Abstract
Sepsis is a dynamic, acute, infectious disease syndrome characterized by dysregulated thrombo-inflammatory responses. The high mortality associated with sepsis has been recognized since the earliest clinicians’ writings. Despite this, advances in the treatment of sepsis have been more modest. This is limited, in part, by the heterogeneity in the definition, population, presentation, and causal factors of infectious syndromes. Given the persistently high morbidity and mortality associated with sepsis, a better understanding of the dysregulated cellular biology underpinning sepsis is needed. Platelets are small, anucleate cells that have hemostatic, inflammatory, and immune-mediating properties. Platelets are the second most common circulating blood cell, and emerging evidence suggests that platelets serve as sentinel and effector cells during infectious syndromes. Nevertheless, the molecular and functional changes that occur in platelets during sepsis, and their impact on the clinical course of infected patients, remain incompletely understood. In this review, we first highlight the complex and dynamic pathophysiology characteristics of acute, systemic infections and we then discuss established and emerging evidence of the roles of platelets in sepsis.
Learning Objectives
Understand the systemic pathophysiology and thrombo-inflammation characteristic of human infectious disease syndromes
Review the role of platelets in sepsis as modulating host hemostatic, inflammatory, and immune activities
Introduction
Early descriptions of sepsis date back to antiquity.1 In about 400 BC, Homer described sepsis as the biological decay of the colon and resultant release of substances that caused “auto-intoxication.” Galen, a Roman physician, is known for practices of blood-letting and drainage of abscesses, as well as use of medications to treat infectious diseases. The collective 19th century works of Semmelweis, Pasteur, and Lister identified some of the causes for the development of sepsis at surgical sites, as well as methods to prevent infection. In the early 20th century Lennhartz is credited with developing the modern definition of sepsis as “present if a focus has developed from which pathogenic bacteria, constantly or periodically, invade the blood stream in such a way that this causes subjective and objective symptoms.” Through each generation of scientific inquiry, the identification and management of sepsis has advanced. We have made substantial progress in understanding the causal pathogens and potentiators of infectious diseases. Not until the 1960s were the first links between thrombosis and inflammation made through the identification of cytokines. Before that, septic patients had been thought to primarily have derangements within the coagulation cascade. Today, factors mediating dysregulated coagulation in sepsis continue to intrigue investigators as a potential therapeutic intervention in sepsis.
Despite decades of molecular, clinical, and translational research, acute infectious disease remains a significant public health burden in the United States and worldwide.2 More than 750 000 patients with sepsis, severe sepsis, or septic shock are admitted into US hospitals annually, and this number continues to rise each decade. Sepsis and septic shock continue to have mortality rates ranging from 20% to 60%. In addition to the acute complications in sepsis (eg, organ failure, hypotension, thrombosis), septic syndromes are associated with long-term complications, including cognitive dysfunction, debilitation, and significant reductions in health-related quality of life.3,4 As the risk and incidence of sepsis increase with age, coupled with forecasts of a sustained rise in the age of the population, sepsis will continue to be a substantial public health issue.
Adverse outcomes after septic syndromes remain only marginally improved. Improvements in outcomes in patients with sepsis are primarily attributed to advances in processes of care with the establishment of management guidelines, “bundles,” ventilator management, and goal-directed therapy. Efforts continue to focus on the early recognition of septic syndromes with highly sensitive and more clinical definitions with the goal of establishing early treatment.2 Although progress has been made in the early identification and initial treatment of sepsis (eg, fluids, antibiotics, and vasopressor support), specific therapies targeting the injurious thrombo-inflammatory cellular responses remain limited. As such, ongoing studies examining the fundamental cellular and biological mechanisms underlying septic physiology are likely to advance our understanding.
