Abstract
Atypical hemolytic uremic syndrome (aHUS) is a thrombotic microangiopathy (TMA) that affects multiple organs and the kidneys in particular. aHUS can be sporadic or familial and is most commonly caused by dysregulation of the alternative complement pathway. The initial attack of aHUS can occur at any age, and is associated with a high rate of progression to end stage renal disease. Many aHUS patients relapse in the native or transplanted kidneys, and require close monitoring and long-term management. Availability of anticomplement therapy has revolutionized the management of aHUS, and can change the natural course of aHUS by inducing hematologic remission, improving or stabilizing kidney functions, and preventing graft failure. As a result, it is important to succeed in the challenging task of differentiating aHUS from other TMAs and initiate adequate treatment early during the course of disease. Considering the high cost of currently available anticomplement therapy, it is important also from a financial point of view to accurately diagnose aHUS early during the course of disease and determine the necessary length of therapy. This highlights the need for development of precise complement functional and genetic studies with rapid turnaround time.
Learning Objectives
To understand the regulation of the complement system and role of complement dysregulation in the pathogenesis of aHUS
To become familiar with a diagnostic algorithm for aHUS
To understand how to treat aHUS and to monitor the therapeutic response of aHUS patients
Definition
Thrombotic microangiopathies (TMA) are a group of disorders characterized by injury and occlusion of microvessels resulting in tissue ischemia and organ damage. TMA can be categorized according to their etiology (hereditary or acquired), age of onset, being sporadic or familial, involved organs, clinical association and manifestations, and prognosis. Overlap between these categories make a definite diagnosis challenging, and justify the initial consideration of different therapeutic approaches.1,2
Thrombotic thrombocytopenic purpura (TTP) is a distinct category of TMA, caused by a severe reduction in the function of ADAMTS13 (a disintegrin and metalloproteinase with thrombospondin type 1 motif 13) (<10% activity), due to blocking ADAMTS13 autoantibodies or in 5% to 10% of patients due to loss-of-function mutations in ADAMTS13 (Upshaw-Shulman syndrome). TTP is a systemic disorder with microthrombi in several organs, including the heart, central nervous system, and to a lesser degree, the kidneys.
Hemolytic uremic syndrome (HUS) is another category of TMA that is associated with microthrombi mainly in the kidneys; and can be classified into typical HUS and atypical HUS (aHUS). Majority of HUS patients are children (mostly >6 months of age), diagnosed with typical HUS as a sequela of an infection with Shiga-toxin (Stx) or Shiga-like toxin-producing enteropathogenic bacteria, most commonly Stx-producing Escherichia coli (STEC). Typical HUS is also known as Stx-HUS or STEC-HUS, and will be referred to as STEC-HUS in the rest of this study. STEC-HUS is an abrupt severe illness. One week after the development of bloody diarrhea, ∼15% of children develop acute renal failure,3 but long-term renal impairment in survivors is not common. Rarely, urinary tract infection by STEC, without diarrhea, can cause STEC-HUS.
In addition to E coli, invasive pneumococcal infections can result in HUS, via generating neuroaminidase that removes Thomsen-Friedenreich antigen (T antigen) from the surface of erythrocytes, platelets, and glomerular endothelial cells. Preformed immunoglobulin M natural antibodies bind to the exposed T antigen and initiate complement-mediated tissue damage. As a result of binding of natural antibodies to exposed T antigen on red blood cells, most patients with pneumococcal HUS have a positive direct Coombs test for C3. Pneumococcal infection- associated HUS has a worse prognosis than STEC-associated HUS with a mortality rate close to 50%.4
aHUS is less common than STEC-HUS, and only ∼10% of HUS cases are aHUS.5,6 In comparison with STEC-HUS, aHUS has a more insidious onset with fluctuating clinical and laboratory abnormalities. aHUS is not associated with STEC infection, but can be triggered by infections (especially in children), pregnancy, or drugs. aHUS can be further classified into the primary aHUS that is associated with dysregulation of the alternative complement pathway, and secondary aHUS (cancer-, chemotherapy-, solid-organ transplant- or hematopoietic stem cell transplant- [HSCT], pregnancy-, or autoimmune disorder-associated). Whether complement dysregulation has a role in the pathogenesis of secondary HUS is an interesting question that requires additional studies. In this study, we will focus on primary aHUS, and on complement-mediated tissue injury as the main pathogenic mechanism of primary aHUS.
