC plus azacitidine 75 mg/m2 per d for 7 d on a 28-d cycle | 40 | Stratified PLT <50 and >50 × 109/L (MDS IPSS INT-1 and INT-2) | * |
| ROM plus decitabine randomized vs PBO plus decitabine65 | 750 μg/wk SC plus decitabine 20 mg/m2 per d for 5 d on a 28-d cycle or 15 mg/m2 IV over 3 h repeated every 8 h for 3 d every 6 wk | 29 | Stratified PLT <50 and >50 × 109/L and also higher-risk MDS | 33 |
| ROM plus lenalidomide66 | 500 or 750 μg/wk SC plus lenalidomide 10 mg/d for 21 d on a 28-d cycle | 39 | Del5q and non-del5q | † |
| Study . | Dose of drugs . | No. Patients . | Inclusion criteria . | Results (ORR) % . |
|---|---|---|---|---|
| ROM phase 3 randomized vs PBO61,62 | 750 μg/wk × 58 wk SC | 250 | PLT <50 × 109/L | 36.5 (IWG HI-P) |
| EL escalating dose phase 2 vs PBO 2:163 | 50-300 mg os daily | Ongoing | PLT <30 × 109/L | 32 (IWG HI-P) |
| ROM plus azacitidine64 | 500 and 750 μg/wk SC plus azacitidine 75 mg/m2 per d for 7 d on a 28-d cycle | 40 | Stratified PLT <50 and >50 × 109/L (MDS IPSS INT-1 and INT-2) | * |
| ROM plus decitabine randomized vs PBO plus decitabine65 | 750 μg/wk SC plus decitabine 20 mg/m2 per d for 5 d on a 28-d cycle or 15 mg/m2 IV over 3 h repeated every 8 h for 3 d every 6 wk | 29 | Stratified PLT <50 and >50 × 109/L and also higher-risk MDS | 33 |
| ROM plus lenalidomide66 | 500 or 750 μg/wk SC plus lenalidomide 10 mg/d for 21 d on a 28-d cycle | 39 | Del5q and non-del5q | † |
EL, eltrombopag; IWG HI-P; International Working Group hematological improvement-PLTs; ORR, overall response rate; ROM, romiplostim.
*The incidence of PLT transfusions was 46%, 36%, and 69% in patients receiving ROM 500 μg, ROM 750 μg, or PBO.
†Thrombocytopenia-related adjustments in lenalidomide dose occurred in 6 (50%) patients in the PBO group, 5 (36%) in the ROM 500 μg group, and 2 (15%) in the 750 μg group. No difference in transfusions was noted.
It is approved for treatment of immune thrombocytopenic purpura. Durable PLT increase was demonstrated in lower-risk MDS: romiplostim 750 μg per week SC increased PLT counts, and decreased the number of bleeding events and PLT transfusions with respect to PBO.61 Although the study was stopped because of an increase of blasts, at 58 weeks, the AML rates and the OS rates were not significantly different between PBO and romiplostim.62 Endogenous levels of TPO, similar to what was observed for EPO, could serve to select patients sensitive to thrombomimetic drugs. Eltrombopag, an orally bioavailable, synthetic nonpeptide TPO-receptor agonist, differs substantially from romiplostim in structure and mechanism of action, and binds to the transmembrane and juxtamembrane domains of the TPO receptor. It is approved for the treatment of immune thrombocytopenic purpura and aplastic anemia. Eltrombopag efficacy is under evaluation in severely thrombocytopenic (PLT <30 × 109/L) IPSS lower-risk MDS patients in an ongoing phase 2, multicenter, prospective, PBO-controlled, single-blind study at escalating doses from 50 to 300 mg per day. Preliminary results showed that 32% of treated patients responded vs none in the PBO arm, with a median PLT rise of 46 ± 1 Gi/L (P = .009).63 Both romiplostim and eltrombopag have been used in combination with HMTs to decrease thrombocytopenia observed during the first cycles. Romiplostim was demonstrated as active in reducing PLT nadir and decreasing bleeding events during azacitidine and decitabine treatments.64,65 The study exploring the efficacy of eltrombopag in inducing PLT transfusion independence within the first 4 cycles of azacitidine therapy in patients with IPSS INT-1, -2, and high-risk MDS (www.clinicaltrials.gov; #NCT02158936) was very recently put on hold because of the results of the futility analysis and for safety concerns. The association of romiplostim and lenalidomide used in the attempt to decrease the frequency of dose reductions/delays due to thrombocytopenia, induced a trend toward lower numbers of transfusions in romiplostim 500 μg and 750 μg during each treatment cycle.66
TPO is active in stimulating very early hematopoietic progenitor cells. It is therefore not surprising that active thrombomimetic drugs do increase the number of blasts in the bone marrow. Until we ascertain that the effect of thrombomimetic agents on blasts is transient, in analogy to what was observed for G-CSF, they should be used with caution. Indeed, it seems at present safer to limit their use to lower-risk MDS patients without marrow blasts.
