Abstract
Immune deficiencies resulting from inherited defects in neutrophil function have revealed important features of the innate immune response. Although sharing an increased susceptibility to bacterial and fungal infections, these disorders each have distinctive features in their clinical manifestations and characteristic microbial pathogens. This review provides an update on several genetic disorders with impaired neutrophil function, their pathogenesis, and treatment strategies. These include chronic granulomatous disease, which results from inactivating mutations in the superoxide-generating nicotinamide dinucleotide phosphate oxidase. Superoxide-derived oxidants play an important role in the control of certain bacterial and fungal species, and also contribute to the regulation of inflammation. Also briefly summarized are updates on leukocyte adhesion deficiency, including the severe periodontal disease characteristic of this disorder, and a new immune deficiency associated with defects in caspase recruitment domain–containing protein 9, an adaptor protein that regulates signaling in neutrophils and other myeloid cells, leading to invasive fungal disease.
Learning Objectives
Review key updates in the pathogenesis and management of chronic granulomatous disease, leukocyte adhesion deficiency, and caspase recruitment domain–containing protein 9, 3 different primary immune deficiencies with defects in neutrophil function
Understand why there are differences in the pathogenesis and clinical manifestations of these 3 disorders
Introduction
Neutrophils and other phagocytic leukocytes are essential effector cells in the innate immune system, which rapidly responds to the presence of invading bacteria, fungi, and parasites. Hence, patients with inherited defects in phagocyte function typically present in infancy or childhood with recurrent, unusual, and/or difficult-to-clear bacterial and sometimes fungal infections. Typical sites of infection include skin or mucosa, lung, lymph nodes, and deep tissue abscesses. Despite this shared propensity, these disorders also have distinctive clinical and microbiologic features related to the particular functional defect. New disorders of neutrophil function continue to be described. This review provides a selected update on chronic granulomatous disease (CGD), leukocyte adhesion deficiency, and caspase recruitment domain–containing protein 9 (CARD9) deficiency.
CGD
CGD is caused by genetic defects in the leukocyte nicotinamide dinucleotide phosphate (NADPH) oxidase (also referred to as the respiratory burst oxidase).1 Superoxide generated during the respiratory burst is converted into reactive oxygen species (ROS), including hydrogen peroxide and myeloperoxidase-catalyzed formation of hypochlorous acid. These oxidants are important microbicidal effectors, as their absence leads to recurrent, often life-threatening bacterial and fungal infections with a distinctive set of pathogens. A second hallmark of CGD is the frequent development of inflammatory granulomas and other inflammatory disorders that are not always related to infection. This feature reflects an increasingly appreciated influence of NADPH oxidase-derived ROS on other cellular processes.2,3
NADPH oxidase and molecular genetics of CGD
The NADPH oxidase is a multisubunit phagosome and plasma membrane–associated enzyme that is expressed in neutrophils, monocytes and macrophages, dendritic cells, and eosinophils.4 The enzyme is also present in B and perhaps T lymphocytes, although its function in lymphocytes is not well understood.
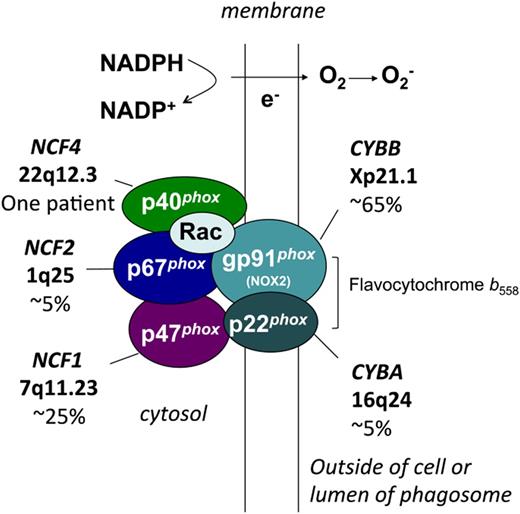
CGD results from inactivating X-linked or autosomal recessive mutations in any 1 of the 5 subunits of the NADPH oxidase (Figure 1).1 These subunits are referred to by their molecular mass (kDa) and the designation “phox,” for phagocyte oxidase. Two subunits are membrane proteins that make up flavocytochrome b558, the redox center of the oxidase. The gp91phox subunit (also known as NOX2) contains flavoprotein and heme-binding domains, and p22phox contains a key docking site for p47phox. In neutrophils, most flavocytochrome b558 resides in specific granules, which fuse with either the phagolysosome or plasma membrane on cellular activation. Three regulatory subunits, p47phox, p67phox, and p40phox, are associated with each other in the cytosol of unstimulated cells. When phosphorylation of p47phox is activated by inflammatory or phagocytic stimuli, this cytosolic phox complex rapidly moves to flavocytochrome b558 to activate superoxide formation via a domain in p67phox. The 40phox subunit plays a specialized role in upregulating oxidant production on phagosome membranes, but is dispensable for plasma membrane enzyme activity.4 The NAPDH oxidase is also regulated by the Rac GTPase, which acts as a molecular switch through conformational changes between the GDP and GTP-bound forms. Rac-GTP binds to p67phox and is required for NADPH oxidase activity. Genetic defects in Rac1 or Rac2 have not been reported in CGD. However, dominant-negative mutations in the hematopoietic-specific Rac2 GTPase are described, which affect both phagocyte and lymphocyte functions, particularly adhesion and motility, but result only in mild NADPH oxidase defects.1
