Abstract
Long-term survivors of Hodgkin lymphoma (HL) experience several late adverse effects of treatment, with second malignant neoplasms (SMNs) and cardiovascular diseases (CVDs) being the leading causes of death in these patients. Other late effects have also been identified, such as pulmonary dysfunction, endocrinopathies (thyroid dysfunction, infertility), neck muscle atrophy, and persistent fatigue. HL survivors have two- to fourfold increased risks to develop SMNs and CVD compared with the general population. With respect to SMNs, radiotherapy is associated with 1.5- to 15-fold increased risk of solid malignancies. The relative risk (RR) of solid tumors increases steadily with increasing follow-up time from 5 to 15 years since radiotherapy, and remains elevated for at least 40 years. The RR of solid SMNs increases strongly with younger age at first treatment. Risks of lung, breast, and gastrointestinal (GI) cancers increase with higher radiation dose. Alkylating agent chemotherapy, especially procarbazine, does not only increase risk of leukemia but also of solid malignancies, in particular, cancers of the lung and GI tract. In contrast, gonadotoxic chemotherapy decreases the risk of radiation-associated breast cancer, through induction of premature menopause. Smoking appears to multiply the radiation- and chemotherapy-associated risks of lung cancer. Both radiotherapy and chemotherapy for HL may cause cardiovascular toxicity. Radiotherapy increases the risk of coronary heart disease, valvular heart disease, congestive heart failure (HF), and pericarditis, whereas anthracycline-containing chemotherapy increases the risks of HF and valvular heart disease. Cardiovascular toxicity following radiotherapy is usually observed from 5 to at least 35 years after therapy, whereas anthracycline-related toxicity is already observed during treatment, up to at least 25 years. The joint effects of anthracyclines, radiotherapy, and conventional cardiovascular risk factors (eg, hypertension, smoking, and physical inactivity) appear to be additive rather than multiplicative. HL survivors need lifelong risk-based screening for selected SMNs and CVDs. Furthermore, preventive strategies should include lifestyle and drug-based interventions to minimize exposure to conventional risk factors for cancer and CVD.
Learning Objectives
Know treatment-related risk factors for the development of second malignancy and CVDs after HL
Be aware of the content of use surveillance guidelines for HL survivors, aimed at reduction of morbidity and mortality from second malignancy and CVDs
Introduction
Since the introduction of modern radiotherapy and combination chemotherapy in the 1960s, Hodgkin lymphoma (HL) has become a highly curable malignancy with 5-year survival rates of more than 80%.1 However, the life expectancy and quality of life of HL survivors are reduced by the occurrence of late adverse treatment effects.2-5 The vast majority of HL survivors experience one or more physical and/or psychosocial problems.6-8 Common late effects include second and subsequent malignant neoplasms (SMNs), several cardiovascular diseases (CVDs), thyroid dysfunction, subfertility, premature menopause, and fatigue.7-17 In addition to this wide range of physical late effects, many HL survivors face psychosocial problems that may or may not be linked to specific treatments, but significantly reduce their quality of life. These include impaired memory and concentration, depression and anxiety, sexual problems, and problems with employment and insurances.8,18,19
This review will focus on the risk of second malignancy and CVD after HL treatment, because these late effects contribute most to the substantial excess mortality after HL treatment.1,2,5,20,21 Although HL is the major cause of excess death in the first 10 to 15 years after diagnosis, excess mortality after this period is mainly due to second malignancies and CVD.2,5
