Abstract
Heavy menstrual bleeding (HMB), which is the preferred term for menorrhagia, affects ∼90% of women with an underlying bleeding disorder and ∼70% of women on anticoagulation. HMB can be predicted on the basis of clots of ≥1 inch diameter, low ferritin, and “flooding” (a change of pad or tampon more frequently than hourly). The goal of the work-up is to determine whether there is a uterine/endometrial cause, a disorder of ovulation, or a disorder of coagulation. HMB manifest by flooding and/or prolonged menses, or HMB accompanied by a personal or family history of bleeding is very suggestive of a bleeding disorder and should prompt a referral to a hematologist. The evaluation will include the patient’s history, pelvic examination, and/or pelvic imaging, and a laboratory assessment for anemia, ovulatory dysfunction, underlying bleeding disorder, and in the case of the patient on anticoagulation, assessment for over anticoagulation. The goal of treatment is to reduce HMB. Not only will the treatment strategy depend on whether there is ovulatory dysfunction, uterine pathology, or an abnormality of coagulation, the treatment strategy will also depend on the age of the patient and her desire for immediate or long-term fertility. Hemostatic therapy for HMB may serve as an alternative to hormonal or surgical therapy, and may even be life-saving when used to correct an abnormality of coagulation.
Learning Objectives
Identify 3 clinical features that correlate with true HMB
Name the first-line therapies for HMB
Heavy menstrual bleeding (HMB), which is the preferred term for menorrhagia, affects ∼90% of women with an underlying bleeding disorder1 and ∼70% of women on anticoagulation.2-5 It is a condition hematologists are expected to understand and, in some cases, to help manage. This paper will describe normal menstruation, define HMB, summarize the causes of HMB, describe the gynecologic and hematologic evaluation of HMB, and discuss the hormonal, surgical, and hemostatic management of HMB.
Normal menstruation
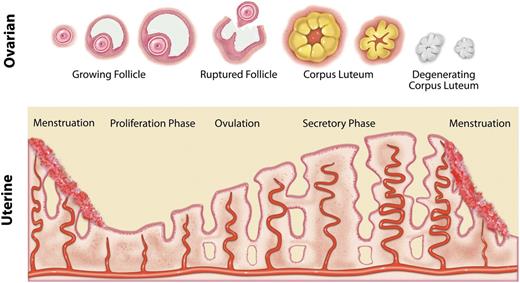
The following description of normal menstruation applies to all reproductive-age women, including adolescents. If fertilization (pregnancy) does not occur, the menstrual cycle begins with the sloughing or shedding of the lining of the uterus (ie, the endometrium). Then, under the influence of estrogen from ovarian follicles, the endometrium increases in thickness due to the development of glands and blood vessels. Approximately mid-cycle (in a normal cycle), ovulation occurs. The dominant ovarian follicle undergoes a process described as luteinization, becomes a corpus luteum, and secretes progesterone. Under the influence of progesterone, the endometrial glands mature and become secretory. In the absence of fertilization, the endometrial vessels constrict and the newly developed portion of the endometrium, including the new glands and blood vessels, becomes ischemic and starts to slough, thereby beginning another cycle (Figure 1).
Diagram of changes in the ovary and endometrium during the normal menstrual cycle.
Diagram of changes in the ovary and endometrium during the normal menstrual cycle.
Definition and estimation of HMB
HMB is defined as >80 mL of blood per cycle.6 The gold standard for the measurement of blood in sanitary products is the extraction of hematin using a 5% sodium hydroxide solution and the estimation of the alkaline hematin content by spectrophotometry.6 This method is not feasible in clinical practice and has only been used in research studies. In a rigorous study in which the alkaline hematin method was used to measure menstrual blood loss, HMB was predicted on the basis of clots ≥1 inch in diameter, low ferritin, and “flooding,” defined as a change of pad or tampon more frequently than hourly.7 When attempting to ascertain if a patient has HMB, these are signs and symptoms that can be queried.
