Abstract
Several genetic syndromes have long been associated with a predisposition to the development of leukemia, including bone marrow failure syndromes, Down syndrome, and Li Fraumeni syndrome. Recent work has better defined the leukemia risk and outcomes in these syndromes. Also, in the last several years, a number of other germ line mutations have been discovered to define new leukemia predisposition syndromes, including ANKRD26, GATA2, PAX5, ETV6, and DDX41. In addition, data suggest that a substantial proportion of patients with therapy related leukemias harbor germ line mutations in DNA damage response genes such as BRCA1/2 and TP53. Recognition of clinical associations, acquisition of a thorough family history, and high index-of-suspicion are critical in the diagnosis of these leukemia predisposition syndromes. Accurate identification of patients with germ line mutations associated with leukemia can have important clinical implications as it relates to management of the leukemia, as well as genetic counseling of family members.
Learning Objectives
Understand the leukemia risk and therapeutic implications in syndromes with phenotypic abnormalities
Recognize that some familial disorders of hematopoiesis may have increased risk of hematologic malignancy
Recognize that certain molecular findings in leukemia may indicate an underlying leukemia predisposition
Introduction
Cancer predispositions due to germ line mutations have long been recognized to contribute to the development of many solid tumors. Until recently, with the exception of Li Fraumeni syndrome (LFS), leukemia susceptibility has been primarily associated with clinical syndromes such as Fanconi anemia (FA), dyskeratosis congenita, and trisomy 21 (Down syndrome). However, in the last several years, population and family studies have identified a number of germ line genetic mutations that increase the risk of leukemia in carriers. Many of these mutations are in genes already implicated in leukemogenesis, shedding further light on their importance, and in some cases the function of these genes in the pathogenesis of leukemia. The development of massively parallel sequencing platforms has facilitated many of these findings. The increasing use of targeted and unbiased sequencing in both research and clinical settings necessitates familiarity with these genetic syndromes in the evaluation and management of patients with hematologic malignancies.
Herein, this review highlights new scientific and clinical insights in well-known syndromes associated with leukemia, as well as new leukemia predisposition disorders caused by germ line mutations. These disorders are loosely categorized as syndromic, with associated phenotypic anomalies; disorders of abnormal hematopoiesis; and isolated cancer/leukemia predispositions (Table 1). A few emerging leukemia predisposition syndromes are also briefly discussed. Due to space limitations, important findings from genome-wide association studies identifying variants with strong association with the development of leukemia are not discussed, as these have been recently, thoroughly reviewed.1
Germ line mutations associated with leukemia
| Predisposition . | Inheritance . | Genes . | Altered pathway/function . | Hematologic malignancies . |
|---|---|---|---|---|
| Syndromic predispositions to leukemia | ||||
| FA | AR, XLR | FANCA, FANCB, FANCC, FANCE, FANCF, FANCG, FANCL, FANCM, and FANCT; | DNA damage/repair | MDS, AML |
| FANCI and FANCD2; | ||||
| BRCA2/FANCD1, BRCA1/FANCS, FANCR/RAD51, FANCJ, FANCN, FANCO, FANCP, and FANCQ | ||||
| Dyskeratosis congenita | AD, AR, XLR | DKC1, TERC, and TINF2 | Telomere maintenance | MDS, AML |
| TERT, NHP2, NOP10, WRAP53, RTEL1, CTC1, PARN | ||||
| Down syndrome | Sporadic | Unknown | Multifactorial | AMKL, ALL |
| RASopathies | AD | NF1, PTPN11, KRAS, NRAS, SOS1, RAF1, CBL, SHOC2, HRAS, BRAF, MEK1, MEK2, and SPRED1 | RAS signaling | NS-MPD, JMML |
