Abstract
Acute myeloid leukemia (AML) is a disease of the elderly, but less than half of these patients are offered therapy despite the evidence of better survival with treatment in this patient population. Assessing fit, vulnerable, and frail older adults with AML remains a challenge for the treating oncologist. A majority of AML patients are elderly and often have significant comorbidities, lack of social support, and older caregivers. Performance status (PS), a subjective measure of how a patient will tolerate cancer chemotherapy, has been strongly correlated with mortality in older AML patients. However, a large portion of older adults have poor PS as a result of their underlying AML, and these patients may end up being undertreated. Conversely, some patients with excellent PS unexpectedly end up with excessive toxicity and mortality. The treating physician thus needs a more objective and comprehensive method to differentiate patients along the fit-frail spectrum irrespective of their chronological age. For more than a decade, comprehensive geriatric assessment has been shown to improve routine oncology assessment by adding information about the functional, emotional, cognitive, and social status of older patients with cancer. In addition to the chronological and functional age, there is an attempt to quantify a patient’s biological age to aid in better decision making. This chapter attempts to review the clinical challenges of AML treatment in the elderly population and to highlight the current literature and future research required to be able to assess fitness and maximize therapeutic options in this heterogeneous patient population.
Learning Objectives
To review methods for assessing patient fitness by using available evidence that may guide the treatment of older patients with AML
To recognize the importance of an individualized treatment approach outside of simple chronological age, along with the importance of clinical trials in this demographic accounting for the heterogeneity of both tumor biology and patient characteristics
Introduction
Acute myeloid leukemia (AML), the most common acute leukemia, is a disease of older adults, presenting at a median age of 68 years. Older patients are generally undertreated, and although selected older adults may benefit from intensive therapies, as a group, they experience increased treatment-related mortality and morbidity, have lower complete remission (CR) rates, are more likely to relapse, and have decreased survival.1 Age-related outcome disparities are attributed to both tumor and host characteristics, requiring an individualized approach to treatment decision making beyond consideration of chronological age alone. Patients can be matched with the right therapy with the help of both disease-specific risk factors (eg, karyotype and genetic mutations) and estimates of treatment tolerance and life expectancy derived from evaluation of functional status and comorbidity.2
Patient profiles, treatment patterns, and outcomes among elderly AML patients
The most recent big data analysis consisted of a retrospective cohort analysis of primary AML patients older than age 66 years in the Surveillance, Epidemiology, and End Results (SEER) Medicare database from 2000 to 2009.3 In all, 3327 patients (40%) received chemotherapy within 3 months of diagnosis. The patients who received therapy were more likely to be younger, male, and married and were less likely to have secondary AML and poor performance indicators and comorbidity score compared with their untreated counterparts. In multivariate analysis (MVA), the 30-day mortality was 33% lower for the treated (9%) compared with the untreated (31%) group. Both intensive and hypomethylating agent (HMA) therapies compared with no therapy led to a significant survival benefit. In addition, younger Medicare patients were noted to have a survival benefit with allogeneic hematopoietic stem cell transplantation (HSCT). That study confirmed what has been known in the literature for at least the last 2 decades regarding AML in the elderly: that despite a known survival benefit with antileukemic therapy, about 60% of elderly AML patients remain untreated after diagnosis. However, treatment rates increased over the time period of the study from 35% in 2000 to 50% in 2009. Another similar analysis of treatment patterns, survival, and costs in elderly patients with primary AML used SEER data between 1997 and 2007.4 In that study, 43% of the 4058 patients received chemotherapy, and 57% received supportive care only. Among patients who received chemotherapy, 69% died within 1 year, and median survival was 7.0 months. Among patients who received supportive care only, 95.0% died within 1 year, and median survival was 1.5 months. Patient age and Charlson Comorbidity Index (CCI) score were directly proportional to receipt of chemotherapy and mortality. The mean all-cause health care cost per older AML patient was $96 078, the largest component of which was utilization of inpatient care and services (76.3%).