Treatment of pancytopenia
Immunosuppressive therapy is not a frequently selected treatment option for lower-risk MDS. Its toxicity impairs application in elderly patients, the need of hospitalization, and its results did not encourage broader use. In fact, ATG plus CSA have been demonstrated to induce hematologic improvement in a good proportion of cases. Of course, patients with 2 to 3 cytopenias remain categorized as lower-risk only if they have normal cytogenetics and no blasts in the marrow. Azacitidine and decitabine are an option in these cases, but ATG-responsive MDS can be quite well identified as those with hypo-cellular marrow, without increased blasts, normal karyotype, HLA-DR 15-positivity, and STAT-3 mutant cytotoxic T cells.16 IPSS scoring has no impact on hematologic improvement.67 Unlike aplastic anemia, results from clinical studies do not always demonstrate that MDS treated with immunosuppression have OS advantage.67,68 These nonconsistent results have to be interpreted with caution because of higher-risk MDS cases in clinical studies and differences in patient populations.69 Other approaches to immunosuppression in MDS have been attempted with anti-CD52 antibody alemtuzumab with some success,70 although this agent is currently not available.
HSCT
Allogeneic stem cell transplant remains the only curative option even for lower-risk MDS patients. Of course, the toxicity and mortality of this therapy renders it less suitable for elderly patients who have a fairly long life expectancy and a reasonable QoL, but it should be proposed to younger patients (in selected cases) when the burden of disease and its risk of progression are high. The features determining the ideal timing for HSCT in lower-risk MDS have been subjected to thorough analysis.
Conclusion
Several agents active in relieving cytopenias compose the treatment armamentarium for lower-risk MDS. Some of these agents have been available for years and the MDS subpopulations selectively responding to them are well identified. The best outcome is obtained when therapy is based on this solid evidence and intentional sequencing of effective therapies is planned (Figure 1). The experimental agents in the development for lower-risk MDS are less empiric and more often tentatively target groups of MDS with defined biological/molecular characteristics, such as the presence of ring sideroblasts, or mutations in splicing factor genes, or increased telomerase activity. In conclusion, as we stand, lower-risk MDS patients may have a notably long survival: they should receive standard sequential treatments wisely chosen according to individual clinical, biological, and molecular parameters, and when relapsing, they should be addressed as much as possible to investigational studies tailored to their characteristics.
Correspondence
Valeria Santini, SODc Hematology, Azienda Ospedaliera Universitaria Careggi, University of Florence, Largo Brambilla 3, 50134 Florence, Italy; e-mail: santini@unifi.it.
References
Competing Interests
Conflict-of-interest disclosure: V.S. is on an advisory committee for Amgen and Astex, has received research funding from Celgene, has consulted for Janssen, and has received honoraria from Janssen, Novartis, and Celgene.
Author notes
Off-label drug use: None disclosed.