NADPH oxidase and molecular genetics of CGD. Shown are the membrane and cytosolic subunits of the leukocyte NADPH oxidase, indicating those involved in the 5 different genetic subgroups of CGD, the approximate incidence, and the gene and chromosomal location. CYBB and CYBA refer to the large and small subunits of flavocytochrome b558, whereas the NCF refers to neutrophil cytosolic factor, used to designate the cytosolic regulatory subunits of the oxidase. Flavocytochrome b558 is the redox center of the enzyme, and is located in plasma, specific granule (in neutrophils), and phagolysosomal membranes. This heterodimer is composed of the gp91phox (phox stands for phagocyte oxidase) and p22phox subunits of the NADPH oxidase, which are affected in X-linked and an autosomal recessive form of CGD, respectively. The gp91phox subunit is sometimes referred to as NOX2. The soluble regulatory proteins p47phox, p67phox, and p40phox are found in the cytosol until leukocyte activation by soluble or particulate inflammatory stimuli, upon which they move to the membrane, where p47phox and p67phox bind flavocytochrome b558. Mutations in the genes encoding p47phox, p67phox, and p40phox account for 3 other autosomal recessive forms of CGD. Another essential regulatory component of the NADPH oxidase is the small GTPase, Rac, which in its active GTP-bound state becomes membrane-bound and associates with p67phox. By a mechanism that is not fully understood, these multiple regulatory subunits activate the flavocytochrome to catalyze the transfer of electrons from cytosolic NADPH across the membrane via FAD and heme redox centers to molecular oxygen, thereby forming superoxide in the extracellular space or within phagosomes.
NADPH oxidase and molecular genetics of CGD. Shown are the membrane and cytosolic subunits of the leukocyte NADPH oxidase, indicating those involved in the 5 different genetic subgroups of CGD, the approximate incidence, and the gene and chromosomal location. CYBB and CYBA refer to the large and small subunits of flavocytochrome b558, whereas the NCF refers to neutrophil cytosolic factor, used to designate the cytosolic regulatory subunits of the oxidase. Flavocytochrome b558 is the redox center of the enzyme, and is located in plasma, specific granule (in neutrophils), and phagolysosomal membranes. This heterodimer is composed of the gp91phox (phox stands for phagocyte oxidase) and p22phox subunits of the NADPH oxidase, which are affected in X-linked and an autosomal recessive form of CGD, respectively. The gp91phox subunit is sometimes referred to as NOX2. The soluble regulatory proteins p47phox, p67phox, and p40phox are found in the cytosol until leukocyte activation by soluble or particulate inflammatory stimuli, upon which they move to the membrane, where p47phox and p67phox bind flavocytochrome b558. Mutations in the genes encoding p47phox, p67phox, and p40phox account for 3 other autosomal recessive forms of CGD. Another essential regulatory component of the NADPH oxidase is the small GTPase, Rac, which in its active GTP-bound state becomes membrane-bound and associates with p67phox. By a mechanism that is not fully understood, these multiple regulatory subunits activate the flavocytochrome to catalyze the transfer of electrons from cytosolic NADPH across the membrane via FAD and heme redox centers to molecular oxygen, thereby forming superoxide in the extracellular space or within phagosomes.
CGD occurs in ≈ 1/200 000 live births, as estimated in a retrospective study using a US registry.5 Approximately two-thirds of patients with CGD have defects in CYBB, the X-linked gene encoding gp91phox, with up to 15% resulting from de novo CYBB mutations.1 Autosomal recessive mutations in NCF1, encoding p47phox, account for ≈25% of CGD. Rarer autosomal recessive forms of CGD are caused by mutations in the CYBA, NCF2, or NCF4 genes encoding p22phox, p67phox, or p40phox, respectively. However, the incidence of autosomal recessive CGD can be higher than X-linked CGD in countries with high rates of consanguineous marriage.6,7
CGD mutations identified in the genes encoding gp91phox, p22phox, and 67phox are heterogenous and include missense mutations, small deletions, splicing, and promoter mutations.1 Almost all result in complete loss of enzyme activity, although some patients with partial expression or function of the affected subunit have up to ≈10% of normal NADPH oxidase activity. Most patients with CGD with p47phox defects are either homozygotes or compound heterozygotes for a GT deletion at the beginning of NCF1 exon 2 that predicts a premature stop codon. The high frequency of this mutation is a result of highly conserved and closely linked NCF1 pseudogenes, which can recombine with the wild-type gene.1
New twists on CGD and NADPH oxidase
Unexpectedly, several missense mutations in the X-linked CYBB gene were discovered in 2 kindreds in which affected patients had mycobacterial infections, but not other bacterial or fungal infections characteristic of CGD.8 The amino acid changes in gp91phox led to a selective reduction in macrophage flavocytochrome b levels and NADPH oxidase activity, highlighting the importance of the macrophage oxidase for controlling mycobacteria. Mechanistic studies showed that the gp91phox mutations impaired flavocytochrome b heterodimer formation, which was partially overcome in neutrophils by their much higher level of gp91phox expression compared with macrophages.