Through ongoing innovative and rigorous studies, we have seen advances in diagnostic and prognostic biomarkers and scoring systems in sepsis, promising preclinical animal studies, and clinical trials testing therapeutic agents targeting thrombo-inflammatory mediators and pathways. Despite these sustained efforts, only a few therapeutic agents made it to phase 3 clinical trials and none have seen continued clinical use. For example, eritoran tetrasodium, an anti–toll-like receptor (TLR) 4 compound failed in a phase 3 clinical trial, and activated protein C (APC) was pulled from the market. Emerging evidence suggested that single therapeutic agents may not be an effective solution for a dynamic, complicated disease like sepsis.5
Sepsis is a dynamic, heterogeneous disease process in humans
Sepsis is a heterogeneous disease process because of differences in pathogens, toxins, host factors, and individual patient responses. Genetic variations, comorbid conditions, immune suppression, and obesity are host factors that can modulate susceptibility to infection. Compounding host and host-pathogen heterogeneity, emerging evidence highlights that sepsis is a much more dynamic process than initially recognized. The first phase, called the systemic inflammatory response syndrome (SIRS), is characterized by injurious, systemic inflammation and lasts several days after the onset of infection. SIRS develops when exaggerated immune cell activation responses damage host tissues and organs and, in doing so, impair adaptive responses by immune and nonimmune cells. The second phase, known as the compensatory antiinflammatory response syndrome (CARS), may last anywhere from days to weeks. During the CARS phase, the immune system is markedly suppressed, leading to secondary infection and organ failure. Recent evidence points to this immune suppression during CARS as a major cause of morbidity and mortality in patients with sepsis.6 These 2 phases of sepsis often overlap, creating a highly complex spectrum of pathophysiologic responses that may not be easily amenable to safe, effective therapeutic interventions.7 Investigations are currently underway to parse out these complexities, and many biomarkers have been identified to describe these phases of treatment.
Platelets in sepsis
Platelets are small anucleate cells highly specialized for hemostasis and vascular wall repair. Emerging data continue to describe platelets effector cells with a repertoire of immune functions in addition to their well-recognized roles in hemostasis. These newly appreciated functions provide mechanisms whereby platelets bridge thrombotic and inflammatory pathways, contributing to systemic inflammatory and immune processes.8-11 The inflammatory and immune specializations of platelets may also represent evolutionarily driven adaptations that augment host defenses to pathogens. Among the dynamic repertoire of platelet functions, platelets secrete pleiotropic immune and inflammatory mediators, factors that orchestrate heterotypic interactions with endothelial cells, monocytes, and neutrophils; participate in TLR-mediated responses; and induce neutrophil extracellular trap (NET) formation (Table 1). Here, we provide a focused review of several emerging aspects of the biology of platelets during septic syndromes. For additional information, the reader is referred to several recent reviews.8,9,12
Platelet inflammatory and immune factors in sepsis
| Functional class . | Factor(s) . | Target cells . |
|---|---|---|
| Adhesion and signaling molecule | P-selectin | PMNs, monocytes, lymphocytes |
| ITIM-containing immunoreceptor | Triggering receptor expressed on myeloid cells–like transcript-1 (TLT-1) | Platelets |
| Inflammatory modulators | Histamine | ECs, monocytes, PMNs, natural killer cells, T and B cells, eosinophils |
| Serotonin (5-HT) | Monocytes, macrophages, DC | |
| Inflammatory and immunomodulatory lipids | TXA2 | Platelets, T-lymphocyte and macrophage subsets |
| PAF | Platelets, PMNs, monocytes, macrophage and lymphocyte subsets | |
| Adaptive immune modulator | CD40L (CD154) | B cells, T lymphocytes, EC, monocytes, DC subtypes, epithelial cells |
| Growth factors | PDGF | Monocytes, macrophages, T lymphocytes |
| TGF-β | Monocytes, macrophages, T and B lymphocytes | |
| Chemokines | PF4 (CXCL4) | PMNs, monocytes, macrophages |
| NAP2 (CXCL7) | PMNs | |
| GRO-α (CXCL1) | PMNs | |
| ENA-78 (CXCL5) | PMNs | |
| SDF-1 (CXCL12) | Bone marrow–derived progenitor cells | |