Risk of second malignancy
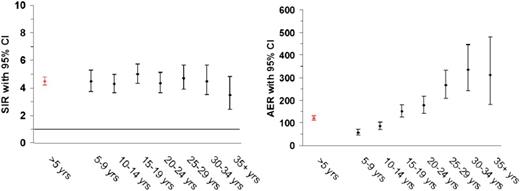
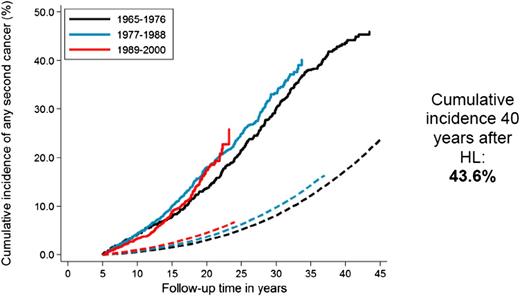
Increased risks of solid tumors in irradiated HL patients and of leukemia in chemotherapy-treated patients have been reported consistently in the literature.14,21,22 In a recent study that included HL patients treated from 1965 to 2000,9 the excess risk of second malignancy remained significantly increased beyond 35 years after HL treatment (Figure 1),9 with a 40-year cumulative incidence of second cancer estimated at 43.6% (Figure 2).9 The largest standardized incidence ratios (SIRs) are observed for leukemia (SIR = 10-30), followed by connective tissue, pleura and thyroid cancer, and non-HL (SIR = 6-20).9,12,21,23 Moderately increased risks (SIR = 2-7) are observed for a large number of solid tumors, such as cancers of the lung, breast, stomach, esophagus, colon/rectum, cervix, mouth and pharynx, and melanoma.9,12,23-25 Absolute excess risk (AER) is the best risk measure to express burden of disease. HL patients experience an excess of about 85 to 125 malignancies per 10 000 patients per year, over and above the background rate. Solid tumors account for the large majority of excess cancers (60 to 100 per 10 000 patients per year), and of those, breast and lung cancer account for the largest proportion of excess malignancies.9,12,21,23-27
Risk (SIR and AER) of new malignancies after HL according to follow-up interval. CI, confidence interval; yrs, years.
Risk (SIR and AER) of new malignancies after HL according to follow-up interval. CI, confidence interval; yrs, years.
Cumulative incidence of solid malignancy after HL according to calendar period of treatment. Solid lines represent the observed incidence; and dashed lines the expected incidence in the general population.
Cumulative incidence of solid malignancy after HL according to calendar period of treatment. Solid lines represent the observed incidence; and dashed lines the expected incidence in the general population.
The SIR of solid tumors is minimally elevated in the 1- to 4-year follow-up period, but becomes significantly increased from 5 to >30 years since first treatment.9,14,24-26 For several tumor sites (breast, thyroid, and esophagus), the excess risk does not become apparent until after 10 to 15 years of observation. Hodgson et al12 modeled relative risks (RRs) and found no indication for increasing or decreasing RRs beyond 10 years of follow up. Due to the rising background incidence of cancer with age, long-term survivors experience strongly increasing AERs of solid malignancy. In a recent report with very long-term follow up, HL survivors in their 60s or 70s experienced 1.7 and 3.1 excess cancers per 100 patients per year, on top of a background cancer incidence of 1.3 and 2.1 per 100 patients per year, respectively.9
The literature uniformly shows that the SIRs of various solid tumors increase strongly with younger age at first treatment.9,12,23,28 The effect is strongest for breast cancer.24,25 Both for breast and nonbreast solid malignancies, AERs strongly increase with older attained age, indicating the increasing burden of excess cancers with advancing age of HL survivors.9,12,24 Thirty years after treatment, at attained ages of ≤51 years, the cumulative incidence of breast cancer in survivors treated before age 21 was as high as 26%,25,28 which is comparable to the risk of breast cancer gene mutation carriers.26,29