The causes of HMB
HMB can result from an abnormality of the uterus (polyps, adenomyosis, leiomyoma, malignancy, or hyperplasia), an abnormality of the endometrium, a disorder of ovulation, an iatrogenic cause, an abnormality of coagulation, or some cause not yet recognized.8 Polyps, adenomyosis, leiomyoma, malignancy, or hyperplasia are acquired conditions and are more likely to affect adult women than adolescents. Polyps are epithelial overgrowths in the endometrium or the lining of the uterine cervix (endocervix). Adenomyosis is the growth of endometrium within the muscular layer of the uterus (myometrium). Leiomyoma or fibroids are benign fibromuscular tumors of the uterus. Malignancy or hyperplasia is more likely to occur in women over the age of 45 or women who have been exposed to unopposed estrogen (estrogen without the benefit of progesterone). An abnormality specifically of the endometrium is hard to diagnose, but chlamydial infection has been associated with abnormalities of the endometrium. Reduced or absent ovulation is more likely to occur with an endocrine abnormality (thyroid disease, polycystic ovarian syndrome, or pituitary adenoma) or at the extremes of reproductive age (adolescence and the perimenopause). Failure of ovulation allows the endometrium to continue to thicken (increase in size). With no corpus luteum to secrete progesterone, the endometrial glands do not mature or become secretory so the endometrium is shed irregularly. If and when ovulation does occur, the subsequent shedding of the thick, abundant endometrium results in HMB. An iatrogenic cause of HMB can be due to unsuccessful hormonal manipulations. An abnormality of coagulation can be due to anticoagulants, antiplatelet medications, thrombocytopenia, other abnormal platelet function, or insufficient clotting factors including von Willebrand factor (VWF).
The gynecologic evaluation of HMB
A woman with HMB usually seeks care from a gynecologist or other women’s health care provider, and it is usually that provider who initiates the work-up. The goal of the work-up is to determine whether there is a uterine/endometrial abnormality, a disorder of ovulation, or a disorder of coagulation. The International Federation of Gynecology and Obstetrics developed a mnemonic for these disorders: PALM-COEIN (polyp; adenomyosis; leiomyoma; malignancy and hyperplasia; coagulopathy; ovulatory dysfunction; endometrial; iatrogenic; and not yet classified).8 The initial work-up includes the history (menstrual, medical, bleeding, medications, and recent trauma) and the physical examination. Speculum and pelvic examinations will depend on the age of the patient and the clinician’s judgment. In a virginal adolescent, an abdominal ultrasound may be substituted for the pelvic examination. Papanicolau test, endometrial biopsy, or endocervical/vaginal swab for Chlamydia and gonorrhea depends on the age of the patient and her other history. A pelvic ultrasound will depend on clinical judgment (based on diagnostic suspicion and the age of the patient). The transabdominal approach is the procedure of choice for any nonsexually active female, and the transvaginal approach is the procedure of choice for those females who are emotionally mature and sexually active.9 Intrauterine saline instillation at the time of transvaginal (endovaginal) ultrasound ( sonohysterography), increases the sensitivity for abnormalities of the uterine cavity, but is usually reserved for the evaluation of acquired uterine abnormalities in perimenopausal bleeding.10 Initial laboratory studies should include a complete blood count (CBC) and ferritin. If the patient also has irregular menstrual bleeding, the patient should have a pregnancy test as well as laboratory tests for underlying pathology that would explain ovulatory dysfunction such as thyroid stimulating hormone, prolactin, and, if signs of hyperandrogenism, serum androgens.
HMB manifest by flooding and/or prolonged menses, or HMB accompanied by a personal or family history of bleeding is very suggestive of a bleeding disorder and should prompt a referral to a hematologist. Philipp et al11 reported on the importance of various signs and symptoms as predictors of a bleeding disorder in women with HMB and developed a screening tool that can be used by women’s health care providers. From 12 pages of questions and using multiple variable logistic regression, the investigators were able to identify 8 questions, subsumed by the following 4 criteria, any one of which was predictive of a bleeding disorder if accompanied by HMB:
Duration of menses ≥7 days and flooding or impairment of daily activities with most periods
History of treatment of anemia
Family history of a diagnosed bleeding disorder
History of excessive bleeding with tooth extraction, delivery or miscarriage, or surgery
The screening tool alone had a sensitivity of 82% for bleeding disorders. Although the results would not be available at an initial visit, adding a pictorial blood assessment chart score >100 increased the sensitivity of the screening tool to 95%. A PFA-100 (Tarrytown, NY) did not increase the sensitivity of the screening tool for all bleeding disorders, but did increase the sensitivity for von Willebrand disease (VWD) to 92%.11
The hematologic evaluation in HMB
The gynecologic and hematologic evaluation of HMB is summarized in Table 1.