Another single-center study compared the direct costs of decitabine and conventional induction therapy in patients with AML (age older than 60 years) by using a semi-Markov model that compiled survival and cost data.5 The estimated cost of a direct hospital stay of 1 day was ∼$2100, and the cost of an infusion clinic visit was ∼$524. The cost-effectiveness was assessed by using an incremental cost-effectiveness ratio. The expected cost was nearly the same in both groups, with $88 325 for patients receiving cytarabine plus daunorubicin vs $91 312 for patients who received decitabine alone. The incremental cost-effectiveness ratio per quality-adjusted life year with decitabine was $38 839. It is important to note that the data accounted for re-induction therapy with idarubicin, fludarabine, cytarabine, and granulocyte colony-stimulating factor and consolidation therapy with 4 cycles of high-dose cytarabine.
Quality of life in elderly AML patients
Quality of life (QOL) is another very important outcome for the older AML patient, and it has been addressed in several studies. One study investigated the association between baseline QOL and physical function (PF) with short-term treatment outcomes in 239 adult and elderly AML patients treated with intensive chemotherapy (IC).6 Sixty-day mortality, intensive care unit admission, and CR occurred in 9 (3.7%), 15 (6.3%), and 167 (69.9%) patients, respectively. QOL and PF at presentation were not predictive of 60-day mortality, intensive care unit admissions, or CR rates. Findings were similar when patients age 60 years or older were examined, thus suggesting that QOL at presentation in an elderly AML patient is likely a result of the disease itself and may not reflect the prediagnosis QOL. Another study from the same institution recruited 237 patients (97 were older than age 60 years) to study whether survivors of AML in remission after IC achieved significant improvements in QOL, fatigue, and PF over time.7 One-year survival was 60% in older patients, and for patients in remission, global QOL, fatigue, certain physical performance measures, and daily function improved significantly over time. Clearly, choosing the right older AML patient for therapy on the basis of disease biology and host-related factors is becoming more important and may lead to better patient outcomes along with a decrease in health care–related costs.
Value of performance status in AML
Performance status (PS) tools, such as Karnofsky performance status (KPS) or Eastern Cooperative Oncologic Group (ECOG) PS, are central to cancer care and to attempts to quantify the general well-being of patients with cancer to determine whether they can receive chemotherapy safely. Good PS is defined as a KPS ≥80% or an ECOG PS <2. In AML, age at diagnosis and ECOG PS correlate with 30-day induction mortality as demonstrated in a now landmark trial by the Southwest Oncology Group.8 In that study (n = 968), patients with ECOG PS of 0 had a 30-day mortality of 11% to 15% irrespective of age (ie, 56 to 65 years or older than 75 years). However, the mortality rate was 50% in patients older than age 75 years with ECOG PS 2 and was 82% for those with ECOG PS 3. Assessing PS is extremely subjective, and it is not always synonymous with functional status. Functional status is defined as the level of autonomy individuals have in their daily activities. The Activity Daily Living (ADL) scale and Instrumental Activity of Daily Living (IADL) scale are complementary tools that have been widely validated to measure functional status. In 1 small study of elderly AML patients (n = 63), it was demonstrated that impairment according to the IADL scale was the single most predictive variable for median survival, even higher than age and unfavorable cytogenetics.9 Thus, measuring function according to the IADL scale adds information to the KPS and may aid in identifying vulnerable patients.
In addition to PS, another important factor that affects treatment decision making in older patients is comorbidity. Comorbidity is typically measured by using standardized indices to assess burden and severity of diseases; the most commonly used indices are the CCI and the Hematopoietic Cell Transplantation Comorbidity Index (HCT-CI).10,11 One study that used SEER claims that data from 5480 AML patients (median age, 78 years) demonstrated that although half of all patients had a favorable CCI score of 0, the score progressively worsened with age.12 Patients age 65 to 69 years with a CCI score of 0 received leukemia therapy more than 3 times more often than patients age 80 years or older with the same CCI score. A recent single-institution study analyzed AML patients receiving IC between 2002 and 2009: 144 patients age 60 years or older and 133 patients younger than age 60 years.13 Older patients had a worse survival and a higher comorbidity burden (CCI score ≥1, 58% vs 26%; P < .001). Prevalent comorbid conditions differed by age (diabetes, 19.2% vs 7.5%; cardiovascular disease, 12.5% vs 4.5% for older vs younger patients, respectively). The CCI was not independently associated with overall survival (OS) or 30-day mortality in either age group. Interestingly, among older patients, diabetes was associated with higher 30-day mortality (33.3% vs 12.0% in diabetic vs nondiabetic patients; P = .006). After, controlling for age, cytogenetic, and other comorbidities, the presence of diabetes increased the odds of 30-day mortality by 4.9 times in older patients receiving IC.