For lung cancer, SIRs do not decrease with increasing age at HL treatment as strongly as for breast and gastrointestinal tract cancers; the SIR was still 5.2-fold increased for patients treated at ages 35 to 51 years.24 Also, increased SIRs for lung cancer appears to become manifest earlier (5 to 9 years from first treatment) than for breast and gastrointestinal tract cancers.9,24
Although alkylating chemotherapy is the main cause of acute myeloid leukemia after HL,30 elevated risks of solid cancers following HL have been largely attributed to radiation therapy (RT).9,12,21,23,27 For a number of solid malignancies (lung, breast, stomach, and pancreas), the risk has been shown to increase strongly and linearly with higher radiotherapy doses.31-35 For example, compared with a dose of <4 Gy to affected breast site, the RR of breast cancer rises from 4.1 for 7 to 23 Gy to an eightfold increase for more than 40.5 Gy.31 For lung cancer, the increased RRs from smoking appeared to multiply the elevated risks from radiotherapy (Table 1),31 implying that there are large AERs for lung cancer among irradiated patients who smoke, whereas nonsmokers experience little excess risk from radiation.31,36 It was estimated that 9.6% of all lung cancers after HL were due to treatment, 24% were due to smoking, and 63% were due to treatment and smoking in combination.31
Risk of lung cancer in patients with HL according to type of treatment and smoking category
| Treatment of Hodgkin disease . | RR (95% CI) by smoking category (# of case patients; control patient)* . | ||
|---|---|---|---|
| Radiation ≥5 Gy . | Alkylating agents . | Non-smoker, light, other† . | Moderate-heavy‡ . |
| No | No | 1.0§ | 6.0 (1.9-20.4) |
| Yes | No | 7.2 (2.9-21.2) | 20.2 (6.8-68) |
| No | Yes | 4.3 (1.8-11.7) | 16.8 (6.2-53) |
| Yes | Yes | 7.2 (2.8-21.6) | 49.1 (15.1-187) |
| Treatment of Hodgkin disease . | RR (95% CI) by smoking category (# of case patients; control patient)* . | ||
|---|---|---|---|
| Radiation ≥5 Gy . | Alkylating agents . | Non-smoker, light, other† . | Moderate-heavy‡ . |
| No | No | 1.0§ | 6.0 (1.9-20.4) |
| Yes | No | 7.2 (2.9-21.2) | 20.2 (6.8-68) |
| No | Yes | 4.3 (1.8-11.7) | 16.8 (6.2-53) |
| Yes | Yes | 7.2 (2.8-21.6) | 49.1 (15.1-187) |
Adapted from Travis et al.31
*Represents estimated tobacco smoking habit 5 years before diagnosis date of lung cancer and corresponding date in control patients, with the use of information recorded up to 1 year before these dates.
†This group includes nonsmokers, light current cigarette smokers (<1 pack per day), former cigarette smokers, smokers of cigar and pipes only, and patients from whom tobacco smoking habit was not stated.
‡Moderate (1 to 2 packs per day) and heavy (>2 packs per day) current cigarette smokers.
§Reference group.
Radiation field size is an important risk factor for solid cancer risk. For breast cancer, it has been shown that smaller radiation volumes than mantle field are associated with substantially lower risk,9,26,28 which is important in view of the smaller field sizes currently used in HL treatment.9,37 Furthermore, alkylating chemotherapy and pelvic radiotherapy appear to reduce the risk of radiotherapy-associated breast cancer, due to the high frequency of premature menopause after chemotherapy.9,26,28,32,33 A long vs short duration of intact ovarian function after radiation was a strong predictor of subsequent breast cancer risk. Women with <10 years of intact ovarian function after radiotherapy had a 70% decreased risk of breast cancer compared with women with 10 to 20 years of ovarian function after irradiation, whereas those with >20 years of intact ovarian function after radiotherapy had 5.3-fold increased risk of breast cancer.28 These results indicate that ovarian hormones are a crucial factor to promote breast tumorigenesis once radiotherapy has produced an initiating event.