Gynecologic and hematologic evaluation of the patient with HMB
| Evaluation | Component |
| Patient history | Menstrual |
| Medical | |
| Bleeding | |
| Medication, including use of hormonal contraceptives, anticoagulants, and antiplatelet agents | |
| Recent trauma | |
| Vital signs | Blood pressure |
| Heart rate | |
| Speculum and pelvic examinations | Depending on the age of the patient and the clinician’s judgment |
| Depending on the age of the patient and her previous history | |
| Papanicolau test (age >21 y) | |
| Endometrial biopsy (age >45 y or unopposed estrogen) | |
| Endocervix or vaginal swab for gonorrhea and Chlamydia (age <25 y) | |
| Pelvic ultrasound | Depending on clinical judgment (based on diagnostic suspicion and the age of the patient) |
| With intrauterine saline instillation for increased sensitivity (sonohysterography) | |
| Laboratory tests | |
| Immediate | CBC |
| Ferritin | |
| If periods are irregular | Pregnancy test |
| TSH | |
| Prolactin | |
| If signs of androgen excess | DHEA; total testosterone |
| If on warfarin | PT/INR |
| If on a direct oral anticoagulant | Creatinine |
| If a bleeding disorder is suspected | PT |
| aPTT | |
| Fibrinogen level | |
| Additional to consider | VWF and FVIII levels |
| Plasma sample for storage | |
| Serum iron and total iron binding capacity | |
| Liver function | |
| Full hemostatic evaluation including the following tests if indicated (ie, past personal or family history to suggest an underlying disorder of hemostasis) | |
| Platelet aggregation and release studies (cannot be done at time of acute event) | |
| FIX, XI, and XIII | |
| Tests of fibrinolysis | |
| Evaluation | Component |
| Patient history | Menstrual |
| Medical | |
| Bleeding | |
| Medication, including use of hormonal contraceptives, anticoagulants, and antiplatelet agents | |
| Recent trauma | |
| Vital signs | Blood pressure |
| Heart rate | |
| Speculum and pelvic examinations | Depending on the age of the patient and the clinician’s judgment |
| Depending on the age of the patient and her previous history | |
| Papanicolau test (age >21 y) | |
| Endometrial biopsy (age >45 y or unopposed estrogen) | |
| Endocervix or vaginal swab for gonorrhea and Chlamydia (age <25 y) | |
| Pelvic ultrasound | Depending on clinical judgment (based on diagnostic suspicion and the age of the patient) |
| With intrauterine saline instillation for increased sensitivity (sonohysterography) | |
| Laboratory tests | |
| Immediate | CBC |
| Ferritin | |
| If periods are irregular | Pregnancy test |
| TSH | |
| Prolactin | |
| If signs of androgen excess | DHEA; total testosterone |
| If on warfarin | PT/INR |
| If on a direct oral anticoagulant | Creatinine |
| If a bleeding disorder is suspected | PT |
| aPTT | |
| Fibrinogen level | |
| Additional to consider | VWF and FVIII levels |
| Plasma sample for storage | |
| Serum iron and total iron binding capacity | |
| Liver function | |
| Full hemostatic evaluation including the following tests if indicated (ie, past personal or family history to suggest an underlying disorder of hemostasis) | |
| Platelet aggregation and release studies (cannot be done at time of acute event) | |
| FIX, XI, and XIII | |
| Tests of fibrinolysis | |
aPTT, activated partial thromboplastin time; DHEA, dehydroepiandrosterone; FIX, factor IX; INR, international normalized ratio; PT, prothrombin time; TSH, thyroid stimulating hormone.