Regardless of the etiology of HUS, the clinical and pathologic manifestations of STEC-HUS and aHUS are similar. Clinical manifestations of HUS include intravascular hemolysis, thrombocytopenia, and kidney failure. Pathologic findings in kidneys are consistent with endothelial injury in microvessels and glomeruli, including endothelial swelling and proliferation, widening of subendothelial space, mesengiolysis, and fibrin-rich thrombi in capillaries and arterioles.7
In the past 3 decades, various mutations in complement and complement regulatory proteins (loss-of-function mutations in Factor H [FH], FI, membrane cofactor protein (MCP), and deletions in FH-related proteins; and gain-of-function mutations in C3 and FB) have been discovered in 50% to 60% of aHUS patients.8-12 The genotype-clinical phenotype correlations in aHUS will be discussed in this study (Table 1).8,11,13,14 However, it is important to emphasize that the anticomplement therapy is effective in aHUS associated with different genotypes, and have changed the natural course of aHUS.
Genotype-phenotype correlation in aHUS
| . | Frequency (%) . | ESRD/death (%) . | Relapse (%) . | Recurrence in transplanted kidney . |
|---|---|---|---|---|
| CFH | 23-27 | 63-80 | 20-40 | 80-90 |
| CFI | 3-8 | 50-70 | 20-40 | 70-80 |
| C3 | 2-8 | 43-60 | 20-40 | 40-50 |
| MCP | 5-9 | 17-20 | 80 | 15-20 |
| CFB | 1-2 | 70 | 70 | 88 |
| Anti-FH Ab | 3-6 | 30 | 60* 10† | 20 |
| THBD | 0-5 | 60 | — | — |
| Combined | 4 | — | — | — |
| No mutation | 33-54 | 27 | 20-40 | 27 |
| . | Frequency (%) . | ESRD/death (%) . | Relapse (%) . | Recurrence in transplanted kidney . |
|---|---|---|---|---|
| CFH | 23-27 | 63-80 | 20-40 | 80-90 |
| CFI | 3-8 | 50-70 | 20-40 | 70-80 |
| C3 | 2-8 | 43-60 | 20-40 | 40-50 |
| MCP | 5-9 | 17-20 | 80 | 15-20 |
| CFB | 1-2 | 70 | 70 | 88 |
| Anti-FH Ab | 3-6 | 30 | 60* 10† | 20 |
| THBD | 0-5 | 60 | — | — |
| Combined | 4 | — | — | — |
| No mutation | 33-54 | 27 | 20-40 | 27 |
Ab, antibody; CFB, factor B; CFH, factor H; CFI, factor I.
*Without immunosuppression.
†With immunosuppression.