The HCT-CI is used in the transplant setting and has been studied in AML patients.11 In 1 study of 177 patients age 60 years or older who received induction chemotherapy, the HCT-CI scores of 0, 1 to 2, and ≥3 corresponded to early death rates of 3%, 11%, and 29%, respectively and OS of 45, 31, and 19 weeks, respectively.14 In another study of 92 AML patients age 80 years or older who had AML, 64% were treated intensively with a variety of regimens.15 In that population of very elderly AML patients, the CCI and the HCT-CI had similar predictive ability for outcome in both groups. Thus, screening for comorbidities should be considered routine clinical practice and should be included in clinical trials as part of the inclusion and exclusion criteria. Integration of PS and comorbidity with other host-related factors may provide a better understanding of the AML patient as a whole and help individualize therapy for this patient population.
Prognostic models and risk assessment
Clinical trial data have been used to create algorithms to improve risk stratification of elderly AML patients. A variety of prognostic models have provided a wide range of estimates for early mortality (16% to 71%), CR (12% to 91%), and 3-year survival (3% to 40%) in older AML patients who receive intensive induction therapy.16-19 One model for predicting OS identified age, karyotype, NPM1 mutational status, white blood cell count, lactate dehydrogenase levels, and CD4 expression as risk factors, and it categorized patients into 4 groups, with 3-year OS ranging from 3% to 40%.16 Another model derived from >1000 intensively treated patients identified cytogenetic risk group, white blood cell count, secondary AML, PS, and age as predictors of OS.17 One Web-based model predicting remission rates and induction mortality used clinical and laboratory variables (body temperature, age, hemoglobin, platelet count, secondary leukemia or antecedent hematologic disease, fibrinogen, and lactate dehydrogenase) and predicted CR rates ranging from 12% to 91%.18 To predict 8-week induction mortality for patients age 70 years or older, another model included age 80 years or older, complex cytogenetics, ECOG PS >1, and creatinine >1.3 mg/dL.19 Patients with risk factors of 0, 1, 2, and ≥3 had 8-week mortality rates of 16%, 31%, 55%, and 71%, respectively. Although each of these models provides the treating oncologist with valuable information and aids decision making, they all rely on tumor characteristics and chronological age. None of these models incorporate the functional, cognitive, or psychosocial factors that are integral to aging. In addition, none of these models are designed to address end points that perhaps are relevant to older AML patients: QOL, days of hospitalization, PF, and rehabilitation or long-term care requirements.
Comprehensive geriatric assessment: the case for the obvious
Comprehensive geriatric assessment (CGA) is a tool used by geriatricians to assess functional status, comorbidity, cognition, social support system, nutrition, and medication use.20 Results from the CGA can help oncologists predict outcomes and select appropriate therapy for their patients. CGA can also help identify and follow up on symptoms in older patients that can affect QOL. The first studies of CGA in older patients with cancer have been around for more than a decade.20,21 Each domain within the CGA has been shown to predict morbidity and mortality in community-dwelling older adults (Table 1). Dependence for ADLs and IADLs is predictive of mortality in geriatric oncology, and the incidence of ADL and IADL deficiencies is higher for older patients with cancer than for age-matched controls. The geriatrics literature supports the use of a directly observed assessment of PF to assess the risk of falls and identify vulnerability in older patients who may otherwise seem fit.22,23 Other geriatric issues like polypharmacy, weight loss, cognitive disorders, depression, and social isolation can increase the risk of adverse events from chemotherapy in the older patient with cancer.