In 2009, the first (and to date only) patient with p40phox defects was reported: a 3-year-old boy who was a compound heterozygote for 2 null NCF4 alleles.9 p40phox is a specialized subunit that stimulates phagosome, but not plasma membrane oxidase activity, via a regulatory domain that binds to phosphatidylinositol 3-phosphate, a membrane lipid present in high concentrations on phagosome membranes. The main clinical finding was granulomatous inflammation of the intestinal tract. However, this patient did not have bacterial and fungal infections characteristic of other forms of CGD. This may reflect the more selective role of p40phox in regulating NADPH oxidase activity and suggests that residual plasma membrane oxidase activity may be sufficient for effective microbial killing.
Clinical features of CGD
Infections
The clinical manifestations of CGD typically begin in infancy or early childhood. The spectrum and relative frequency of infectious and inflammatory complications in recent registry-based studies6,7,10-14 are similar to older reports, which were based on registries from North America and Europe,1,5 although certain infections are more frequent in other regions of the world.
Patients with CGD are particularly susceptible to Staphylococcus aureus, Burkholderia cepacia, Nocardia species, and certain gram-negative enteric bacilli including Serratia marcescens and Salmonella species.15 Patients with CGD also have increased risk for infection with mycobacterial species and can develop severe local or systemic disease with BCG, an attenuated strain of Mycobacterium bovis, after administration of the BCG vaccine.7,11 Invasive fungal infections with molds and yeast are also a major threat in CGD, with Aspergillus fumigatus and Aspergillus nidulans as the most commonly isolated fungal species.16,17 Some fungal pathogens in CGD, including A. nidulans and Paecilomyces, are rarely encountered in other conditions. Many unusual bacterial species that seldom affect other individuals also cause serious disease in CGD.1 Some microbes that cause infections in CGD contain catalase, which prevents CGD phagocytes from using microbe-generated hydrogen peroxide to promote killing of ingested organisms. However, the reason why other microbial species require the NADPH oxidase for effective control is not well understood.
Frequent sites of infection include the lungs, followed by lymph nodes; skin and soft tissues; the gastrointestinal tract, including Staphylococcus liver abscesses (which are almost unique to CGD); and osteomyelitis.1,5 In contrast to infections with bacteria and Nocardia, those caused by Aspergillus and other fungi, especially A. nidulans, can be indolent with mild symptoms.16 However, acute inhalation exposure to decayed organic material, such as mulch or hay, can cause a fulminant fungal pneumonia referred to as “mulch pneumonitis.” Although S. aureus is the most frequently isolated organism in CGD infections overall, the most common causes of death continue to be pneumonia and/or sepsis resulting from Aspergillus or B. cepacia.15
Inflammatory complications
Inflammatory manifestations are common in CGD, often associated with granulomatous inflammation. In some instances, chronic inflammation develops in response to active infection or as sequelae. However, other conditions appear to reflect a dysregulated inflammatory response in the absence of respiratory burst-derived ROS. The basis for this propensity is multifactorial. Likely mechanisms include impaired digestion of microbes or debris, increased pro-inflammatory cytokine production reflecting changes in redox-regulated signaling pathways, and altered antigen presentation.2,3
The most frequent inflammatory disease of significance is gastrointestinal disease that is somewhat reminiscent of Crohn’s disease. Gastrointestinal disease occurs in all genetic subgroups of CGD, and most commonly involves the anus and rectum.18,19 Upper gastrointestinal tract disease is also frequent. Analysis of 140 patients with CGD followed at the National Institutes of Health revealed inflammatory involvement of the gastrointestinal tract in ≈1 in 3 patients.20 Microgranulomas, pigmented macrophages, and tissue eosinophilia are common biopsy findings and are often not associated with acute inflammation.19 Granulomatous obstruction of either the gastric outlet or urinary tract can also occur. There appears to be no correlation of the occurrence of gastrointestinal disease with a higher risk for severe bacterial and fungal infections.15
A recent single-institution study retrospectively examined the frequency of inflammatory episodes in 98 patients with CGD.21 Almost 70% had symptoms associated with inflammatory manifestations. Gastrointestinal symptoms were present in 60 patients, including abdominal pain, diarrhea, and oral aphthous ulcers. Forty-four patients underwent biopsy, and chronic gastrointestinal inflammatory lesions were identified in 22. Pulmonary findings, often associated with dyspnea, were reported in 18 patients. Imaging studies revealed pleural thickening, interstitial lung disease, and/or fibrosis. Twelve patients had episodes of urogenital tract inflammation, including bladder wall granulomata. Chorioretinitis and ocular granulomata were reported in 6 patients, and 7 patients had discoid lupus, leukocytoclastic vasculitis, arthritis, or dermatomyositis. Overall, these findings are consistent with reports based on multi-institution registry data (eg, Winkelstein et al5 ).