| RANTES (CCL5) | Monocytes, eosinophils, basophils, natural killer cells, T-lymphocyte and DC subsets | |
| MIP-1α (CCL3) | Monocytes, eosinophils, basophils, natural killer cells, lymphocyte and DC subsets | |
| MCP-3 (CCL7) | Monocytes, basophils, natural killer cells, lymphocyte and DC subsets | |
| Cytokines | IL-1β and IL-1α | Monocytes, DC and macrophage subsets, T-cell lines, EC, vascular smooth muscle cells, synoviocytes |
| MIF | Monocytes, macrophages | |
| HMBG1 | Macrophages, PMNs, ECs | |
| GM-CSF | Eosinophils | |
| Antimicrobial peptides | Platelet microbicidal proteins (several classes) | No human cellular targets identified; microbicidal for several bacteria and fungi |
| β-defensin 1 | PMNs (NET formation) |
| Functional class . | Factor(s) . | Target cells . |
|---|---|---|
| Adhesion and signaling molecule | P-selectin | PMNs, monocytes, lymphocytes |
| ITIM-containing immunoreceptor | Triggering receptor expressed on myeloid cells–like transcript-1 (TLT-1) | Platelets |
| Inflammatory modulators | Histamine | ECs, monocytes, PMNs, natural killer cells, T and B cells, eosinophils |
| Serotonin (5-HT) | Monocytes, macrophages, DC | |
| Inflammatory and immunomodulatory lipids | TXA2 | Platelets, T-lymphocyte and macrophage subsets |
| PAF | Platelets, PMNs, monocytes, macrophage and lymphocyte subsets | |
| Adaptive immune modulator | CD40L (CD154) | B cells, T lymphocytes, EC, monocytes, DC subtypes, epithelial cells |
| Growth factors | PDGF | Monocytes, macrophages, T lymphocytes |
| TGF-β | Monocytes, macrophages, T and B lymphocytes | |
| Chemokines | PF4 (CXCL4) | PMNs, monocytes, macrophages |
| NAP2 (CXCL7) | PMNs | |
| GRO-α (CXCL1) | PMNs | |
| ENA-78 (CXCL5) | PMNs | |
| SDF-1 (CXCL12) | Bone marrow–derived progenitor cells | |
| RANTES (CCL5) | Monocytes, eosinophils, basophils, natural killer cells, T-lymphocyte and DC subsets | |
| MIP-1α (CCL3) | Monocytes, eosinophils, basophils, natural killer cells, lymphocyte and DC subsets | |
| MCP-3 (CCL7) | Monocytes, basophils, natural killer cells, lymphocyte and DC subsets | |
| Cytokines | IL-1β and IL-1α | Monocytes, DC and macrophage subsets, T-cell lines, EC, vascular smooth muscle cells, synoviocytes |
| MIF | Monocytes, macrophages | |
| HMBG1 | Macrophages, PMNs, ECs | |
| GM-CSF | Eosinophils | |
| Antimicrobial peptides | Platelet microbicidal proteins (several classes) | No human cellular targets identified; microbicidal for several bacteria and fungi |
| β-defensin 1 | PMNs (NET formation) |
This list is not comprehensive and platelet factors with other immune or inflammatory activities have been identified (modified from Rondina et al.8 ).
DC, dendritic cell; EC, endothelial cell; IL, interleukin; MCP-3, monocyte chemotactic protein-3; NET, neutrophil extracellular traps; PF4, platelet factor 4; PMNs, polymorphonuclear neutrophils; RANTES, regulated on activation, normal T cell expressed and secreted.
Platelet surface ligands sense and respond to pathogens
The platelet surface is replete with numerous receptors that not only regulate hemostatic responses but also trigger proinflammatory and immune cascades (Figure 1; Table 1). Glycoprotein VI (GPVI), which is only found on platelets, triggers platelet microparticle release and subsequent inflammatory signaling through IL-1.13 GPVI also amplifies platelet activation by thrombin, thus providing a mechanism to coordinate signaling with G-protein–coupled pathways.14 Interestingly, GPVI may be a receptor for hepatitis C, mediating viral transport and replication mechanisms.15 C-type lectin-like receptors, including DC-SIGN, mediate HIV-1 capture by platelets16 and platelet activation in dengue infection.17
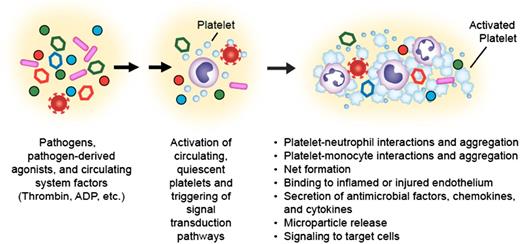
Upon activation, platelets mediate responses central to inflammatory and immune activities. Pathogens, pathogen-derived agonists, and circulating systemic factors present in the septic milieu such as thrombin, adenosine diphosphate (ADP), and others activate circulating human platelets (indicated by blue cells). Platelet activation results in upregulation of surface integrins, such as integrin αIIbβ3, binding of plasma fibrinogen, the formation of platelet-platelet and platelet-leukocyte aggregates, display and release of p-selectin, CD40L and other mediators, secretion of antimicrobial factors, and signaling to other target cells. These responses can be both adaptive and maladaptive to the host during infectious syndromes (modified from Rondina et al.8 ).