Domains for cancer-specific comprehensive geriatric assessment
| Domain with measure . | No. of items . | Description . |
|---|---|---|
| Functional status | ||
| MOS physical health | 10 | Measures limitations in a wide range of physical functions from bathing and dressing to vigorous activities such as running |
| Instrumental Activities of Daily Living (subscale of the OARS) | 7 | Measures ability to complete activities required to maintain independence in the community (ie, preparing meals, shopping, making telephone calls, managing money) |
| Karnofsky performance status (rated by the health care professional) | 1 | Global indicator of patient function determined by the health care professional on a scale of 0 to 100 |
| No. of falls in the last 6 months | 1 | No. of times patient has fallen in the last 6 months |
| Timed up and go | 1 | Performance-based measure of functional status: amount of time it takes for seated patient to rise from a chair, walk 10 feet, walk back, and sit down |
| MOS social activities | 4 | Measures ability to participate in social activities and degree to which health status limits normal social activities |
| Comorbid medical conditions | ||
| Physical health section (subscale of the OARS) | 15 | List of comorbid illnesses and the degree to which they impair daily activities; patient can add additional comorbid illnesses not listed; rating of eyesight and hearing |
| Psychological state | ||
| Hospital Anxiety and Depression Scale | 14 | Measures of anxiety and depression |
| Social support | ||
| MOS social support survey: emotional information and tangible subscales | 12 | Perceived availability of social support |
| Nutritional status | ||
| Body mass index | 1 | Weight and height |
| Percent unintentional weight loss in the past 6 months | 1 | Unintentional weight loss in last 6 months/baseline body weight × 100 |
| Cognition | ||
| Blessed Orientation-Memory-Concentration test | 6 | Gross measure of cognitive function |
| Medications | ||
| Comprehensive list of medications | 1 | List of medications including prescribed, herbal, and over-the-counter |
| Domain with measure . | No. of items . | Description . |
|---|---|---|
| Functional status | ||
| MOS physical health | 10 | Measures limitations in a wide range of physical functions from bathing and dressing to vigorous activities such as running |
| Instrumental Activities of Daily Living (subscale of the OARS) | 7 | Measures ability to complete activities required to maintain independence in the community (ie, preparing meals, shopping, making telephone calls, managing money) |
| Karnofsky performance status (rated by the health care professional) | 1 | Global indicator of patient function determined by the health care professional on a scale of 0 to 100 |
| No. of falls in the last 6 months | 1 | No. of times patient has fallen in the last 6 months |
| Timed up and go | 1 | Performance-based measure of functional status: amount of time it takes for seated patient to rise from a chair, walk 10 feet, walk back, and sit down |
| MOS social activities | 4 | Measures ability to participate in social activities and degree to which health status limits normal social activities |
| Comorbid medical conditions | ||
| Physical health section (subscale of the OARS) | 15 | List of comorbid illnesses and the degree to which they impair daily activities; patient can add additional comorbid illnesses not listed; rating of eyesight and hearing |
| Psychological state | ||
| Hospital Anxiety and Depression Scale | 14 | Measures of anxiety and depression |
| Social support | ||
| MOS social support survey: emotional information and tangible subscales | 12 | Perceived availability of social support |
| Nutritional status | ||
| Body mass index | 1 | Weight and height |
| Percent unintentional weight loss in the past 6 months | 1 | Unintentional weight loss in last 6 months/baseline body weight × 100 |
| Cognition | ||
| Blessed Orientation-Memory-Concentration test | 6 | Gross measure of cognitive function |
| Medications | ||
| Comprehensive list of medications | 1 | List of medications including prescribed, herbal, and over-the-counter |
MOS, Medical Outcomes Study; OARS, Older Americans Resources and Services [Program].
A recent systematic review evaluated the value of geriatric assessment (GA) in elderly patients with hematologic malignancies in 15 studies; 3 of those studies were in AML patients.24 The median age of patients was 73 years, and despite generally good PS, the prevalence of geriatric impairments was high. Geriatric impairments were noted in IADLs in 55%, nutritional status in 67%, cognitive capacities in 83%, and objectively measured physical capacity in 100% of patients, all associated with a shorter OS in a relevant proportion of studies. Comorbidity, physical capacity, and nutritional status were more significantly and frequently predictive of toxicity and mortality than were age and PS.