Excessive immune activation leading to macrophage activation syndrome and hemophagocytic lymphohistiocytosis (HLH) can be another complication of CGD. A recent analysis identified 63 patients with primary immunodeficiencies that met criteria for HLH, but did not have cytotoxicity defects or X-linked lymphoproliferative disorders.22 Twenty-two patients had CGD, 18 had common variable immunodeficiency, 12 had severe combined immunodeficiency, and the remainder had other defects. In common variable immunodeficiency and severe combined immunodeficiency, HLH was typically associated with viral infections. However, episodes of HLH in CGD were mainly triggered by infections caused by fungi, bacteria (especially B. cepacia), or Leishmania in areas where this is endemic. In 1 case, an HLH-like picture was even the presenting manifestation of CGD in an infant who was subsequently diagnosed with an underlying Candida infection.
Up to ≈10% of patients with X-linked CGD develop discoid lupus-like lesions or aphthous ulcers.6,7,10-14 The occurrence of discoid lupus, photosensitivity, and aphthous ulcers may be even more common in carrier females, with incidence of the latter 2 manifestations reported to be as high as ≈50%.23 Less commonly, systemic autoimmune manifestations are reported in patients with CGD and X-linked CGD carriers and include lupus, juvenile idiopathic arthritis, antiphospholipid syndrome, idiopathic thrombocytopenic purpura, and immunoglobulin A nephropathy.6,7,10-14
Liver disease with noncirrhotic portal hypertension has recently been identified as an independent risk factor for mortality in CGD.24 This was based on a study of 194 patients at the National Institutes of Health, 24 of whom died during the observation period, all of infectious complications. A higher frequency of decreased platelet counts, elevated liver enzymes, and/or a history of liver abscesses were identified as factors associated with mortality. Liver damage likely reflects injury to the liver microcirculation as a result of repeated systemic and liver infections.
Correlation between genetic defect and clinical manifestations
It has long been observed that patients with X-linked CGD and those with autosomal recessive mutations in CYBA and NCF2 as a group tend to have a more severe clinical course compared with patients with NCF1 defects.1,5 This was affirmed in a recent comprehensive study examining outcomes for 287 patients followed at the National Institutes of Health.25 p47phox−/− neutrophils have a small amount (≤2%) of residual superoxide formation, as this subunit functions as an adaptor protein, rather than directly catalyzing electron transfer. Some X-linked patients with a partially functional gp91phox also have a milder clinical course. However, despite the fact that more than 90% of patients with non-p47phox-deficient forms of CGD have undetectable levels of O2− production, there is still a surprising clinical heterogeneity.1,5 At one end of the spectrum are those who develop severe infections beginning during infancy, and at the other are patients who are well for many years and then develop a serious infection typical of CGD. Polymorphisms in oxygen-independent antimicrobial systems and other components of innate immunity are likely to play important roles in modifying disease severity. These remain to be fully defined, although variants in the myeloperoxidase, mannose binding lectin, and FcgfRIIa genes were associated with a higher risk for granulomatous or autoimmune/rheumatologic complications in CGD.26
Patients with p47phox−/− CGD are distinctive in having a higher incidence of diabetes, renal glomerular disease, and cardiovascular disease with dyslipidemia, as reported in a study of 64 patients with p47pphox−/− CGD followed at the National Institutes of Health compared with 165 patients with X-linked CGD followed over the same period of time.27 Diabetes occurred in 10% of patients with p47phox−/−.
Treatment of CGD
Care by an experienced specialist who can also coordinate care with a multidisciplinary team is important for optimal management of patients with CGD. The use of prophylactic antibiotics and interferon-γ (IFN-γ), coupled with aggressive treatment of acute infections and prolonged courses of antimicrobial treatment, has markedly improved the clinical course.1,28 Trimethoprim/sulfamethoxazole (or, in sulfa-allergic patients, dicloxacillin or trimethoprim) is standardly used for antibacterial prophylaxis. Itraconazole is effective for prophylaxis for fungal infections, as evaluated in a randomized, double-blind, placebo-controlled study.29 Voriconazole is an alternative agent for patients with CGD who cannot tolerate itraconazole, for example, because of hepatitis. Prophylactic IFN-γ is another mainstay of current management. Use of IFN-γ is not accompanied by any measurable improvement in phagocyte NADPH oxidase activity in the majority of patients with CGD, and its clinical benefit is probably related to enhanced phagocyte function and killing by nonoxidative mechanisms, and perhaps the NOS2, and xanthine oxidase pathways. A multicenter trial first established that recipients of IFN-γ had 70% fewer and less severe infections.30 A follow-up study reinforced these findings, reporting only 0.30 serious bacterial infections and 0.12 serious fungal infections per patient-year in patients observed for up to 9 years, with a total observation period of 328.4 patient-years.31 The most common adverse effects were fever and influenza-like symptoms. Importantly, there was no increase in the incidence of chronic inflammatory complications of CGD in patients receiving IFN-γ. Hence, the current recommendation for patients with CGD is to use prophylaxis with trimethoprim-sulfamethoxazole, itraconazole, and IFN-γ. Use of prophylactic IFN-γ is less frequent in Europe.