The patient with a suspected underlying bleeding disorder
The patient’s evaluation will include a review of the history (menstrual, medical, bleeding, and medications), a review of the gynecologic evaluation, and a laboratory assessment for an underlying bleeding disorder. A previously undiagnosed underlying bleeding disorder accompanies 5% to 24% of women with HMB1 and an even higher proportion of adolescents with HMB.12 In a meeting of experts in the fields of hematology, and obstetrics and gynecology, a consensus was achieved on what should comprise the laboratory assessment for an underlying bleeding disorder.1 Evaluation should include:
CBC
Ferritin (if not performed already)
Activated partial thromboplastin time
PT
VWF (measured with ristocetin cofactor activity and antigen)
FVIII
Fibrinogen
Although testing appears to be most sensitive during menstruation when coagulation factor levels, most notably VWF and FVIII, are potentially at their lowest,13 testing should not be delayed to coincide with menstruation, but repeat testing during menses should be considered if initial VWF levels are at the lower limit of normal.1 Patients should not be removed from hormonal contraception to permit testing.1 Nonetheless, it should be remembered that women with mild type 1 VWD may have normal results when combined hormonal contraceptives are used, estrogens being sometimes, but not always, associated with normalization of coagulation test results.14 If the preceding tests are normal, studies of platelet aggregation and platelet release should be considered, because data suggest that defects in platelet aggregation and/or release may be associated with HMB.15 Although it is well documented that blood group affects plasma VWF levels, the identification of blood group is not essential, because bleeding symptoms in VWD are determined by VWF levels, independent of blood group.1 Hematologic assessments should be repeated as necessary to confirm the diagnosis of a bleeding disorder.
The patient on anticoagulation or antiplatelet medication
The patient’s evaluation will include a review of the history (menstrual, medical, bleeding, and medications), a review of the gynecologic evaluation and a basic laboratory assessment to include a CBC and ferritin (if not already performed), an international normalized ratio if the patient is on warfarin, and a creatinine if the patient is on a direct oral anticoagulant. The evaluation will also include an assessment as to whether antiplatelet medication or anticoagulation is necessary for a particular patient, or whether antiplatelet medication or anticoagulation can be safely discontinued or the dose reduced.
Hormonal management of HMB
In women with bleeding disorders
The goal of treatment is to reduce HMB. Not only will the treatment strategy depend on whether there is ovulatory dysfunction, uterine pathology, or an abnormality of coagulation, the treatment strategy will also depend on the age of the patient and her desire for immediate or long-term fertility. In a woman who desires future fertility, who is not trying to conceive, and who does not have contraindications to estrogen, first-line therapy is combined hormonal contraceptives. Combined hormonal contraceptives containing an estrogen (usually ethinyl estradiol), and a progestin (a synthetic progesterone) suppress ovulation (preventing hemorrhagic ovarian cysts), regulate the menstrual cycle, and reduce menstrual blood flow.16 Combined hormonal contraceptives are available in the form of oral contraceptive pills (taken daily), patches (changed weekly), and vaginal rings (changed monthly), but only oral contraceptive pills have been included in randomized trials of HMB.17 Oral contraceptive pills reduce menstrual blood flow 35% to 69%.17 Bleeding patterns appear to be improved with continuous as opposed to cyclic administration,18 and with pills containing more than 20 μg of ethinyl estradiol vs pills with 20 μg or less.19 In a randomized trial of continuous administration, combined oral contraceptives containing 1 mg of norethindrone resulted in fewer days of bleeding and spotting than preparations containing 0.1 mg of levonorgestrel.20 An alternative to either continuous administration, which is associated with breakthrough bleeding,21 or the traditional cyclic administration with 21 days of hormones and 7 days of placebo, is a shortened hormone-free interval of 2, 3, or 4 days instead of 7. A shortened hormone-free interval is associated with greater ovarian suppression (with reduced risk of hemorrhagic ovarian cysts) and less menstrual bleeding than traditional cyclic administration.21 Pills with a single hormonal formulation (monophasic pills), as opposed to pills with formulations that vary during the cycle, can be used in tapering regimens and are otherwise easier to manipulate.