Table 2 summarizes 3 studies of CGA in older patients with AML.25-27 One multi-institution study (n = 195; median age, 71 years) assessed patients age 60 years or older with myelodysplastic syndrome and AML who were grouped according to treatment intensity (nonintensive, n = 107; IC/HSCT, n = 75) and who underwent GA.25 GA consisted of 8 instruments that evaluated ADLs, depression, mental functioning, mobility, comorbidities, KPS, and QOL. Patients who received IC/HSCT were younger and significantly less often affected by geriatric symptoms. To focus on a homogenous cohort and to avoid the confounding effect of treatment, the authors conducted the GA only in patients who were not treated intensively (n = 107). After MVA, a prognostic model was created that included disease-related factors such as poor risk cytogenetics and bone marrow blasts along with GA/QOL factors such as KPS, ADL, and fatigue; and it was noted that these factors were independently associated with OS in patients who received nonintensive therapy. Three GA variables (KPS, ADL, and fatigue) were used to create a risk score that correlated with OS. Patients who were low risk (0 risk features), intermediate risk (1 to 2 features), or high risk (all 3 features) had a median OS of 774, 231, or 51 days, respectively (P < .001). Another study in 101 patients age 65 years or older with newly diagnosed AML explored whether GA variables in addition to known prognostic factors predicted mortality.26 Baseline comorbidity score, difficulty with strenuous activity, and pain were independent prognostic factors for greater risk of death after MVA that included the cytogenetic risk group. They remained independent predictors, even in the subset of patients with baseline ECOG PS 0 to 1, confirming the notion that GA adds value to traditional PS measurement.
Studies in older AML adults using CGA
| Reference . | Disease and no. of patients . | Therapy . | Variables . | CGA variables . | Outcomes: impact on OS . | ||||
|---|---|---|---|---|---|---|---|---|---|
| Clinical . | Disease . | Treatment . | Risk groups . | OS . | P . | ||||
| 25 | MDS (n = 63) AML (n = 132) | Non-IC* (n = 120); IC (n = 65) | BM blast >20%, cytogenetics, HCT-CI ≥3 | KPS <80, impaired ADLs (Barthel index >100), high fatigue score (EORTC QLQ-C30 ≥50) | Low risk (0 risk features) | 744 d | |||
| Intermediate risk (1 to 2 risk features) | 231 d | ||||||||
| High risk (3 risk features) | 51 d | <.001 | |||||||
| 28 | AML (n = 74) | IC | Hemoglobin, age, sex, ECOG PS, cytogenetics | Prior MDS | Cognitive impairment (CI) (Modified MMSE < 77), impaired PF (SPPB <9) | CI vs no CI | 5.2 vs 15.6 mo | .002 | |
| Impaired PF vs not impaired PF | 6 vs 16.8 mo | .018 | |||||||
| 26 | AML (n = 101) | Non-IC* (n= 65); IC (n = 35) | Age >72 y, ECOG PS >1, cytogenetics, HCT-CI >1 | Secondary AML | CR; allogeneic HSCT | Strenuous activity difficulty, pain (more often vs less often) | Less vs more difficulty with strenuous activity | 11.8 vs 4.4 mo | <.001 |
| Less vs more pain | 1.3 vs 4.1 mo | <.002 | |||||||
| HCT-CI score ≤1 vs >1 | 11.8 vs 4.4 mo | <.001 | |||||||
| Reference . | Disease and no. of patients . | Therapy . | Variables . | CGA variables . | Outcomes: impact on OS . | ||||
|---|---|---|---|---|---|---|---|---|---|
| Clinical . | Disease . | Treatment . | Risk groups . | OS . | P . | ||||
| 25 | MDS (n = 63) AML (n = 132) | Non-IC* (n = 120); IC (n = 65) | BM blast >20%, cytogenetics, HCT-CI ≥3 | KPS <80, impaired ADLs (Barthel index >100), high fatigue score (EORTC QLQ-C30 ≥50) | Low risk (0 risk features) | 744 d | |||
| Intermediate risk (1 to 2 risk features) | 231 d | ||||||||
| High risk (3 risk features) | 51 d | <.001 | |||||||
| 28 | AML (n = 74) | IC | Hemoglobin, age, sex, ECOG PS, cytogenetics | Prior MDS | Cognitive impairment (CI) (Modified MMSE < 77), impaired PF (SPPB <9) | CI vs no CI | 5.2 vs 15.6 mo | .002 | |
| Impaired PF vs not impaired PF | 6 vs 16.8 mo | .018 | |||||||
| 26 | AML (n = 101) | Non-IC* (n= 65); IC (n = 35) | Age >72 y, ECOG PS >1, cytogenetics, HCT-CI >1 | Secondary AML | CR; allogeneic HSCT | Strenuous activity difficulty, pain (more often vs less often) | Less vs more difficulty with strenuous activity | 11.8 vs 4.4 mo | <.001 |
| Less vs more pain | 1.3 vs 4.1 mo | <.002 | |||||||
| HCT-CI score ≤1 vs >1 | 11.8 vs 4.4 mo | <.001 | |||||||
BM, bone marrow; EORTC QLQ-C30, European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire-C30; MDS, myelodysplastic syndrome; MMSE, Mini-Mental State Examination.