Upon activation, platelets mediate responses central to inflammatory and immune activities. Pathogens, pathogen-derived agonists, and circulating systemic factors present in the septic milieu such as thrombin, adenosine diphosphate (ADP), and others activate circulating human platelets (indicated by blue cells). Platelet activation results in upregulation of surface integrins, such as integrin αIIbβ3, binding of plasma fibrinogen, the formation of platelet-platelet and platelet-leukocyte aggregates, display and release of p-selectin, CD40L and other mediators, secretion of antimicrobial factors, and signaling to other target cells. These responses can be both adaptive and maladaptive to the host during infectious syndromes (modified from Rondina et al.8 ).
Human platelets and megakaryocytes also express mRNA and/or protein for TLRs 1, 2, 3, 4, 5, 6, and 9 18,19 (Adrianna Vieira de Abreu, Robert Campbell, and Guy Zimmerman, unpublished studies). TLRs bind diverse ligands from many infectious pathogens, including bacteria, viruses, parasites, and protozoa. Thus, the discovery that human platelets possess TLRs established direct mechanisms by which platelets may function as pathogen “sensors.”19 Moreover, by recognizing endogenous ligands as well as microbial pathogen-associated molecular pattern (PAMP) motifs, platelet TLRs provide pathways by which human platelets can respond to PAMPs, thereby mediating both infectious and noninfectious immune syndromes.20,21
Among the TLRs identified on platelets, TLR2 and TLR4 have been most extensively studied to date.18,19 TLR2 is expressed on the cell surface of platelets and serves as a receptor for PAMPs, particularly lipoproteins and peptidoglycans of bacterial cell walls. TLR2 forms heterodimers with TLR1 or TLR6 and stimulates platelet activation and aggregation and promotes platelet-neutrophil aggregates. TLR4 is abundantly expressed on the platelet surface. TLR4 is the receptor for lipopolysaccharide (LPS), an endotoxin component on the outer membrane of gram-negative organisms that elicits strong immune responses. Although still incompletely understood and potentially less robust than TLR2 responses, LPS-induced platelet responses include aggregation, degranulation, and secretion. LPS activates human platelets both in vitro and during in vivo settings, where healthy volunteers are challenged with low doses of LPS, although activation patterns are variable.22-24 However, murine platelets exposed to LPS have demonstrated increased adherence to fibrinogen despite no increase in activation, a lack of fibrin clot formation, and no evidence of degranulation by electron microscopy. Interestingly, LPS has also been shown to lead to pre-mRNA splicing and protein synthesis,24 alterations in surface CD40L,25 and trafficking of platelets to the pulmonary capillaries in murine models of endotoxemia.26 Moreover, LPS-induced platelet activation triggers formation of NETs by PMNs, enhancing bacterial capture and killing.26 The role of platelet trafficking to the pulmonary capillaries is unclear but remains an area of active investigation. Platelets may also mediate key aspects of acute lung injury.
TLR9, although less well examined, is intriguing given its unexpected cellular localization patterns.22,27 TLR9 is expressed on the plasma membrane and in the cytoplasm of quiescent human platelets, and its surface expression increases upon activation of platelets with agonists such as thrombin.27,28 TLR9 is a ligand for unmethylated CpG islands in viral and bacterial DNA, suggesting a previously unrecognized system of pathogen sensing by human platelets. Moreover, TLR9 binds a carboxyalkylpyrrole protein adduct that may serve as a PAMP, resulting in platelet activation, aggregation, and granule secretion and thrombosis.29 Though it is incompletely characterized, TLR9 may represent another functional platelet receptor linking immune, inflammatory, and thrombotic pathways.
Expression of surface receptors and ligands allows platelets to sense and respond to pathogens as well as directly interact with immune effector cells and interact with the vascular endothelium. For example, activated platelets can express GP1bα and can bind both neutrophils via Mac-1 and activated endothelial cells via P-selectin and von Willebrand factor.30,31 Platelets were also recently identified as “collaborating” with Kupffer cells, liver immune cells that constitutively express von Willebrand factor via GP1b.32 In addition, platelets may also encase bacteria and aggregate on the Kupffer cells to aid in pathogen clearance and protection against organ damage. These findings further confirm that platelets not only act as sensors but, in some settings, can directly act on pathogens (bacteria, parasites, and viruses). These recent discoveries build on earlier works, including those of Youssefin and colleagues demonstrating that activated platelets can engulf Staphylococcus aureus and HIV.32-34
Platelets secrete mediators that augment host immune mechanisms
Activated platelets have numerous direct and indirect mechanisms for delivering signals to target cells involved in immune and inflammatory interactions. These diverse mechanisms include platelet secretion of chemokines, cytokines, and other mediators (Table 1).12 In sepsis, activation of platelets may contribute to injurious micro- and macrovascular thrombosis (Figure 2). There are >300 proteins secreted by platelets, the majority of which are stored in at least 1 of 3 types of storage organelles: α granules, dense granules or bodies, and lysosomes. Recently, a fourth electron-dense tubular system compartment, called the T-granule, was discovered and, intriguingly, colocalizes with TLR9.27 Many of these proteins translocate to the platelet surface and are released upon platelet activation. Other secreted proteins are located basally on the platelet membrane and/or within other subcellular stores (Table 1).