| GATA2 deficiency | AD | GATA2 | TF network | MDS, AML, CMML |
| Disorders of abnormal hematopoiesis | ||||
| FPDMM | AD | RUNX1 | TF network | MDS, AML, T-ALL |
| THC2 | AD | ANKRD26 | MAPK signaling | MDS, AML, CML |
| THC5 | AD | ETV6 | TF network | MDS, B-ALL, MP-ALL |
| Isolated cancer/leukemia predisposition | ||||
| LFS | AD | TP53 | DNA damage/repair | AML, ALL, sAML |
| CMMRD | AR | MLH1, MSH2, MSH6, and PMS2 | DNA damage/repair | T-NHL, B-NHL, T-ALL, B-ALL, MDS, AML |
| CEBPA mutation | AD | CEBPA | TF network | AML |
| DDX41 mutation | AD | DDX41 | RNA splicing and/or telomere maintenance? | MDS, AML, CML, HL, NHL |
| PAX5 mutation | AD | PAX5 | TF network | B-ALL |
| Emerging leukemia predisposition syndromes | ||||
| ATG2B/GSKIP duplication | AD | ATG2/GSKIP | Unknown | MPN, AML |
| ACD mutation | AD | ACD | Telomere maintenance? | BMF |
| SRP72 mutation | AD | SRP72 | Unknown | MDS |
| SH2B3 mutation | AR | SH2B3 | JAK/STAT signaling | B-ALL |
| RBBP6 mutation | AD | RBBP6 | DNA damage/repair? | MPN |
| LAPTM5 and HCLS1 mutations | AD | LAPTM5 and HCLS1 | Unknown | WM |
| Predisposition . | Inheritance . | Genes . | Altered pathway/function . | Hematologic malignancies . |
|---|---|---|---|---|
| Syndromic predispositions to leukemia | ||||
| FA | AR, XLR | FANCA, FANCB, FANCC, FANCE, FANCF, FANCG, FANCL, FANCM, and FANCT; | DNA damage/repair | MDS, AML |
| FANCI and FANCD2; | ||||
| BRCA2/FANCD1, BRCA1/FANCS, FANCR/RAD51, FANCJ, FANCN, FANCO, FANCP, and FANCQ | ||||
| Dyskeratosis congenita | AD, AR, XLR | DKC1, TERC, and TINF2 | Telomere maintenance | MDS, AML |
| TERT, NHP2, NOP10, WRAP53, RTEL1, CTC1, PARN | ||||
| Down syndrome | Sporadic | Unknown | Multifactorial | AMKL, ALL |
| RASopathies | AD | NF1, PTPN11, KRAS, NRAS, SOS1, RAF1, CBL, SHOC2, HRAS, BRAF, MEK1, MEK2, and SPRED1 | RAS signaling | NS-MPD, JMML |
| GATA2 deficiency | AD | GATA2 | TF network | MDS, AML, CMML |
| Disorders of abnormal hematopoiesis | ||||
| FPDMM | AD | RUNX1 | TF network | MDS, AML, T-ALL |
| THC2 | AD | ANKRD26 | MAPK signaling | MDS, AML, CML |
| THC5 | AD | ETV6 | TF network | MDS, B-ALL, MP-ALL |
| Isolated cancer/leukemia predisposition | ||||
| LFS | AD | TP53 | DNA damage/repair | AML, ALL, sAML |
| CMMRD | AR | MLH1, MSH2, MSH6, and PMS2 | DNA damage/repair | T-NHL, B-NHL, T-ALL, B-ALL, MDS, AML |
| CEBPA mutation | AD | CEBPA | TF network | AML |
| DDX41 mutation | AD | DDX41 | RNA splicing and/or telomere maintenance? | MDS, AML, CML, HL, NHL |
| PAX5 mutation | AD | PAX5 | TF network | B-ALL |
| Emerging leukemia predisposition syndromes | ||||
| ATG2B/GSKIP duplication | AD | ATG2/GSKIP | Unknown | MPN, AML |
| ACD mutation | AD | ACD | Telomere maintenance? | BMF |
| SRP72 mutation | AD | SRP72 | Unknown | MDS |
| SH2B3 mutation | AR | SH2B3 | JAK/STAT signaling | B-ALL |
| RBBP6 mutation | AD | RBBP6 | DNA damage/repair? | MPN |
| LAPTM5 and HCLS1 mutations | AD | LAPTM5 and HCLS1 | Unknown | WM |
AD, autosomal dominant; ALL, acute lymphoblastic leukemia; AMKL, acute megakaryocytic leukemia; AML, acute myeloid leukemia; AR, autosomal recessive; B-ALL, B-cell acute lymphoblastic leukemia; B-NHL, B-cell NHL; CML, chronic myeloid leukemia; CMML, chronic myelomonocytic leukemia; CMMRD, constitutional mismatch repair deficiency; FPDMM, familial platelet disorder with associated myeloid malignancy; HL, Hodgkin lymphoma; JMML, juvenile myelomonocytic leukemia; MAPK, mitogen-activated protein kinase; MDS, myelodysplastic syndrome; MP-ALL, mixed phenotype acute lymphoblastic leukemia; MPN, myeloproliferative neoplasm; NF1, neurofibromatosis type 1; NHL, non-Hodgkin lymphoma; NS-MPD, Noonan syndrome-associated myeloproliferative disease; sAML, secondary AML; T-ALL, T-cell acute lymphoblastic leukemia; TF, transcription factor; THC, thrombocytopenia; T-NHL, T-cell NHL; WM, Waldenström macroglobulinemia; XLR, X-linked recessive.