Non-IC includes best supportive care, HMAs such as decitabine and azacitidine, and other agents such as single-agent 6-mercaptopurine.
Is GA feasible in the inpatient setting? This question was answered by a small pilot study in 54 AML patients age 60 years or older who were receiving induction chemotherapy.27 Ninety-three percent of this cohort completed the entire GA battery (ie, the modified Mini-Mental State Examination, Center for Epidemiologic Studies Depression Scale, Distress Thermometer, Pepper Assessment Tool for Disability [self- reported ADLs], instrumental ADLs, mobility questions, the Short Physical Performance Battery [SPPB, which includes timed 4-minute walk, chair stands, standing balance], grip strength, and HCT-CI) in a mean time of 44 ± 14 minutes. Interestingly, in this study, for the 38 patients who rated their PS as good (ie, ECOG ≤1), impairments in individual GA measures ranged from 23.7% to 50%. This pilot exploratory study highlighted two poignant issues. First, patients with more aggressive tumor biology had more depressive symptoms and poorer self-reported PF, which may reflect the emotional and physical consequences of rapidly progressive symptoms. Second, a broad range of cognitive, psychological, and physical function was found despite stratification according to cytogenetic risk group. The same authors further evaluated the predictive value of GA on OS in older AML patients (n = 74; median age, 70 years) admitted to the inpatient service for IC.28 Pretreatment GA included evaluation of cognition, depression, distress, PF (self-reported and objectively measured), and comorbidity. Objective PF was assessed using the SPPB (timed 4-minute walk, chair stands, standing balance) and grip strength. OS was significantly shorter for participants who screened positive for impairment in cognition and objectively measured PF after adjusting for several other patient and disease-related factors. In addition, the study highlighted the importance of GA and noted that SPPB was a better predictor of treatment outcome than recalled functional status, which supports the investigation of interventions to target physical vulnerability.
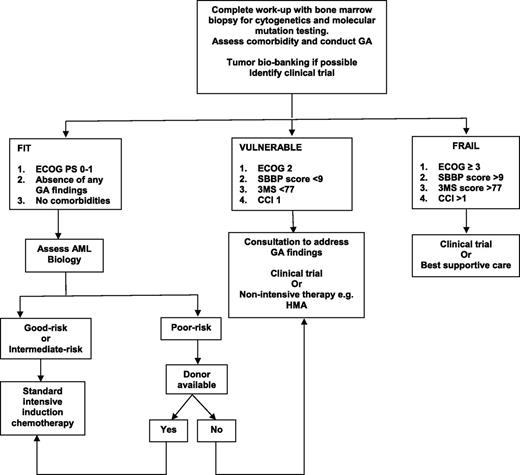
Despite all this emerging data, there is no firm recommendation regarding which GA should be incorporated into clinical practice, at what time points during the treatment trajectory GA should be performed, and how to use that information to have a positive impact on the patient. Although GA can be conducted by a nurse or physician extender, it is time-consuming and requires additional personnel to perform. Will consultations based on GA findings have an impact on survival and QOL? If elderly AML patients who have poor PF are referred to physical therapy, or patients with underlying depression are offered psychotherapy, or patients with weight loss are referred to a nutritionist, will these recommendations lead to better outcome and lower mortality? These are all important questions that need to be addressed in future clinical trials. At this time, there is no evidence from a formal randomized controlled trial that compares induction therapy with IC to an HMA in the elderly patient with AML. In addition, there is no consensus on the role, choice, or value of postremission therapy in this patient population. Figure 1 provides a general algorithm on how the treating oncologist may combine measures of GA and AML biology28 to aid in treatment decision making, but readers should be cautioned to make thoughtful choices for each elderly AML patient they encounter.
Proposed algorithm for treating the elderly AML patient. 3MS, 100-point Modified Mental State Examination.
Proposed algorithm for treating the elderly AML patient. 3MS, 100-point Modified Mental State Examination.