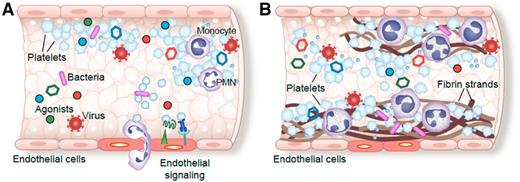
Platelet activation, homotypic and heterotypic binding to other platelets and leukocytes, and sequestration of platelets and myeloid leukocytes within thrombi are common complications of septic syndromes in humans. Pathogens, pathogen-derived factors, or agonists present in the septic milieu (A) induce platelet activation and aggregation, signaling to areas of injured or inflamed endothelium, and (B) orchestrating deposition of fibrin, platelets, and myeloid leukocytes within micro- and macrovascular thrombi (modified from Rondina et al.8 ). In the figure, platelets are indicated as blue, irregularly- or spherically-shaped cells.
Platelet activation, homotypic and heterotypic binding to other platelets and leukocytes, and sequestration of platelets and myeloid leukocytes within thrombi are common complications of septic syndromes in humans. Pathogens, pathogen-derived factors, or agonists present in the septic milieu (A) induce platelet activation and aggregation, signaling to areas of injured or inflamed endothelium, and (B) orchestrating deposition of fibrin, platelets, and myeloid leukocytes within micro- and macrovascular thrombi (modified from Rondina et al.8 ). In the figure, platelets are indicated as blue, irregularly- or spherically-shaped cells.
Protein products released by platelets orchestrate intercellular signaling for dynamic immune and inflammatory responses.8,12 For example, quiescent human platelets possess pre-mRNA for IL-1β but almost no mature IL-1β mRNA or IL-1β protein.35 Upon activation with agonists often present in the infectious milieu, including thrombin and LPS, platelets synthesize pro–IL-1β protein.35 Components of the inflammasome, which regulate host defense mechanisms to pathogens,36,37 are present within platelets and mediate conversion of pro–IL-1β to the mature IL-1β cytokine.18 Mature IL-1β, the gatekeeper of inflammation, can be released by platelets into the systemic circulation and/or packaged within platelet-derived microparticles (MPs). IL-1β within platelet MPs allows for communication with and activation of extravascular cells and induction of endothelial permeability.13,38 Platelet shedding of IL-1β–rich MPs also may contribute to enhanced vascular permeability in hemorrhagic viral infections such as dengue.39 Recent evidence demonstrates that IL-1β also acts through an autocrine loop to amplify platelet activation responses.40 Other proteins secreted by platelets, including PF4, RANTES, PAI-1 (plasminogen activator inhibitor 1), and CD40L are involved in key pathways for innate immune regulation, inflammation, and adaptive immune responses.8,22,41 Additional well-known platelet functions, including clot retraction and the formation of heterotypic aggregates, may mediate host responses and outcomes of infectious syndromes.
Clinical implications of altered platelet functions in sepsis
Emerging and established evidence demonstrates that platelets participate in diverse inflammatory and immune clinical syndromes. Although a detailed discussion is beyond the scope of this review, the reader is referred to several recent articles summarizing these activities.8,9,18,19,22,42 In the next section, we briefly discuss several of these clinical implications.
Platelets are key mediators of bacterial, viral, and parasitic infectious syndromes. Among these infectious syndromes, bacterial sepsis, dengue, and malaria are particularly important to highlight given their commonness and high risk of significant morbidity and mortality.43 Alterations in platelet number and function are integral to each of these, yet the mechanisms and roles of these alterations remain incompletely defined. Moreover, the extent to which platelets may augment host defense mechanisms or induce injurious systemic responses is unclear.