Progestin-only contraceptives are an alternative to combined hormonal contraceptives for the reduction of menstrual blood flow. Progestin-only contraceptives are available in the form of low-dose pills (taken daily), intramuscular or subcutaneous injections (administered every 3 months), subcutaneous implants (changed every 3 years), and the levonorgestrel intrauterine device (IUD) (changed every 3 or 5 years depending on the size). An advantage of the levonorgestrel IUD is that there is very little systemic absorption of the progestin. Nonetheless, menstrual blood flow is reduced 71% to 95%.17 Reduced menstrual blood flow has been confirmed in women with bleeding disorders22 and in women on anticoagulation.23 In the 2 randomized controlled trials of the levonorgestrel IUD vs oral contraceptive pills, the levonorgestrel IUD was superior in reducing menstrual blood flow,17 and, therefore, it should also be considered another first-line therapy for HMB. A disadvantage of the levonorgestrel IUD, however, is that it does not suppress ovulation and does not suppress formation of hemorrhagic ovarian cysts.24 Progestin-only pills incompletely suppress ovulation.24
Specific hormonal contraceptives for the treatment of HMB are summarized in Table 2.
Specific hormonal contraceptives for the treatment of HMB
| . | Estrogen: EE . | Progestin . | Comments . |
|---|---|---|---|
| Combined hormonal contraceptives | |||
| Combined oral contraceptive pills | Packaged as 21 d of active pills or 21 d of active pills + 7 d of placebo pills | ||
| 20 μg EE | 1 mg norethindrone | Available in an extended cycle regimen with 24 d of active pills + 4 d of placebo | |
| 30 μg EE | 1.5 mg norethindrone | — | |
| 35 μg EE | 1 mg norethindrone | — | |
| 20 μg EE | 0.1 mg levonorgestrel | — | |
| 20 μg EE | 90 μg levonorgestrel | Marketed as a continuous regimen | |
| 30 μg EE | 1.5 mg levonorgestrel | Available in an extended cycle regimen with 84 d of active pills + 7 d of placebo or 10 μg of EE | |
| Patch | 20 μg EE daily | 150 μg of norelgestromin daily | Applied weekly for 3 wk out of 4 |
| Ring | 15 μg EE daily | 120 mcg of etonogestrel daily | Worn 3 wk out of 4 |
| Progestin-only contraceptives | |||
| Pills | — | 0.35 mg norethindrone | Daily |
| Intramuscular injection | — | 150 mg DMPA | Every 3 mo |
| Subcutaneous injection | — | 104 mg DMPA | Every 3 mo |
| Subcutaneous implant | — | 68 mg etonogestrel | Slowly released over ≥3 y; ∼60 μg daily after 3 mo, which slowly decreases to 30 μg daily at the end of 2 y |
| Intrauterine device | — | 52 mg levonorgestrel | Release rate of 20 μg daily, FDA-approved for 5 y of use |
| — | 13.5 mg levonorgestrel | Release rate of 14 μg daily, FDA approved for 3 y of use | |
| . | Estrogen: EE . | Progestin . | Comments . |
|---|---|---|---|
| Combined hormonal contraceptives | |||
| Combined oral contraceptive pills | Packaged as 21 d of active pills or 21 d of active pills + 7 d of placebo pills | ||
| 20 μg EE | 1 mg norethindrone | Available in an extended cycle regimen with 24 d of active pills + 4 d of placebo | |
| 30 μg EE | 1.5 mg norethindrone | — | |
| 35 μg EE | 1 mg norethindrone | — | |
| 20 μg EE | 0.1 mg levonorgestrel | — | |
| 20 μg EE | 90 μg levonorgestrel | Marketed as a continuous regimen | |
| 30 μg EE | 1.5 mg levonorgestrel | Available in an extended cycle regimen with 84 d of active pills + 7 d of placebo or 10 μg of EE | |
| Patch | 20 μg EE daily | 150 μg of norelgestromin daily | Applied weekly for 3 wk out of 4 |
| Ring | 15 μg EE daily | 120 mcg of etonogestrel daily | Worn 3 wk out of 4 |
| Progestin-only contraceptives | |||
| Pills | — | 0.35 mg norethindrone | Daily |
| Intramuscular injection | — | 150 mg DMPA | Every 3 mo |
| Subcutaneous injection | — | 104 mg DMPA | Every 3 mo |
| Subcutaneous implant | — | 68 mg etonogestrel | Slowly released over ≥3 y; ∼60 μg daily after 3 mo, which slowly decreases to 30 μg daily at the end of 2 y |
| Intrauterine device | — | 52 mg levonorgestrel | Release rate of 20 μg daily, FDA-approved for 5 y of use |
| — | 13.5 mg levonorgestrel | Release rate of 14 μg daily, FDA approved for 3 y of use | |
DMPA, depot medroxyprogesterone acetate; EE, ethinyl estradiol; FDA, Food and Drug Administration.