Several studies have observed that not only radiotherapy but also alkylating chemotherapy can substantially increase the risk of solid malignancy, in particular risks of lung cancer,31,38 stomach, and pancreatic cancer.9,34,35 Lung cancer risk after HL is increased two to greater than fourfold with increasing number of cycles of alkylating agent-containing chemotherapy, particularly nitrogen mustard, vincristine, procarbazine, and prednisolone.31,38,39 For stomach cancer risk, a strong association with cumulative procarbazine dose was observed.34 Although additive effects of chemotherapy and radiotherapy have been observed for lung cancer,31 supramultiplicative effects were recently reported for stomach cancer. Radiation doses to the stomach of ≥25 Gy combined with exposure to high-dose procarbazine (≥5600 mg/m2) were associated with a 78-fold increased risk of stomach cancer, compared with RRs of 2.8 and 1.2 for exposure to ≥25 Gy of radiation alone and exposure to high-dose procarbazine (≥5600 mg/m2) alone, respectively.34 A recent study on risk factors for pancreatic cancer after HL also showed increasing risk with a larger number of alkylating agent-containing cycles of chemotherapy. The RR of pancreatic cancer for patients treated with both subdiaphragmatic radiation (≥10 Gy to affected subsite of pancreas) and ≥6 alkylating agent cycles, compared with patients with neither treatment, was 17.9. The joint effect of these 2 treatments was significantly greater than additive and nonsignificantly greater than multiplicative. High risks were especially observed among patients receiving ≥8400 mg/m2 of procarbazine with nitrogen mustard or ≥3900 mg/m2 of cyclophosphamide.35
A recent Dutch study examined whether HL patients treated in the 1989 to 2000 period, when less toxic treatments had been introduced, had a lower risk of second malignancy than patients treated in the 1965 to 1988 period.9 Although the cumulative incidence of leukemia was significantly lower in the most recent treatment era, no such decrease was observed for solid malignancies, even though smaller radiotherapy volumes were associated with lower risk in multivariable analysis, especially for breast cancer. The surprising absence of a declining overall risk of solid malignancy was attributed to a number of factors, such as later than expected wide application of changes in radiotherapy policy and changes in chemotherapy regimens. Trends in breast cancer risk were examined in more detail. Although a larger proportion of more recently treated female HL survivors had received less extensive supradiaphragmatic irradiation, there was little evidence that women treated in the most recent period (1989 to 2000) experienced lower breast cancer risk. Interpretation of the surprising absence of a decline in second breast cancers with less radiation exposure was complicated. Firstly, apparently the changes in RT policies were not applied widely enough yet to lower breast cancer risk. Secondly, the absence of a decrease in breast cancer incidence could be partly attributed to earlier breast cancer detection in more recently treated women because of increased screening.9 Furthermore, the introduction of less gonadotoxic chemotherapy appeared to have influenced breast cancer risk. In the earlier periods, high doses of alkylating agents were frequently used, often causing premature menopause, which is associated with lower risk of radiation-associated breast cancer.
Risk of CVD
Both radiotherapy involving the heart and anthracycline-containing chemotherapy can increase the risk of CVD in HL survivors. Radiation-induced CVD includes coronary heart disease (CHD), valvular heart disease (VHD), myocardial dysfunction, electrical conduction abnormalities, and pericardial disease14,20,40 Anthracyclines can, depending on the cumulative dose, lead to both acute cardiomyopathy and chronic cardiac complications (especially heart failure [HF]).41,42 Radiation- and anthracycline-associated cardiac damage have a different pathogenesis, which also appears to differ from the general population. Radiation may damage the endothelium of blood vessels.43 In large arteries, this damage may lead to accelerated atherosclerosis and an increased risk of vascular stenosis and thromboembolism.44 Animal studies have shown that radiation predisposes to the formation of unstable plaques, that are more likely to rupture and cause a fatal heart attack or stroke. Cardiotoxicity following anthracyclines is typically associated with loss of myocardial mass, leading to progressive cardiac remodelling and dysfunction.40
Prospective screening studies among HL survivors demonstrate that clinically significant cardiovascular abnormalities, like coronary artery stenosis, coronary artery calcifications, reduced left ventricular dimensions, VHD, and conduction defects, are very common, even in asymptomatic survivors.45-48
Large cohort studies of HL survivors show a two- to sevenfold increased risk of cardiac death (mainly myocardial infarction [MI]), depending on the age of the patients (stronger risk increases for radiotherapy at younger ages), treatment regimens used, and follow-up time.2,5,49,50 Furthermore, three- to sixfold increased SIRs of CHD, VHD, and HF are observed in patients treated for HL relative to the general population, also after long-term follow up.11,51,52 The persistence of increased SIRs over prolonged follow-up times is of concern because they imply increasing AERs over time, due to the rising incidence of CVDs with age.