Corticosteroids are used to treat clinically significant granulomatous or other inflammatory complications, including CGD-associated gastrointestinal disease, as well as “mulch pneumonitis,” although with caution, given the underlying microbial killing defect.1,28 Tumor necrosis factor-α inhibitors often improve symptoms in CGD inflammatory bowel disease, but are not recommended because of their predisposing risk for severe infections.28 Activated CGD myeloid cells have increased production of the pro-inflammatory cytokine interleukin 1 (IL-1), and treatment with Anakinra, an IL-1 receptor antagonist, was reported to relieve symptoms in several patients with CGD with colitis.32 However, responses to Anakinra were either poor or not sustained in another 5 patients with CGD colitis.33 Macrophage activation syndrome and HLH in CGD have been treated with steroids and IV Ig.22 Because of its immunomodulatory and anti-tumor necrosis factor-α effects, thalidomide has been used in a few patients with CGD-associated inflammation.34 Intriguingly, pioglitazone, a peroxisome proliferator-activated receptor γ agonist and a drug currently approved for type 2 diabetes, increased mitochondrial oxidant production and bactericidal function in human and mouse CGD phagocytes.35 Although additional evaluation is clearly needed, partial restoration of oxidant production using this agent could potentially benefit both defects in host defense and inflammation in CGD.
The prognosis of CGD has improved dramatically in the last few decades with the advent of prophylactic antimicrobials and IFN-γ and, more recently, new azole compounds for treating Aspergillus.1 The overall mortality rate in the above-mentioned study on long-term interferon-γ therapy was 1.5% per patient-year,31 and may be even lower in newly diagnosed children managed with optimal supportive care. It will be important to continue to monitor the changing outlook for patients with this disease to weigh the risks and benefits of approaches using stem cell transplantation.
Stem cell transplantation in CGD
Allogeneic hematopoietic stem cell transplantation (HSCT) is curative for CGD. Reduced-intensity conditioning regimens for allogeneic transplantation have emerged as an effective approach, with fewer acute and long-term adverse effects resulting from lower exposure to genotoxic agents such as irradiation and alkylating drugs. Two recent large, multigroup studies with centers in Europe and North America reported promising outcomes for reduced-intensity conditioning before transplantation of either marrow, mobilized peripheral blood (PB) CD34+ cells or cord blood from HLA-matched related and unrelated donors.36,37 Immunosuppression included fludarabine, antithymocyte globulin, and alemtuzumab. As a myeloablative agent (given at reduced doses), 1 trial used busulfan36 and the other treosulfan,37 but outcomes were generally similar. Patients were mainly children or adolescents, as well as some young adults. The majority had X-linked CGD, and most had either ongoing and/or previous significant infection or inflammatory complications. Overall survival was ≈90%, and the incidence of acute grade III to IV GvHD was 4% to 8%, and of chronic GvHD it was 7% to 12%. High-level (>90%) donor chimerism was achieved in almost all patients, although a few had graft failure and received a second transplant. A retrospective study from the United Kingdom compared clinical outcomes for 30 children with CGD treated with HSCT compared with outcomes for 32 treated with supportive care.38 Although survival was similar during the period of observation, children not undergoing transplantation had more serious infections, episodes of surgery, and admissions compared with post-HSCT children (0.71 per CGD life-year compared with 0.15 per transplant-year). Children receiving HSCT also had better height for age.
Gene therapy of CGD
Gene therapy targeted at autologous HSCs is under active development as a treatment of CGD.39-41 It could be used for patients for whom a suitable donor cannot be identified, and also has no risk for GVHD. Observations on female carriers of X-linked CGD and preclinical studies in murine CGD suggest that correction of respiratory burst activity in ≈10% of circulating neutrophils would lead to clinically relevant improvements in host defense.1 Either autologous marrow or mobilized PB CD34+ cells are targets for ex vivo gene correction before reinfusion. Mobilization of PB CD34+ cells can be reduced in some patients with CGD in association with chronic inflammation or infection, and a combination of G-CSF and plerixafor was more effective for mobilization than G-CSF alone.42 There is not an intrinsic advantage of NADPH oxidase-corrected HSC for engraftment, and autologous transplantation lacks the “graft vs donor” effect that promotes engraftment in reduced-intensity conditioning–based allogeneic transplants. Thus, significant myeloablation before reinfusion of transduced autologous cells is required in CGD to facilitate engraftment of gene-corrected stem/progenitor cells.