In women with a history of thrombosis who are on anticoagulation
There has been a question as to whether women who are on anticoagulation should receive combined hormonal contraceptives to help manage their HMB. An argument against their use in this situation is that combined hormonal contraceptives may increase the risk of recurrent thrombosis. An argument for their use is that anticoagulation mitigates the risk of recurrent thrombosis. Also, untreated HMB contributes to anemia, a reduction or interruption of anticoagulant therapy, and surgery, all of which increase the risk of recurrent thrombosis. In women with a history of thrombosis who are on anticoagulation, the US Centers for Disease Control and Prevention (CDC) Medical Eligibility Criteria for Contraception considers that the risks of combined hormonal contraceptives are unacceptable or outweigh the benefits.25 However, in recognition of the need to balance risks in women with HMB, the CDC Medical Eligibility Criteria also state, “When a contraceptive method is used as a therapy, rather than solely to prevent pregnancy, the risk/benefit ratio may differ and should be considered on a case-by-case basis.”25 New data from the EINSTEIN deep vein thrombosis and pulmonary embolism anticoagulation trial found that women who used combined hormonal contraceptives (with a recurrence rate of venous thromboembolism [VTE] of 3.7%) were no more likely to suffer recurrent VTE than were women who used progestin-only contraceptives (with a recurrence rate of 3.8% per year), or women who did not use any hormonal contraceptives (with a recurrence rate of 4.7% per year). The investigators concluded that in women who are on therapeutic anticoagulation, hormonal therapy did not increase the risk of recurrent VTE.5
In women with a history of thrombosis who are not on anticoagulation
There has generally been agreement that women with a history of thrombosis who are not on anticoagulation should not receive combined hormonal contraceptives to help manage their HMB. The risk of VTE with combined hormonal contraceptives is increased two- to eightfold.26,27 The component of combined hormonal contraceptives most closely associated with an increased risk of thrombosis is estrogen, but progestins, depending on their formulation or their dosage, have also been implicated. Different progestins appear to have a differential effect on the risk of thrombosis. There is new evidence that the injectable contraceptive, DMPA increases the risk of thrombosis, whereas other lower-dose progestin-only contraceptives (pills, subcutaneous implants, and the levonorgestrel IUD) do not. A recently published systematic review of progestins and thromboembolism completed by the CDC included 2 studies that found that the use of DMPA among healthy women was associated with increased odds of VTE.28 In the Thrombo Embolism Hormone Study in Sweden, DMPA was associated with an increased risk of VTE (adjusted odds ratio [OR] 2.2; 95% confidence interval [CI], 1.3-4.0). In the Multiple Environmental and Genetic Assessment study in the Netherlands,29 DMPA was also associated with an increased risk of VTE (OR, 3.6; 95% CI, 1.8-7.1). In both studies, the progestin-containing levonorgestrel IUD was not associated with an increased risk of VTE, nor was there an increased risk of VTE with progestin-only pills and subcutaneous implants in the Swedish study.30 Similarly, in national cohort studies from Denmark, the levonorgestrel IUD, progestin-only pills, and subcutaneous implant were not associated with increased risk of VTE, stroke, or myocardial infarction.27,31,32 There are no Danish data on DMPA.
Sometimes women with HMB are treated with noncontraceptive progestin-formulations, such as medroxyprogesterone or norethindrone, which generally contain higher doses than those used for contraception and which may increase the risk of VTE. For instance, a contraceptive dose of norethindrone is 0.35 mg daily, whereas a typical therapeutic dose of norethindrone for abnormal uterine bleeding is 5.0 mg daily, which is nearly 15 times the contraceptive dose. There are limited data on the increased risk of VTE when progestins are used for the treatment of abnormal uterine bleeding (presumably in higher doses) as opposed to when progestins are used for contraception, but 1 nested case-control study found an adjusted OR of 5.3 (95% CI, 1.5-18.7).33 The formulation of the progestin(s) in this study was not specified.