van Nimwegen et al recently examined long-term CVD risk in 2524 5-year survivors of HL who were treated in The Netherlands between 1965 and 1996. After 35 years of follow up, HL survivors still had a four- to sixfold increased SIR of CHD or HF compared with the general population, corresponding to 81 865 excess events of CHD and HF per 10 000 person-years.11 Within the cohort, 40-year cumulative incidence of CVD was 50%. In patients treated before 25 years of age, the highest RRs were seen (for CHD and VHD, as well as HF), but substantial AERs were also observed for patients treated at older ages. For patients treated before 25 years of age, cumulative incidences at 60 years were 20%, 31%, and 11% for CHD, VHD, and HF as first events, respectively. Patients treated before 25 years of age reached a given cumulative incidence 10 to 20 years earlier than patients treated at an older age. For example, a 50-year-old survivor treated before 25 years of age experienced the same absolute risk as a 61-year-old survivor treated at 35 to 50 years of age.11
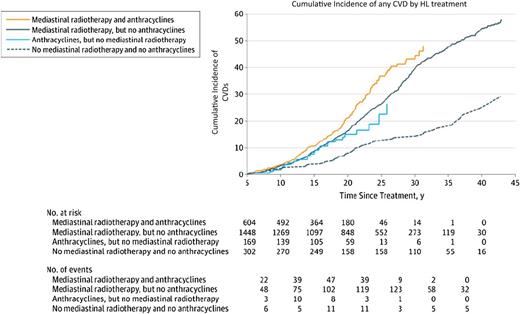
In the Dutch HL cohort, mediastinal radiotherapy increased the risks of developing CHD (2.7-fold), VHD (6.6-fold), and also HF (2.7-fold), as first cardiovascular events.11 Anthracycline-containing chemotherapy increased the risks of VHD (1.5-fold) and HF (threefold) as first cardiovascular events. Patients treated with mediastinal radiotherapy had a 40-year cumulative incidence of any CVD of 54.6% compared with 24.7% in patients not treated with mediastinal radiotherapy or anthracyclines. The cumulative incidence of CVD after HL according to treatment exposure is shown in Figure 3.11 After 20 years of follow up, anthracycline-associated risks of VHD and HF (as first events) were still significantly elevated.
Cumulative incidence of CVDs after HL according to treatment, with death from any cause as a competing risk.
Cumulative incidence of CVDs after HL according to treatment, with death from any cause as a competing risk.
Another large study assessed CVD risk in 9 trials conducted by the European Organization for Research and Treatment of Cancer in the period from 1964 to 2004.15 In multivariable analysis, mean heart radiation dose and the cumulative dose of anthracyclines had a statistically significant effect on CVD risk. The hazard ratio for radiotherapy was 1.015 per 1 Gy increase in mean heart dose. For the cumulative dose of anthracyclines in doxorubicin-equivalents, the hazard ratio was 1.077 per 50 mg/m2 anthracycline.
Data on the possible interaction between chemotherapy and radiotherapy, and risk of cardiac diseases are scarce. Several studies suggest that anthracycline-containing chemotherapy further increases the radiotherapy-related risk of VHD and HF by two- to threefold compared with radiotherapy alone.11,51,53 This effect was additive in a recent report11 and more than additive in another study.53
Three recent case-control studies addressed the shape of the radiation dose-response curve for CHD and VHD after HL treatment.54-56 The first study included 325 patients diagnosed with CHD as their first cardiovascular event after HL.56 Radiation charts and simulation radiographs were used to estimate mean heart dose for all cases and 1204 matched controls. The median interval between HL and CHD was 19 years. Risk of CHD increased linearly with increasing mean heart dose (excess RR per Gy: 7.4%), with a 2.5-fold increased risk for patients receiving a mean heart dose of 20 Gy (compared with no mediastinal RT). In the study of risk factors for VHD after HL, the radiation dose-response relationship was linear with upward curvature.54 A similar case-control study of cardiomyopathy and HF after HL showed a linear relationship with the mean left ventricular dose (MLVD). Relative to 0 Gy, HF rates following MVLDs of 1-15, 16-20, 21-25, and ≥26 Gy were 1.27, 1.65, 3.84, and 4.39, respectively (P < .001). Anthracycline-containing chemotherapy increased the HF rate by a factor of 2.83, and there was no significant interaction with MLVD. Twenty-five year cumulative risks of HF following MLVDs of 0-15 Gy, 16-20 Gy, and ≥21 Gy were 4.4%, 6.2%, and 13.3%, respectively, in patients treated without anthracycline-containing chemotherapy, and 11.2%, 15.9%, and 32.9%, respectively, in patients treated with anthracyclines.55
The establishment of a clear radiation dose-response for different CVDs implies substantially lower CVD risks for more recently treated HL patients who received involved-node or involved-site radiotherapy and lower radiation doses.