Early studies used γ-retroviral vectors for gene delivery, which have a risk for genotoxicity as a result of activation of oncogenes near vector insertion sites by enhancer elements in the vector promoter region.39-41 Only partial myeloablation was used, so there was no significant sustained engraftment of gene-corrected HSC. However, even a transient increase in oxidase-positive neutrophils in the first weeks after transplantation resulted in clinical benefit with resolution of infections.43,44 One trial used a vector that incorporated a potent enhancer of myeloid gene expression, which was associated with clonal outgrowth of myeloid leukemia cells.43,45 Current efforts are now focused on “self-inactivating” γ retroviral vectors or lentiviral vectors that lack vector enhancer sequences. Several phase I/II CGD gene therapy clinical trials using either autologous bone marrow CD34+cells or cytokine-mobilized PB CD34+ cells are ongoing, with enrollment at sites both in Europe and the United States (NCT01906541, NCT02757911, NCT01855685, NCT02234934). These trials involve conditioning with myeloablative doses of busulfan before infusion of vector-transduced cells.
Alternative approaches for gene correction are also being investigated. These include insertion of a functional copy of the therapeutic gene into a genomic “safe harbor” site in which there is low risk of activating neighboring oncogenes. One such “safe harbor” site is the AAVS1 locus on chromosome 19. Induced pluripotent stem cells from different genetic subgroups of CGD were successfully used as targets for insertion of a CGD genotype-specific minigene into the AAVS1 site, using zinc finger nucleases.46 This approach was optimized to efficiently insert donor constructs into the AAVS1 locus in human hematopoietic stem and progenitor cells from X-linked patients with CGD, achieving gp91phox expression in 4% to 11% human cells after xenotransplantation into mice.47
Hypomorphic variants in NADPH oxidase genes are associated with inflammatory and autoimmune diseases
As already mentioned, CGD is associated with aberrant inflammation not always related to infection. These observations were the first clues that that NADPH oxidase-derived ROS may have immunoregulatory effects, which curb responses that otherwise promote inflammation, and even autoimmunity, in certain genetic backgrounds. Multiple recent studies identified variant alleles of NADPH oxidase genes as risk factors for inflammatory and autoimmune diseases, including lupus (NCF2), rheumatoid arthritis (NCF1, NCF4), Crohn's disease (NCF2, NCF4, Rac2), and early-onset inflammatory bowel disease (NCF1, NCF2, NCF4, CYBB, Rac2).3,48,49 These variants result in reduced, but not absent, NADPH oxidase activity, and thus are not associated with microbial infections.
Other disorders of phagocyte function
Disorders of leukocyte adhesion and motility
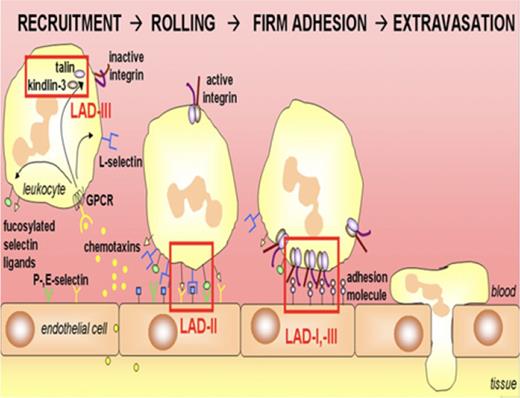
Adhesion of neutrophils to the endothelium, tissue matrix, and microbes is essential for their ability to emigrate into sites of infection and eliminate pathogens. Many of these adhesive interactions are mediated by the integrin and selectin families of cell surface glycoproteins. Leukocyte adhesion deficiency (LAD) is a group of autosomal recessive immunodeficiencies resulting from defects in this important function (Figure 2; Table 1), leading to severe bacterial infections. The most common and first described LAD subtype is LAD-1, caused by genetic defects in CD18, the common chain of the β2 integrin family; more than several hundred patients have been reported.1,50 LAD-1 neutrophils have profound adhesive and motility defects. Patients present with recurrent severe infections, including deep tissue abscesses caused by Staphylococcus aureus or gram-negative enteric organisms, and also have neutrophilia, but impaired formation of pus. New subgroups include LAD type 2 (LAD-2) and type 3 (LAD-3), both of which have been described only in small numbers of patients. LAD-2 results from mutations in the Golgi GDP-fucose membrane transporter, leading to a generalized loss of expression of fucosylated glycans on the cell surface.1,50 Thus, LAD neutrophils are unable to bind to E- and P-selectin receptors on endothelium. Most patients are from the Middle East. Infections are less severe than LAD-1 and LAD-3, and patients also have the Bombay (hh) erythrocyte phenotype because of a lack of fucosylation, as well as developmental delay. LAD-3 has been described in ≈20 families thus far, and is characterized by functional defects in the “inside-out” activation of multiple classes of β integrins in blood cells. In 2009 and 2010, LAD-3 was shown by several groups to result from mutations in FERMT3, which encodes kindlin-3, a protein that binds β integrin tails.50 LAD-3 patients have severe recurrent infections and leukocytosis, similar to LAD-1, and a bleeding tendency similar to Glanzmann thrombasthenia. HSC transplantation is generally recommended for LAD-1 and LAD-3, whereas LAD-2 is managed by supportive care; fucose supplementation is reported to be helpful in some patients.1,50
Leukocyte recruitment and defects in LAD. Leukocytes migrate to the site of inflammation after a gradient of chemoattractants. The cells slow down within the vasculature because of transient interactions between selectins and their glycosylated ligands, which are defective in LAD-II. Next, stable adhesion by leukocyte integrins, absent in LAD-I, to ligands on the endothelium results in leukocyte arrest. Activation of blood cell integrins is decreased in LAD-III. Normal neutrophils extravasate into tissues after firm adhesion. From van de Vijver et al,50 with permission.