Syndromic predispositions to leukemia
Several syndromes have been recognized to be associated with the development of leukemia for decades. For example, FA is primarily an autosomal recessive syndrome that includes bone marrow failure (BMF), short stature, and abnormal thumbs among other anomalies, and a predisposition to leukemia, primarily AML. Studies from the National Cancer Institute’s Inherited Bone Marrow Failure Syndrome Cohort have estimated observed to estimated ratios of AML incidence at over 300 in patients with FA. FA is caused by mutation in one of at least 19 different genes that contribute to a DNA repair mechanism.2 Importantly, many patients with FA do not have overt clinical syndromic features. However, those with bi-allelic mutation in FANCD1/BRCA2 have more phenotypic anomalies, including imperforate anus, renal anomalies, and cardiac defects, and are at the highest risk of AML, with a reported cumulative incidence of ∼80% by age 10 years.3 BRCA2 and BRCA1 are both FA genes (FANCD1 and FANCS, respectively); and although most notably associated with Hereditary Ovarian-Breast Cancer syndrome, germ line mutations in DNA damage response genes, including BRCA1 and BRCA2, are found in ∼20% of patients with therapy-related leukemias.4,5 Similarly, patients with dyskeratosis congenita, due to mutations in telomere maintenance genes (DKC1, TERC, and TINF2 among others), have BMF and much higher than expected rates of AML.6 Patients with other inherited BMF syndromes such as Diamond-Blackfan anemia, Shwachman-Diamond syndrome, severe congenital neutropenia and thromobocytopenia, and absent radius syndrome may also have an increased risk of AML, although the relative risk in these diseases has not been determined, in part due to the later age of leukemia onset, in addition to the rarity of diagnosis of these diseases.7
Patients with Down syndrome, due to trisomy of chromosome 21 (T21), also share characteristic phenotypic anomalies, congenital defects, and a risk for leukemia, both AML and ALL. The incidences of ALL and AML in children with T21 are 33 and 150× that of age-matched disomic individuals. The myeloid leukemias of Down syndrome, as classified by the World Health Organization, are unique in harboring somatic mutations in GATA1, are more likely to be of M7 phenotype (acute megakaryoblastic leukemia), and are often preceded by transient abnormal myelopoiesis in neonates.8 Although patients with Down syndrome are at high risk of toxicity from conventional chemotherapy, cooperative groups report high cure rates of myeloid leukemias of Down syndrome with event-free survival rates of ∼80%.9 Similarly, ALL in those with Down syndrome is biologically distinct from ALL in those without T21. For example, the ETV6-RUNX1 translocation, which typically confers a good prognosis, is found about three- to 10-fold less in those with T21,10 whereas aberrant expression of CRLF2 occurs ∼10-fold more.11 Importantly, outcomes in those with ALL and T21 are not as good as in disomic individuals, in part because of toxicity during remission induction, and perhaps due to differences in the molecular underpinnings of the disease.9 However, the molecular drivers in ALL in those with T21 may ultimately serve as biomarkers indicating targeted therapy, such as JAK inhibition in cases with CRLF2 overexpression. Interestingly, in stark contrast to FA and dyskeratosis congenita, in which the risk of solid tumors is also quite high, patients with T21 rarely develop solid tumors, a pattern that may help in understanding carcinogenesis in the general population.