An important question is whether conventional cardiovascular risk factors influence CVD risk in survivors who received cardiotoxic treatments and whether such factors modify treatment-related risk of CVD. Several studies show that hypertension, hypercholesterolemia, diabetes, and recent smoking do increase CVD risk in HL and childhood cancer survivors,51-53,56,57 but few studies could examine risk modification. Two recent reports show additive effects of smoking on the risks of CVD from mediastinal radiotherapy and anthracyclines.11,56 The above case-control study of risk factors for CHD after HL treatment showed that hypertension was an independent risk factor for CHD (RR = 1.85), which added to the radiotherapy-associated risk but did not modify it.56 However, a recent study in childhood cancer survivors showed that the combined effect of chest radiotherapy plus hypertension resulted in potentiation of risk for major cardiac events beyond that anticipated on the basis of an additive effect.57 Two studies examined the effects of physical inactivity on CVD risk after HL.56,58 Jones et al found a lower risk of treatment-related cardiac events in childhood HL survivors who reported ≥9 metabolic equivalent hours per week, which is equivalent to ∼2 to 2.5 hours of cycling or walking.58 van Nimwegen et al also showed that patients with a high level of physical activity (≥4 hours a week of walking, cycling, or sports) had a considerably lower risk of developing CHD than patients who were inactive (<1 hour a week) (RR = 0.52).56
The above findings underline the importance of control of conventional CVD risk factors, including maintenance or adoption of a healthy lifestyle after HL treatment. Both additive and supra-additive effects of conventional CVD risk factors and treatment imply that early diagnosis and appropriate management of CVD risk factors may substantially reduce the risk of premature cardiac disease.
Treatment of HL has changed dramatically over time. The risk of radiotherapy-related CVD after HL is expected to decrease significantly over time, because fewer patients receive combined modality treatment and radiotherapy policies have changed.37,59 If radiotherapy is applied, the target volumes are smaller, 3-dimensional conformal radiotherapy planning is used, and the applied dose is lower.37 Additional advanced techniques such as deep-inspiration breath-hold and intensity modulated RT with butterfly techniques further significantly reduce doses to the heart and cardiac substructures.60,61 The risk of anthracycline-related HF, however, likely increases because of the increased use of anthracyclines. A recent report showed that cumulative incidence curves of CVD were similar for HL patients treated from 1965 to 1974, 1975 to 1984, and 1985 to 1995, implying that a large population of survivors remains at increased CVD risk for many years to come.11
Surveillance for late effects
The need for long-term follow up of HL survivors is increasingly recognized, as illustrated by the publication of surveillance guidelines for HL and childhood HL survivors, with special attention to key late effects such as SMNs and CVD. The National Comprehensive Cancer Network (NCCN) and the Dutch Better Care After HL: Evaluation of Long-Term Treatment Effects and Screening Recommendations (BETER) consortium provide guidelines for monitoring for late effects specifically for HL survivors 5 years after initial treatment, including types of testing and their timing.62,63 The long-term follow-up guidelines from the Children’s Oncology Group present detailed recommendations according to specific treatment exposures and potential impact to body sites, several of which are also relevant to HL survivors.64 Table 2 shows surveillance recommendations from the 3 groups according to late effect types. It should be realized that the evidence underlying most screening guidelines is rather limited. Although much has been published about the magnitude of the risks and treatment-related risk factors for late effects, much less is known about the diagnostic value, efficacy, and cost-effectiveness of different screening methods. For several guidelines, evidence from other relevant high-risk groups was used, which might be inappropriate because the pathogenesis of treatment-associated SMNs and CVD may differ from that in the general population. Because many guidelines are in fact based on expert opinion, there are large differences between them. Although all agree on annual breast cancer screening with mammography and MRI, starting 8 years after chest RT, recommendations regarding screening for lung and colorectal cancers and CVD differ greatly. Evaluation of the current screening guidelines in terms of diagnostic value and cost-effectiveness is therefore crucially important, as well as timely updates of the guidelines. Also, as new agents and novel radiotherapy techniques are introduced for HL, additional time is needed for their late effects to be fully appreciated, and current follow-up recommendations for HL survivors need to be updated accordingly.