Leukocyte recruitment and defects in LAD. Leukocytes migrate to the site of inflammation after a gradient of chemoattractants. The cells slow down within the vasculature because of transient interactions between selectins and their glycosylated ligands, which are defective in LAD-II. Next, stable adhesion by leukocyte integrins, absent in LAD-I, to ligands on the endothelium results in leukocyte arrest. Activation of blood cell integrins is decreased in LAD-III. Normal neutrophils extravasate into tissues after firm adhesion. From van de Vijver et al,50 with permission.
Characteristics of LAD subgroups
| Section 1.01 . | LAD I . | LAD II . | LAD III . |
|---|---|---|---|
| Skin infection | ++ | + | ++ |
| Severe infections | +++ | + | +++ |
| Periodontitis | ++ | ++ | ? |
| Delayed umbilical cord separation | +++ | + | |
| Developmental abnormalities | +++ | ||
| Bleeding tendencies | +++ | ||
| Neutrophilia : baseline | + | +++ | ++ |
| Neutrophilia : with infection | +++ | +++ | +++ |
| Genetic defect | CD18 subunit of β2 integrins (ITGB2) | Fucosylation of selectin ligands (eg, SLeX) because of deficiency in a fucose transporter (SLC35C1) | Inside-out signaling involving kindlin-3 for activation of blood cell β integrins (FERMT3) |
| Section 1.01 . | LAD I . | LAD II . | LAD III . |
|---|---|---|---|
| Skin infection | ++ | + | ++ |
| Severe infections | +++ | + | +++ |
| Periodontitis | ++ | ++ | ? |
| Delayed umbilical cord separation | +++ | + | |
| Developmental abnormalities | +++ | ||
| Bleeding tendencies | +++ | ||
| Neutrophilia : baseline | + | +++ | ++ |
| Neutrophilia : with infection | +++ | +++ | +++ |
| Genetic defect | CD18 subunit of β2 integrins (ITGB2) | Fucosylation of selectin ligands (eg, SLeX) because of deficiency in a fucose transporter (SLC35C1) | Inside-out signaling involving kindlin-3 for activation of blood cell β integrins (FERMT3) |
LAD-1 is associated with an aggressive periodontitis, which was historically attributed to impaired neutrophil surveillance of periodontal tissue. However, new studies in LAD-1 patients and mouse models identified a dysregulated IL-23/IL-17 inflammatory axis as the critical mediator of periodontal destruction, rather than bacterial load.51 This in part appears to reflect increased production of IL-23 and downstream IL-17 and G-CSF as a result of a defective “neutrostat” because senescent neutrophils emigrating into tissues are reduced in LAD-1. Moreover, compared with healthy individuals and those with aggressive periodontitis not associated with LAD-1, the subgingival microbiome of LAD-1 patients has a unique composition, with microbial products that triggered IL-23-related inflammation.52
CARD9 deficiency
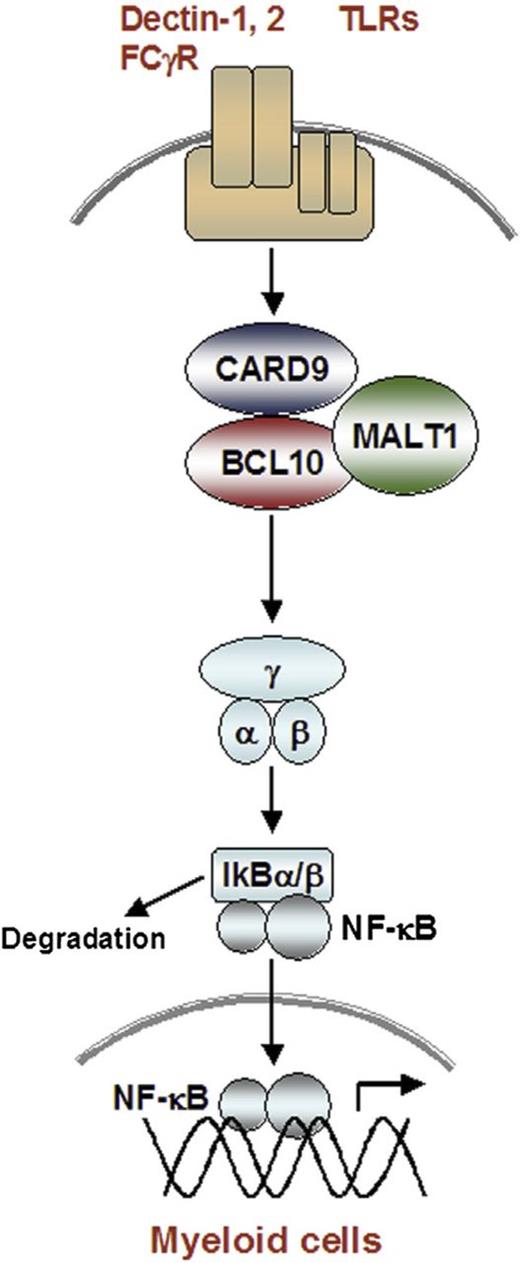
Autosomal recessive defects in CARD9 were first described in 2009,53 and 46 patients are now reported.54,55 All exhibit a selective susceptibility to fungal diseases, including central nervous system or digestive tract infections with Candida species; invasive Exophiala species infection; superficial, extensive, or deep dermatophytosis; and mucocutaneous candidiasis. Pulmonary infections have not been reported. Some patients present early in life, whereas others do not develop a serious fungal infection until adulthood. CARD9 is a signaling molecule expressed in myeloid cells, which acts downstream of C-type lectin receptors and Toll-like receptors that recognize fungal cell wall components, including the Dectin-1 receptor for β-glucan. CARD9 forms a trimeric complex with B-cell lymphoma 10 and mucosa-associated lymphoid tissue lymphoma translocation gene 1 (Figure 3) to activate NF-ΚΒ, leading to pro-inflammatory gene expression.54,56 Both granulocyte-macrophage colony-stimulating factor and granulocyte-colony-stimulating factor have been used as adjunctive therapy to antifungal antibiotics for fungal meningoencephalitis.54,55