ALL in disomic individuals often demonstrates somatic trisomy of chromosome 21, suggesting unique contributions from this chromosome in leukemogenesis. More infrequently (∼2% of childhood ALL), there is intrachromosomal amplification of chromosome 21 (iAMP21), which defines a unique subgroup of ALL. Recently, it was found that those with constitutional Robertsonian translocation of chromosomes 15 and 21 are at over 2000-fold risk of developing leukemia with iAMP21.12 Robertsonian translocations are those that involve the short arms of acrocentric chromosomes, and are found in ∼1 in 1000 newborns, although the t(15;21) is quite rare. Importantly, patients with iAMP21 ALL have inferior outcomes when treated as standard risk, but fare relatively well when treated as high risk.13 Current cooperative group protocols stratify iAMP21 ALL as high risk, necessitating intensified therapy and highlighting the importance of identifying these patients up front. The RUNX1 gene is within the region most commonly amplified; therefore, iAMP21 ALL can be diagnosed by fluorescence in situ hybridization using the same probes for identifying ETV6-RUNX1 fusions, when there are 5 or more copies of the RUNX1 gene per cell.14
A number of syndromes with overlapping phenotypes caused by germ line mutations in the RAS/mitogen-activated protein kinase pathway are also associated with a high risk of leukemia. These “RASopathies” include NF1, and the NS, Costello, Noonan-like CBL, Legius, and cardio-facial-cutaneous syndromes.15 Approximately 10% of patients with NS have a transient NS-MPD during infancy.15 Analogous to transient abnormal myeloipoiesis in those with T21, NS-MPD usually resolves, but can cause significant morbidity and even mortality. NS-MPD infrequently progresses to JMML. Indeed, the identification of mutations in PTPN11 being responsible for NS, led to the identification of somatic mutations in the same gene in ∼35% of cases of nonsyndromic JMML.16 Subsequent studies of RASopathies and JMML have solidified the genetic link between RAS activation via mutation in the NF1, NRAS, KRAS, and CBL genes, and JMML.16 This link has clinical implications, because targeted inhibition of MEK and/or the phosphatidylinositol 3-kinase/AKT pathway have demonstrated promising results in preclinical models of JMML,17,18 and planning of clinical trials of MEK inhibition in children with RAS activated cancer is underway.16
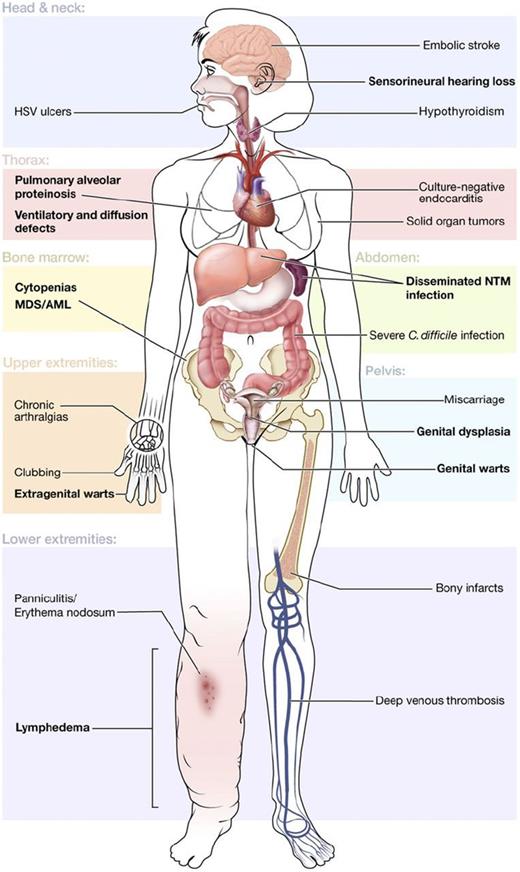
More recently, another group of overlapping syndromes has been defined by germ line mutations in GATA2. In addition to MDS and/or AML, patients may have immunodeficiency, sensorineural hearing loss, lymphedema, and dermatologic or pulmonary manifestations, among others (Figure 1).19 The immunodeficiency is associated with particular susceptibility to human papillomavirus and nontuberculous mycobacteria, leading some to advocate for early human papillomavirus vaccination and antibiotic prophylaxis.19 Germ line mutation in GATA2 is also found in 7% of all children with MDS and about two-thirds of adolescents with monosomy 7 MDS, making it the most common germ line defect predisposing to pediatric MDS.20 Evaluation of GATA2 in children with MDS is important, even in the absence of a family history, because it may inform supportive care strategies and/or accelerate planning for hematopoietic stem cell transplantation.