Summary of follow-up recommendations for HL survivors according to NCCN, COG, and the Dutch BETER Consortium according to types of major late effects
| Treatment exposures . | NCCN . | COG . | Dutch BETER consortium . |
|---|---|---|---|
| Second malignancy | Breast cancer: Annual mammography and breast MRI screening, to start 8- to 10-y post -treatment, or at age 40, whichever comes first, for women with a history of chest RT between ages 10 and 30 y | Breast cancer: Annual breast self-examination beginning at puberty until age 25, then every 6 mo Annual mammogram and breast | Breast cancer: Screening only recommended for women with a history of RT to chest and/or axillae before age 40: Age 25-30: annual clinical breast examination and MRI |
| Lung cancer: Consider chest imaging for survivors with >30 pack-y history of smoking | MRI, beginning 8 y after radiation or at age 25, whichever occurs last Lung cancer: Imaging and surgery and/or oncology consultation, as clinically indicated | Age 30-60: annual clinical breast examination, mammography, and MRI Ago 60-70: biennial clinical breast examination and mammography Age 70-75: biennial mammagraphy through population screening | |
| Colorectal cancer: Colonoscopy every 10 y for survivors age ≥50, or by the age 40 for survivors at increased risk for colorectal cancer due to treatment history Skin cancer: Counseling on skin cancer risks | Colorectal cancer: Colonoscopy every 5 y, beginning at 10 y after radiation or at age 35, whichever comes first, for patients with RT of ≥30 Gy to the abdominal and/or pelvic region Thyroid nodule/cancer: Yearly thyroid examination Skin cancer: Annual dermatologic examination and monthly skin self-examination in patients with prior RT exposures | Thyroid nodule/cancer: See screening for thyroid dysfunction At this moment, screening for lung cancer, colorectal cancer, and skin cancer are not recommended, as evidence is lacking that this is effective in reducing morbidity and mortality | |
| CVD | Cardiac disease: Consider stress test and echocardiogram at 10-y intervals after treatment of patients with a history of chest RT | Cardiac disease: Periodic echocardiogram and ECG with frequency dependent on age at treatment exposure and cumulative doses in patients with a history of treatment with anthracyclines or chest RT | Screening only recommended after: Cardiotoxic CT with cumulative doses equivalent to doxorubicin ≥300 mg/m2 Chest RT only or combined with cardiotoxic CT, independent of dose |
| Carotid disease: Consider carotid ultrasound at 10-y intervals in patients with a history of neck RT CVD risk factors: Annual blood pressure, lipids, and aggressive management of cardiovascular risk factors | Carotid disease: Examination for diminished carotid pulses and carotid bruits in patients treated with neck RT | CVD: Echocardiogram every 5 y if treated with cardiotoxic CT; only once, 15 y after diagnosis, when treated with RT only Every 5 y, up to age 70: physical examination (eg, blood pressure), lipids, glucose, biomarkers (BNP or NTproBNP) ECG once 5 y after diagnosis | |
| Endocrinopathies | Hypothyroidism: Annual TSH for patients with a history of neck irradiation Infertility: | Hypothyroidism: Annual TSH, free T4 Infertility: Periodic follicle-stimulating FSH LH, and estradiol screening in patients with exposure to alkylating agents or pelvic RT | Thyroid dysfunction: For patients with a history of neck RT: Every 1-3 y palpation of thyroid gland |
| Reproductive counseling | Annual TSH, if abnormal: free T4 Infertility: When treated with alkylating CT or RT to gonadal region (before age 40 in women: counseling about reduced fertility span Men: testosterone if hypogonadism is suspected; women: LH, FSH, and estradiol |
| Treatment exposures . | NCCN . | COG . | Dutch BETER consortium . |
|---|---|---|---|
| Second malignancy | Breast cancer: Annual mammography and breast MRI screening, to start 8- to 10-y post -treatment, or at age 40, whichever comes first, for women with a history of chest RT between ages 10 and 30 y | Breast cancer: Annual breast self-examination beginning at puberty until age 25, then every 6 mo Annual mammogram and breast | Breast cancer: Screening only recommended for women with a history of RT to chest and/or axillae before age 40: Age 25-30: annual clinical breast examination and MRI |