Biomarkers of fitness: evaluating the physiologic reserve
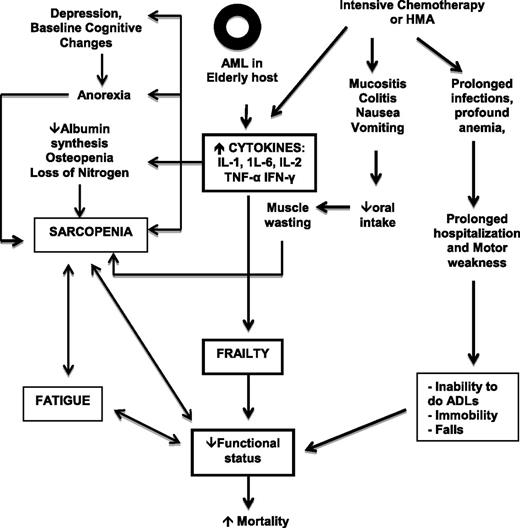
The elderly AML patient who commits to IC, HMA, or allogeneic HSCT has several reasons for a progressive decline in functional reserve that may lead to significant morbidity and mortality. Figure 2 illustrates how the interaction between the older host, underlying AML, and treatment effects can lead to loss of homeostasis and mortality. CGA can provide information on the general health status of individuals but is far from perfect as a prognostic or predictive tool for individual patients. Are there genomic and proteomic markers of aging that can help predict fitness in an elderly AML patient?29 It is well known that aging is associated with numerous events at the molecular, cellular, and physiological levels that increase susceptibility to carcinogens, promote carcinogenesis, and decrease protective mechanisms.30 In addition, cellular senescence, loss of function of tumor suppressor genes, and excessive exposure of the cell to oxidative stress can also lead to a similar response to that observed with replicative exhaustion, resulting in a permanently growth-arrested senescent status.31
Interaction between host, disease, and treatment-related factors. IFN-γ, interferon gamma.
Interaction between host, disease, and treatment-related factors. IFN-γ, interferon gamma.
Telomere length and telomerase activity, gene expression of aging related genes, and plasma microRNA expression are all potential biomarkers of aging.32 Lymphocyte senescence,33 and thus aging of the immune system, is reflected by increased messenger RNA expression of the cell cycle regulator p16INK4a. In addition, “inflammaging” (ie, inflammation in the process of aging) has been well studied in AML, and activation of pro-inflammatory cytokines (eg, interleukin-6 [IL-6], tumor necrosis factor α [TNF-α], and IL-8) and chemokines can alter the disease course, affect the bone marrow microenvironment, and play a deleterious role in the progression of AML.34 The geriatric host is particularly vulnerable as these cytokines and chemokines are also implicated in the development of frailty, fatigue, and declining cognitive function. One study has examined the relationship between circulating cytokines and cancer-related fatigue in 74 older AML patients before and after the first cycle of induction chemotherapy.35 Plasma levels of 13 cytokines were measured via electrochemiluminescence. At baseline, clinically significant correlations were seen between TNF-α and fatigue. Over time, correlations with fatigue were noted with TNF-α, and the chemokine interferon-inducible protein 10 (IP-10).
The European Organisation for Research and Treatment of Cancer (EORTC) Elderly Task Force (ETF) has initiated an aging biomarker program. Biological material will be collected in patients participating in EORTC-ETF clinical trials. Candidate biomarkers cover different aspects of the biology of aging and include leukocyte telomere length, p16INK4a expression in T lymphocytes, immunosenescence markers, oxidative stress markers, circulating inflammatory mediators, genetic variability in aging- and longevity-related genes, and microRNA expression. The purpose of this program is to evaluate the feasibility of this approach and validate the ability of this panel of candidate aging biomarkers to determine a person’s biological age, provide important information on life expectancy, and determine the reserve capacities of patients as well as their chance of tolerating therapy or the risk of suffering severe toxicity.