Clinical features of GATA2 deficiency. Common manifestations are shown by organ system, with primary features in bold. Reprinted from Spinner et al.19 C difficile, Clostridium difficile; HSV, herpes simplex virus; NTM, nontuberculous mycobacteria.
Clinical features of GATA2 deficiency. Common manifestations are shown by organ system, with primary features in bold. Reprinted from Spinner et al.19 C difficile, Clostridium difficile; HSV, herpes simplex virus; NTM, nontuberculous mycobacteria.
Disorders of abnormal hematopoiesis
In the last decade or so, more subtle disorders associated with leukemia have been genetically defined. These disorders have been identified by astute clinical observations of unique familial diseases, followed by thorough laboratory investigation, initially through linkage analysis and more recently with high throughput genome sequencing methods. Several of these leukemia predisposition syndromes are associated with abnormal hematopoiesis, particularly thrombopoiesis. Affected family members harbor germ line mutations in one of several genes, most of which are recurrently somatically mutated in sporadic cancers. In addition to shedding light on the mechanisms of leukemogenesis, identification of familial germ line mutations has clinical implications, particularly as it relates to genetic counseling of family members and donor identification in those for whom hematopoietic stem cell transplantation is indicated.
The first of these to be defined at the gene level was Familial Platelet Disorder with Associated Myeloid Malignancy, caused by a mutation in the RUNX1 gene. Affected family members generally have moderate thromobocytopenia, mild bleeding tendency, and some develop leukemia.21 Most cases of leukemia are myeloid, but a few cases of lymphoid malignancies have been observed.21 Most mutations in RUNX1 have been classified as being dominant negative or haplo-insufficient, leading to different levels of RUNX1 inactivation. Although both types of mutations are associated with platelet disorders, clinical and experimental data suggest that dominant negative RUNX1 mutations have distinct effects on hematopoiesis and may confer a higher risk of leukemia.21,22
Another disorder of abnormal thrombopoiesis (Online Mendelian Inheritance in Man THC2) is caused by germ line mutation in ANKRD26.23 In ANKRD26-related thromobocytopenia (ANKRD26-RT), the bleeding risk is fairly low and the thromobocytopenia tends to be moderate with normal platelet size, and with no consistent defect in in vitro aggregation studies. Careful examination of case and family histories indicate that patients with ANKRD-RT are at increased risk of MDS and leukemia.24,25 Although not fully penetrant, the observed/expected rates for AML, MDS, and CML are 23, 12, and 34, whereas the risks of lymphoid malignancy and nonhematologic cancers do not appear to be increased.25
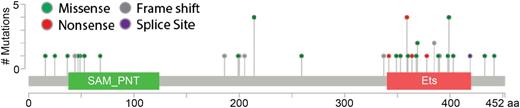
Recent examinations of families with autosomal dominant thromobocytopenia and leukemia predisposition have defined a new syndrome of thromobocytopenia, and susceptibility to malignancy (Online Mendelian Inheritance in Man THC5) caused by germ line mutation in ETV6.26-29 Although there is some concentration of mutations in the DNA binding domain of ETV6, mutations in ETV6 are distributed throughout the gene (Figure 2). Most that have been examined cause a loss of normal transcriptional repression by ETV6, probably in a dominant negative manner. Although variable in presentation, patients had mild to moderate thromobocytopenia and bleeding tendency, red cell macrocytosis, and multilineage dysplasia with hypolobulated megakaryocytes, and about one-third of reported cases had hematologic malignancy. Although germ line ETV6 mutations are most closely associated with B-cell ALL, other hematologic malignancies have been observed in carriers, including MDS, multiple myeloma, and AML. Examination of samples from the Pediatric Cancer Genome Project did not reveal germ line mutation in children with cancers other than leukemia,28 but some affected family members had solid tumors in adulthood,26 and ETV6 variants were recently reported to be associated with colorectal cancer susceptibility,30 raising the possibility that germ line mutations in ETV6 contribute to a more general cancer predisposition syndrome. Importantly, in a population-based study, ∼1% of children with apparently sporadic ALL harbor germ line mutations in ETV6,29 suggesting that germ line predispositions to leukemia may be more common than previously believed.