| Lung cancer: Consider chest imaging for survivors with >30 pack-y history of smoking | MRI, beginning 8 y after radiation or at age 25, whichever occurs last Lung cancer: Imaging and surgery and/or oncology consultation, as clinically indicated | Age 30-60: annual clinical breast examination, mammography, and MRI Ago 60-70: biennial clinical breast examination and mammography Age 70-75: biennial mammagraphy through population screening | |
| Colorectal cancer: Colonoscopy every 10 y for survivors age ≥50, or by the age 40 for survivors at increased risk for colorectal cancer due to treatment history Skin cancer: Counseling on skin cancer risks | Colorectal cancer: Colonoscopy every 5 y, beginning at 10 y after radiation or at age 35, whichever comes first, for patients with RT of ≥30 Gy to the abdominal and/or pelvic region Thyroid nodule/cancer: Yearly thyroid examination Skin cancer: Annual dermatologic examination and monthly skin self-examination in patients with prior RT exposures | Thyroid nodule/cancer: See screening for thyroid dysfunction At this moment, screening for lung cancer, colorectal cancer, and skin cancer are not recommended, as evidence is lacking that this is effective in reducing morbidity and mortality | |
| CVD | Cardiac disease: Consider stress test and echocardiogram at 10-y intervals after treatment of patients with a history of chest RT | Cardiac disease: Periodic echocardiogram and ECG with frequency dependent on age at treatment exposure and cumulative doses in patients with a history of treatment with anthracyclines or chest RT | Screening only recommended after: Cardiotoxic CT with cumulative doses equivalent to doxorubicin ≥300 mg/m2 Chest RT only or combined with cardiotoxic CT, independent of dose |
| Carotid disease: Consider carotid ultrasound at 10-y intervals in patients with a history of neck RT CVD risk factors: Annual blood pressure, lipids, and aggressive management of cardiovascular risk factors | Carotid disease: Examination for diminished carotid pulses and carotid bruits in patients treated with neck RT | CVD: Echocardiogram every 5 y if treated with cardiotoxic CT; only once, 15 y after diagnosis, when treated with RT only Every 5 y, up to age 70: physical examination (eg, blood pressure), lipids, glucose, biomarkers (BNP or NTproBNP) ECG once 5 y after diagnosis | |
| Endocrinopathies | Hypothyroidism: Annual TSH for patients with a history of neck irradiation Infertility: | Hypothyroidism: Annual TSH, free T4 Infertility: Periodic follicle-stimulating FSH LH, and estradiol screening in patients with exposure to alkylating agents or pelvic RT | Thyroid dysfunction: For patients with a history of neck RT: Every 1-3 y palpation of thyroid gland |
| Reproductive counseling | Annual TSH, if abnormal: free T4 Infertility: When treated with alkylating CT or RT to gonadal region (before age 40 in women: counseling about reduced fertility span Men: testosterone if hypogonadism is suspected; women: LH, FSH, and estradiol |
BNP, B-type natriuretic peptide; COG, Children’s Oncology Group; CT, chemotherapy; ECG, electrocardiogram; FSH, follicle-stimulating hormone; LH, luteinizing hormone; MRI, magnetic resonance imaging; T4, thyroxine; TSH, thyroid stimulating hormone.
Although survivorship care for childhood cancer survivors has become well organized in many countries over the past decade,65-67 structured survivorship care for HL survivors is, unfortunately, largely lacking. In some countries, (subgroups of) HL survivors have been recalled for screening. For example, in the United Kingdom, female HL survivors treated with mantle field radiotherapy at a young age have been recalled and invited for breast cancer screening,68 and in The Netherlands, all HL survivors are invited to visit a special survivorship clinic.62 Evaluation of adherence (by survivors and physicians) to such programs is important. Furthermore, preventive strategies should include lifestyle and drug-based interventions to minimize exposure to conventional risk factors for cancer and CVD.
Correspondence
Flora E. van Leeuwen, Department of Epidemiology, Division of Psychosocial Research and Epidemiology Netherlands, Cancer Institute Plesmanlaan 121, 1066 CX Amsterdam, The Netherlands; e-mail: f.v.leeuwen@nki.nl.
References
Competing Interests
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Author notes
Off-label drug use: None disclosed.