Surgical management of HMB
For women who have completed childbearing, surgical management of HMB is an option. Reduction of the uterine lining by dilation of the cervix and curettage (D&C) (ie, scraping) of the uterus may be temporarily effective in controlling acute HMB, but D&C is ineffective long term and may cause more bleeding. The role of D&C in the management of HMB is usually used for diagnostic purposes when endometrial biopsy is unsuccessful or uninformative. As opposed to D&C, endometrial ablation (destruction of the uterine lining) is effective in controlling menstrual blood loss in 82% to 97% of women.34 In a study comparing the procedure in 41 women with bleeding disorders with 111 women without, the procedure was found to be equally successful in both groups.35 Disappointingly, 8.5% of women required further surgery.34 Also, in a population-based retrospective cohort study, endometrial ablation was found to be no more effective than the levonorgestrel IUD, and both were found to be less effective than hysterectomy.36 Uterine artery embolization, which has primarily been used to promote the resolution of uterine fibroids through ischemia and necrosis, is also a less invasive technique than hysterectomy. It has not been studied as a method to reduce menstrual blood flow in the absence of uterine fibroids, but in women with fibroids, it has been shown to reduce menstrual blood loss by 80% to 90%.37,38
Hysterectomy is the definitive treatment of HMB. Hysterectomy by any route is associated with the complications of major surgery, including bleeding, infection, and thrombosis, as well as complications specific to hysterectomy such as urinary and sexual problems.34 Consequently, patients and providers may reject hysterectomy as initial treatment in HMB because it is invasive and requires time for recovery, but in a randomized trial of hysterectomy vs medical therapy for abnormal uterine bleeding, hysterectomy was superior to medical treatment of improving health-related quality of life.39 As for the safety in women with bleeding disorders, data from the Nationwide Inpatient Sample, a US discharge database, found that 7% percent of women with VWD who underwent hysterectomy received a transfusion compared with 2% of all women who underwent hysterectomy, which is not an unreasonable difference.40
Hemostatic management of HMB
Hemostatic therapy for HMB may serve as an alternative to hormonal or surgical therapy, or be used to correct a coagulation defect. In women who have failed hormonal therapy and desire to preserve fertility, hemostatic therapy is the next step in treatment. For women who are trying to conceive and cannot use hormonal therapy, it is the preferred treatment. Hemostatic therapy may be specific to a particular disorder or be nonspecific, as is the case with antifibrinolytic medication. Out of 6 randomized controlled trials of antifibrinolytic medication in the treatment of HMB cited in a recent systematic review, 5 used tranexamic acid (or a pro-drug of tranexamic acid) and only 1 used aminocaproic acid (and it was published in 1965).17 In the 4 randomized controlled trials that used tranexamic acid, tranexamic acid was administered in doses of 1.0 g 4 times per day or 1.3 g 3 times per day for a total of 3.9 g to 4.0 g per day for up to 5 days of the menstrual cycle (Table 3).41-44 All recent medical literature on the use of antifibrinolytic medication to treat HMB, including in adolescents,45 involves the use of tranexamic acid. There are no studies on the safety of tranexamic acid in women with a history of thrombosis and there are no studies on the safety of tranexamic acid as an adjunct to hormonal therapy. Multiple studies, however, have demonstrated no increased risk of VTE with the use of tranexamic acid,46 although studies have excluded women with a history of thrombosis or those using hormonal contraception.
Dosing of tranexamic acid for treatment of HMB in randomized trials
| Study . | Dose . | Frequency . | Duration . |
|---|---|---|---|
| 41 | 1 g | Every 6 h | 5 days starting on day 1 of the cycle |
| 42 | 1 g | 4 times per day | 4 days starting on day 1 of the cycle |
| 43 | 1.3 g (2650 mg tablets) | 3 times per day | Up to 5 days per cycle |
| 44 | 0.65 g* vs 1.3 g† | 3 times per day | Up to 5 consecutive days per cycle |
| Study . | Dose . | Frequency . | Duration . |
|---|---|---|---|
| 41 | 1 g | Every 6 h | 5 days starting on day 1 of the cycle |
| 42 | 1 g | 4 times per day | 4 days starting on day 1 of the cycle |
| 43 | 1.3 g (2650 mg tablets) | 3 times per day | Up to 5 days per cycle |
| 44 | 0.65 g* vs 1.3 g† | 3 times per day | Up to 5 consecutive days per cycle |
Met 2 end points.