Distribution and frequency of reported germ line mutations in ETV6. The ETV6 protein is diagrammed with its pointed (SAM_PNT) and canonical ETS family DNA binding (Ets) domains. Germ line mutations associated with leukemia are clustered in the Ets domain, but spread throughout the protein.
Distribution and frequency of reported germ line mutations in ETV6. The ETV6 protein is diagrammed with its pointed (SAM_PNT) and canonical ETS family DNA binding (Ets) domains. Germ line mutations associated with leukemia are clustered in the Ets domain, but spread throughout the protein.
Nonsyndromic cancer/leukemia predisposition
Some inherited predispositions to cancer do not have associated syndromic findings or signs of abnormal hematopoiesis. LFS is caused by mutations in TP53 and confers a lifetime risk of cancer, including leukemia, of ∼70% for men and ∼100% for women. Leukemia is among the core cancers associated with LFS, accounting for 3% to 6% of LFS tumors.31 Virtually all hematologic malignancies are represented in the International Agency for Research on Cancer TP53 database, including therapy related MDS and AML.32 Whether patients with LFS have lower remission rates with therapy remains to be determined; however, somatic mutations in TP53 in leukemia are associated with poor outcomes in childhood and adult leukemias.33-35 Making a diagnosis of LFS or other cancer predisposition syndromes is critical, not only as it relates to genetic counseling and donor selection but also in considering tumor surveillance studies after successful treatment of a primary tumor. Importantly, as much as 40% of children with low hypodiploid ALL harbor germ line mutation in TP53,36,37 suggesting that low hypodiploid ALL is a manifestation of LFS. Thus, one should consider genetic counseling and testing for germ line TP53 mutation in children with hypodiploid ALL.
Like LFS, Lynch syndrome is another general cancer predisposition syndrome, primarily associated with colorectal and endometrial cancer. It is caused by mutations in mismatch repair genes MLH1, MSH2, MSH6, and PMS2. Although those with heterozygous mutations in these genes do not carry a high risk of leukemia, hematologic malignancies are among the most common cancers in those with CMMRD, an autosomal recessive disorder caused by bi-allelic mutation in one of these genes.38 Over half of hematologic malignancies are of T-cell origin, although B-cell lymphoma and leukemia, and myeloid leukemias also occur at high rates.39 Patients with CMMRD may have features associated with NF1, including cafe-au-lait spots and neurofibromas, a family history of gastrointestinal or gynecologic cancer, and/or parental consanguinity, and diagnostic criteria have been suggested.38 As with other cancer predisposition syndromes, accurate diagnosis has implications not only for immediate family members, but may influence treatment decisions because mismatch repair-deficient cells may have altered the sensitivity to certain chemotherapeutics.38
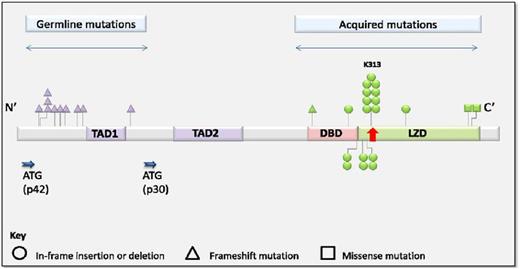
Other disorders are associated more specifically with the development of leukemia, in the absence of other tumor types. One gene that is recurrently somatically mutated in AML that is also involved in familial AML is CEBPA.40 Germ line mutations in CEBPA cause a highly penetrant predisposition to AML in the absence of other clinical features like thromobocytopenia or preceding MDS. In contrast to ETV6, germ line mutations in CEBPA cluster at the N-terminus (Figure 3).41 Additional somatic mutations in the C-terminus were detected in all AML samples.41 Although remission was achieved in >90% of patients and overall survival at 10 years was 67%, recurrent disease was frequent in those with CEBPA germ line mutation. Interestingly, the somatic mutations in AML at recurrence were usually distinct, suggesting the development of new leukemia, rather than the relapse of chemotherapy resistant clones.41 Interestingly, those with AML that harbor a double-somatic mutation in CEBPA and those with a germ line mutation, have a similar, better prognosis, than those with a single-somatic mutation,41,42 suggesting a clinically significant difference in the biology of these diseases.