Future directions: exercise as an intervention and the concept of biological age
It is evident that older AML patients benefit from therapy, be it IC, HMA, or even an allogeneic HSCT. How we assess fitness for the type of therapy prescribed is paramount. CGA, a time-tested measure adopted from geriatricians, clearly helps predict for OS in older patients with AML by identifying vulnerable patients as elucidated in the studies by Klepin et al.28 There are several mechanisms by which impaired physical performance may lead to worse survival in AML and these include long periods of therapy leading to inactivity and immobility, increased risk of infectious complications, falls, and accelerated deconditioning that prohibits delivery of consolidation therapy needed for cure. The advice to rest and avoid intensive exercises is still common practice and is given, partly because of severe anemia and thrombocytopenia in this patient population. Exercise during and after chemotherapy could decrease risks associated with low physical performance at presentation and potentially improve outcomes. A Cochrane Database Systematic Review of aerobic exercise in adults with hematologic malignancies included 9 randomized controlled trials that involved 818 participants.36 There were a fair number of AML studies, and the authors concluded that there is no evidence for differences in mortality between the exercise and control groups. Physical exercise added to standard care can improve QOL, especially physical functioning, depression, and fatigue. Currently, the evidence is inconclusive regarding anxiety, physical performance, adverse events, and serious adverse events. In the AML population, there have been a few small trials. One small pilot study of 40 patients age 40 years or older concluded that a home-based exercise program for posttreatment AML patients can be safely delivered with reasonable recruitment and high retention.37 However, feasibility was hampered by low adherence. Another study enrolled 24 older adults with a mean age of 65 years who were hospitalized for AML chemotherapy.38 Patients were enrolled in a 4-week exercise intervention that included stretching, walking, and strength exercises. Feasibility measures included recruitment, retention, and number of exercise sessions. Unlike the home-based exercise program, this inpatient exercise program demonstrated that 87.5% of participants completed baseline measures, 70.8% attended ≥1 exercise sessions, and 50.0% completed postintervention assessment. Among baseline characteristics, only higher physical performance was associated with a greater number of exercise sessions attended, and postintervention QOL and depressive symptoms improved as a result of the exercise.
CGA in older AML patients leads to the discovery of a broad range of cognitive and psychological issues, and prior knowledge of this may have an impact on issues such as understanding and signing an informed consent for a clinical trial, timing and use of steroids as premedication, aiding caregivers, and the proactive use of psychotherapy and antidepressants. One study has demonstrated the utility of a clinico-genomic assessment of PS.39 The authors used clinically annotated microarray data (messenger RNA expression from marrow leukemic blasts) from 2 data sets with 377 AML patients (GSE1159 and GSE12417). A frailty profile was developed by using a set of previously characterized gene sets and pathways that define the following cytokines: IL-6, IL-1, IL-2, TNF-α, and C-reactive protein. The frailty profile was then queried in the 377 AML patients and by unsupervised clustering methods, two cohorts (n = 58) that represented extremes of survival (cohort 1: median survival, 4.9 months; cohort 2: median survival, 46.3 months; P < .001) were identified. OS for these 58 AML patients, based on clinical ECOG PS only, was not statistically significant (14 months for ECOG PS 0 to 1 and 8.8 months for ECOG PS 2 or greater; P = .29). The authors further stratified these patients into 3 risk groups: low, intermediate, and high risk depending on a cohort based on its frailty profile and ECOG PS. This combined analysis revealed that low-risk patients (cohort 2 plus ECOG PS 0 to 1) had a statistically significantly higher survival of 56.1 months compared with intermediate-risk (9.85 months) and high-risk (8.35 months) patients; P = .02.6 This suggests that gene expression for cytokines from leukemic blasts does add information to a subjective clinical parameter such as the ECOG PS.
Conclusions
AML is a very heterogeneous malignancy and although the response rates to chemotherapy have increased over the past 3 decades, this improvement has been limited to patients age 65 to 74 years. Survival rates have not improved in patients age 75 years or older, and in the oldest old (the fastest growing segment of the US population [ie, patients age 85 years or older]), the survival rates are the lowest with no improvement over time.40 Because the population is aging, we expect an increase in the incidence of AML. We hope that we can move past using only chronological age and instead use functional age and biological age to tailor the right therapy for each AML patient. In the future, clinical trials in elderly AML patients should perhaps be geriatricized to account for the heterogeneity of AML biology and the aging patient, and in addition, end points for trials should include outcomes addressing QOL, maintenance of independence, and use of health care services.
Correspondence
Arati V. Rao, Gilead Sciences, Inc., 333 Lakeside Dr, Foster City, CA 94404; e-mail: arati.rao@gilead.com.
References
Competing Interests
Conflict-of-interest disclosure: The author is an employee of and has equity ownership in Gilead Sciences.
Author notes
Off-label drug use: None disclosed.