Thrombocytopenia is common in septic syndromes, with clinical studies demonstrating an inverse correlation between platelet number and adverse outcomes.19,44,45 Although many studies have focused on alterations in “traditional” platelet activation patterns (eg, adhesion, secretion, platelet-monocyte aggregation), emerging evidence highlights the new biology of platelets in septic syndromes. For example, in response to in vitro stimulation, LPS, thrombin, bacteria, and bacterial toxins, human platelets process constitutively present tissue facture (TF) pre-mRNA, resulting in a mature TF transcript, synthesis of TF protein, and generation of TF-dependent procoagulant activity.24 Moreover, platelets from septic patients, but not healthy controls, express the mature, spliced TF mRNA, demonstrating that the septic milieu may alter the platelet transcriptome.24 Preliminary analyses of next-generation RNA sequencing data (“deep sequencing”) performed on platelets from septic patients identified numerous transcripts that are significantly altered compared with matched, healthy control subjects. Many of these altered transcripts mediate key immune and inflammatory pathways and augment host defenses to bacterial invaders (M.T.R. and J.R. Rowley, unpublished observations). In addition, heterotypic platelet-leukocyte aggregate formation may be linked to adverse outcomes in sepsis, particularly in vulnerable populations such as the elderly.46
Platelets are also likely early responder and effector cells in malaria infections. Human platelets inhibit the growth of some malaria species and also kill the malarial pathogen in parasitized red blood cells, experimental evidence that platelets may serve as early host defense responses to malarial infection in the vasculature.47-49 In contrast to these protective innate immune activities, platelets may also contribute to injurious vasculopathy in severe malaria. Although the mechanisms are still not entirely understood, murine studies demonstrate that platelet signaling via PF4 release mediates T-cell recruitment and monocyte activation within the central nervous system.50 These processes may contribute to central nervous system injury in severe malaria. Recent studies of patients infected with Plasmodium vivax suggest that the thrombocytopenia that commonly occurs in malaria may be caused by platelet phagocytosis.51 Taken together, these studies and others provide experimental and clinical evidence that platelets are key immune effector cells in malaria.52,53
Dengue, a mosquito-borne viral infection, has variable clinical manifestations. Nevertheless, especially in more severe cases, marked thrombocytopenia, hemorrhage, “capillary leak,” and shock may occur. Although the dengue flavivirus targets endothelial cells and leukocytes, interactions of the virus with platelets and megakaryocytes is likely a central feature of the pathophysiology and course of infection.43 Cytokines synthesized by platelets or in response to platelet-dependent signaling of monocytes, including IL-1β, IL-8, tumor necrosis factor–α, and MCP-1,22 are increased in plasma from patients with dengue and correlate with the degree of thrombocytopenia.54 Increased IL-1β in platelets and platelet MPs was recently reported to occur through a mechanism dependent on mitochondrial reactive oxygen species–triggered NLRP3 inflammasomes.39 Dengue virus also alters l-arginine transport and nitric oxide (NO) generation in platelets in vitro, resulting in impaired aggregation.55 It is feasible that this impairment in the NO generation may be protective because NO is known to be a potent vasodilator and contribute to the hemodynamic instability in shock. In addition, platelets isolated from patients with dengue have evidence of mitochondrial dysfunction with activation of apoptosis pathways, mediated through DC-SIGN.17 Intriguingly, dengue virus has recently been demonstrated to enter and replicate with human platelets.56 These findings suggest that platelet dysfunction may contribute to the thrombocytopenia and associated hemorrhagic complications of dengue infection.
Conclusions
Although traditionally thought of as primary hemostatic cells, platelets are emerging as dynamic effector cells that sense and respond to invading pathogens. Ongoing studies in experimental models of infection and human studies will further elucidate the mechanisms, pathways, and processes governing the platelet’s broad repertoire of immune functions. These previously unrecognized findings may also lead to the development of novel therapies targeting the platelet to inhibit or augment host defense mechanisms.
Acknowledgments
The authors thank Diana Lim for outstanding figure preparation.
This work was supported by the National Institutes of Health, National Heart, Lung, and Blood Institute (HL112311 and HL126547) (M.T.R.) and National Institute on Aging (AG048022) (M.T.R.).
Correspondence
Matthew T. Rondina, University of Utah, Department of Internal Medicine, Eccles Institute of Human Genetics, 15 North 2030 East, Building 533, Suite 4220, Salt Lake City, UT 84112; e-mail: matt.rondina@u2m2.utah.edu.
References
Competing Interests
Conflict-of-interest disclosures: The authors declare no competing financial interests.
Author notes
Off-label drug use: None disclosed.