Distribution and frequency of germ line and acquired mutations in CEBPA. Reprinted from Tawana et al.41
Distribution and frequency of germ line and acquired mutations in CEBPA. Reprinted from Tawana et al.41
Germ line mutations in DDX41 were more recently described to define another disorder in which cancer predisposition seems to be limited to the hematopoietic system.43-46 Patients with a germ line mutation in DDX41 tend to be older at the time of presentation of hematologic malignancy, as compared with other leukemia predisposition disorders, with an average age of 62 years, and have poor prognosis compared with those with wild-type (WT) DDX41.45 Malignancies include MDS, AML, CML, Hodgkin lymphoma, and non-Hodgkin lymphoma. Most patients have normal complete blood counts prior to the diagnosis of hematologic malignancy.44 Although the role of DDX41 in hematopoietic progenitor cells is not fully understood, an intriguing observation is that those with a DDX41 mutation have significantly shorter telomeres in peripheral blood cells.43 Importantly, somatic mutations in DDX41 are found in AML, even in some patients without germ line DDX41 mutation, highlighting its role in leukemogenesis.45 Nonetheless, predicted loss-of-function DDX41 variants are found in public databases at rates higher than other leukemia predisposition variants (eg, CEBPA, ETV6), leading some to argue that these variants are risk factors for hematologic malignancies, rather than a causal Mendelian link.43 Regardless of its contribution to leukemia predisposition, patients with DDX41 mutations responded better to lenalidomide treatment as compared with those with WT DDX41, which if replicated prospectively, could provide a therapeutic option for those with this high-risk disease.45
Unique among leukemia predisposition syndromes is that caused by germ line mutation in PAX5, in that to date, the leukemia risk appears to be highly specific to pre–B-cell ALL.47,48 Outside of the nervous system, PAX5 expression is restricted to B cells, in which it plays a critical role in normal B-cell development. It is somatically mutated in one-third of pre–B-ALL cases, highlighting a major role in leukemogenesis. In all familial cases, the WT allele was deleted in leukemia samples, whereas the mutant allele was retained. Notably, only 1 germ line PAX5 mutation has been described (c.547G>A) from all 3 kindreds reported, resulting in lower but not absent PAX5 transcriptional activity, whereas somatic alterations in PAX5 have more profound dysfunction. These data and preclinical experimental models indicate that PAX5 and other B-cell development transcription factors have complex roles in leukemogenesis that remain to be elucidated.
Emerging leukemia predisposition syndromes
There have been other reports of germ line mutations of genes causing a predisposition to leukemia that have not been replicated or reported in additional kindreds. These include the duplication of ATG2B and GSKIP, which seems to predispose to MPNs49 ; mutations in RBB6 associated with MPNs50 ; mutations in ACD that cause a BMF syndrome51 ; mutations in SRP72 that lead to MDS52 ; mutations in LAPTM5 and HCLS1 that are associated with familial Waldenström macroglobulinemia53 ; and mutations in SH2B3 that appear to predispose to ALL.54 Subsequent work will determine the extent to which germ line mutations in these genes have implications outside of the reported families.
Conclusion
The ready availability of high-throughput sequencing technology has helped to define several new leukemia predisposition syndromes over the last few years, and more are expected. In some cases, these findings have helped to better understand the mechanisms of leukemogenesis that should eventually impact clinical care. With increasing use of sequencing in clinical practice, more patients will be identified with mutations in these genes in leukemia samples. A challenge for physicians will be to recognize when these mutations may represent germ line, rather than somatic mutations. It is imperative to be cognizant of the possibility of identifying these mutations with currently available targeted and unbiased sequencing platforms, and to consider plans for genetic counseling and germ line testing in the event that they are identified.
Correspondence
Christopher C. Porter, 12800 East 19th Ave, RC1N 4101, Mail Stop 8302, Aurora, CO 80045; e-mail: chris.porter@ucdenver.edu.
References
Competing Interests
Conflict-of-interest disclosure: The author declares no competing financial interests.
Author notes
Off-label drug use: None disclosed.