Signaling through the CARD9-containing caspase recruitment domain–B-cell lymphoma 10–mucosa-associated lymphoid tissue lymphoma–translocation gene 1 complex. In myeloid cells, activation of C-type lectin receptors for Dectin1 and Dectin2, as well as Toll-like receptors, leads to phosphorylation of the CARD9 protein and formation of a complex with B-cell lymphoma 10 and mucosa-associated lymphoid tissue lymphoma translocation gene 1. This CARD9-containing caspase recruitment domain–B-cell lymphoma 10–mucosa-associated lymphoid tissue lymphoma–translocation gene 1 complex is necessary to activate NF-ΚΒ and pro-inflammatory gene expression downstream of C-type lectin receptors. From Pérez de Diego et al,54 with permission.
Signaling through the CARD9-containing caspase recruitment domain–B-cell lymphoma 10–mucosa-associated lymphoid tissue lymphoma–translocation gene 1 complex. In myeloid cells, activation of C-type lectin receptors for Dectin1 and Dectin2, as well as Toll-like receptors, leads to phosphorylation of the CARD9 protein and formation of a complex with B-cell lymphoma 10 and mucosa-associated lymphoid tissue lymphoma translocation gene 1. This CARD9-containing caspase recruitment domain–B-cell lymphoma 10–mucosa-associated lymphoid tissue lymphoma–translocation gene 1 complex is necessary to activate NF-ΚΒ and pro-inflammatory gene expression downstream of C-type lectin receptors. From Pérez de Diego et al,54 with permission.
Mechanisms underlying the susceptibility of CARD9-deficient patients to fungal diseases are not fully understood. Production of neutrophil chemoattractants and pro-inflammatory cytokines by CARD9-deficient patient leukocytes stimulated in vitro with fungal cell wall is impaired. In Candida central nervous system infection, CARD9 appears to be required for recruitment of neutrophils, according to studies of CARD9-deficient patients and Card9−/− mice.55,57 In mice, CARD9-dependent production of neutrophil chemoattractants by neutrophils themselves was shown to be critical for their sustained recruitment in CNS candida infection.57 CARD9-deficient neutrophils also have impaired in vitro killing of unopsonized Candida spores after their ingestion, with outgrowth of hyphae.57,58 The mechanism is unknown, but may be related to defective phagosome maturation in CARD9-deficient neutrophils.58
Concluding remarks
Inherited disorders affecting neutrophil function provide unique insights into cellular pathways important for effective control of bacterial and fungal pathogens. Each has distinctive clinical features that can guide the diagnostic evaluation of patients who present with recurrent and/ or unusual infections. CGD and LAD I are readily diagnosed by flow cytometry assays for oxidative burst activity (dihydrorhodamine 123 assay) or expression of CD11b/CD18 (Mac1), respectively.1 These are available in clinical reference laboratories, or sometimes locally. Other forms of leukocyte adhesion deficiency and CARD9 deficiency require specialized laboratory studies.1 In the future, early use of gene sequencing will likely be incorporated into the diagnostic approach for patients strongly suspected to have an inherited neutrophil defect.
Correspondence
Mary C. Dinauer, Department of Pediatrics, Washington University School of Medicine in St. Louis, 660 S. Euclid Ave., Campus Box 8208, St. Louis, MO 63110; e-mail: dinauer_m@kids.wustl.edu.
References
Competing Interests
Conflict-of-interest disclosures: The author declares no competing financial interests.
Author notes
Off-label drug use: None disclosed.