Met 3 end points.
Desmopressin is considered specific therapy for type 1 VWD, but it has also been used as nonspecific hemostatic therapy. The dosing of desmopressin for the treatment of HMB is 300 μg administered on days 2 and 3 of the menstrual cycle (one puff in each nostril each day). In a randomized crossover study of intranasal desmopressin vs placebo in 39 women with inherited bleeding disorders, desmopressin was more effective than placebo in reducing menstrual blood loss as measured by pictorial blood assessment chart scores.47 In another randomized crossover study of tranexamic acid vs intranasal desmopressin in 116 women with HMB, a negative gynecological evaluation, and abnormal laboratory hemostasis, tranexamic acid was found to be more effective than desmopressin in reducing menstrual blood loss as measured by pictorial blood assessment chart scores.48
A number of single plasma-derived or recombinant coagulation factors, as well as 3 and 4 factor prothrombin complex concentrates are available to treat specific coagulation factor defects. VWD is the most common inherited bleeding disorder, accounting for 90% of women seen at the CDC-designated Hemophilia Treatment Centers,49 and is the condition for which women with HMB are most likely to receive specific hemostatic therapy. Of 1321 women with VWD seen at Hemophilia Treatment Centers from 2011 to 2014, 816 (61.8%) had HMB. VWF was used to treat 13 women (1.6%).50
Management of acute HMB
The general approach to the management of acute HMB, defined as HMB requiring emergency treatment, in a patient with a disorder of hemostasis requires the combined efforts of the gynecologist and the hematologist. The gynecologist can attempt to control uterine bleeding hormonally or surgically, whereas the hematologist attempts to correct deficiencies of clotting factors, correct abnormalities of platelet number or function, or correct excessive anticoagulation. In a randomized trial of IV conjugated equine estrogen vs placebo to treat acute HMB, 25 mg was administered initially with a second dose after 3 hours, if cessation of bleeding had not been achieved. After 5 hours, 64% of those who received conjugated equine estrogen vs 11% of the controls had cessation of bleeding.51 Oral hormonal regimens do not work as quickly. In a randomized trial comparing oral medroxyprogesterone acetate 20 mg, and a monophasic combination oral contraceptive containing 1 mg norethindrone and 35 μg of ethinyl estradiol, each administered 3 times per day, the median time to cessation of bleeding was 3 days.52 Tapering of a 3-pill per day regimen to a 1-pill per day maintenance regimen can begin when the patient is stable, usually within 24 to 48 hours, by reducing the number of pills by 1 pill per day. The relative importance of hemostatic, hormonal, and surgical treatment options will vary in each clinical situation. When bleeding is uncontrolled and other therapies have not yet been initiated or a response to them has not yet been obtained, cessation of bleeding can usually be achieved with uterine tamponade from a suitably sized Foley catheter and a Foley balloon inflated to the point of resistance, which is then left in place from 2 hours to 2 days.53,54 There are few data regarding the use of balloon tamponade in gynecology relative to its use in obstetrics, but one series reported complete cessation of acute HMB in 17/20 patients and partial cessation in another 2 patients.53
Conclusion
HMB, which is the preferred term for menorrhagia, affects ∼90% of women with an underlying bleeding disorder and ∼70% of women on anticoagulation. The goal of the work-up is to determine whether there is a uterine/endometrial cause, a disorder of ovulation, or a disorder of coagulation. The goal of treatment is to reduce HMB. Not only will the treatment strategy depend on whether there is ovulatory dysfunction, uterine pathology, or an abnormality of coagulation, the treatment strategy will also depend on the age of the patient and her desire for immediate or long-term fertility. The general approach to the management of acute HMB in a patient with a disorder of hemostasis requires the combined efforts of the gynecologist and the hematologist.
Correspondence
Andra H. James, Department of Obstetrics and Gynecology, Duke University, DUMC 3967, Durham, NC 27710; e-mail: andra.james@duke.edu.
References
Competing Interests
Conflict-of-interest disclosure: The author has received honoraria from Baxalta and Novo Nordisk.
Author notes
Off-label drug use: None disclosed.