Alternative complement pathways
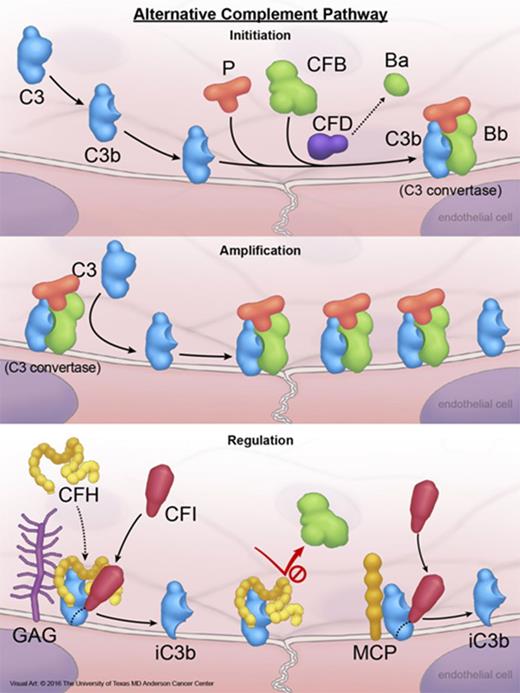
There are 3 complement pathways: classical, alternative, and lectin pathways. The 3 pathways differ in their initiation steps, but all converge on the formation of a C3 convertase that is essential for propagation of complement activation. The classical or lectin pathways require recognition of an antigen by an antibody or mannose-binding lectin, respectively, and subsequent activation of C4 by either C1 complex (classical pathway) or a C1-like complex (lectin pathway). The alternative complement system is a component of innate immunity and comprises several plasma and cell surface proteins.15 For activation of the alternative pathway, there is no need for a specific antigen recognition, and merely the presence of a “permissive” surface that does not actively inhibit complement activation suffices to activate the alternative pathway (Figure 1). Complement activation products C3a and C5a are generated by cleavage of C3 by the C3 convertase and cleavage of C5 by C5a convertase. C3a and C5a are anaphylatoxins with discrete cellular receptors, that when occupied by their cognate ligand, trigger a number of biological responses including chemotaxis of myeloid cells and degranulation of mast cells. The other complement activation product is MAC (C5b-9) that perforates the cell membrane, and after reaching an adequate density on cell membrane, lyses the targeted cells. CD59 is a membrane protein that prevents the assembly of C5b-9 on the cell surface, a negative complement regulatory function that is also performed by soluble proteins clusterin and vitronectin. The final results of complement attack are opsonization of targeted cells by covalently bound C3b for phagocytosis, membrane perforation and cell lysis by C5b-9, and chemotaxis of neutrophils and macrophages guided by the gradient of anaphylatoxins. The extensive damage that can be inflicted by the alternative complement pathway on cells explain the necessity for a tight complement regulation under physiologic conditions (Figure 1); and the pathologic consequences (endothelial damage and platelet activation) of complement dysregulation.
Activation and regulation of the alternative complement pathway. A small amount of plasma C3 is continuously converted to C3b that is highly active and binds to cell surface via forming thiol bonds. Under normal conditions, C3b is rapidly degraded in plasma by FI and FH (FI cofactor), or on the cell surface by FI and glycosoaminoglycan-bound FH, membrane protein CD46 (or MCP), or CD35. Degradation of C3b to an inactive product (iC3b) on the cell membrane by membrane-bound FH, MCP, or CD35 requires FI, and is known as cofactor activity. IC3b cannot participate in any further complement activation. If not inactivated, C3b is able to bind to an activation product of FB (Bb) produced by FD-mediated cleavage of FB, and generate C3bBb (C3 convertase). C3 convertase is the engine of the complement pathways and deposit additional C3b molecules on membrane, which in turn amplify complement activation. C3 convertase is an important target for complement regulatory proteins. FH prevents the formation of C3 convertase and dissociate C3 convertase by competing with FBb for binding to C3b. This negative regulatory activity of FH is known as decay accelerating activity. If C3bBb remains intact, the complex is stabilized by FP (properdin) and binds to additional C3b to generate C3bBbC3b, which is also known as C5 convertase. C5 convertase activates C5 to generate C5b, which in turn binds to C6, C7, and C8, forming the C5b-8 complex that stably inserts into the lipid bilayer of the cell. Next, the C5b-8 complex binds and polymerizes multiple molecules of C9 (C5b-9n), forming cytolytic MAC of complement on the cell surface. CFB, factor B; CFD, factor D; CFH, factor H; CFI, factor I; GAG, glycosoaminoglycans; MAC, membrane attack complex; P, properdin. Reprinted from The University of Texas MD Anderson Cancer Center with permission.
Activation and regulation of the alternative complement pathway. A small amount of plasma C3 is continuously converted to C3b that is highly active and binds to cell surface via forming thiol bonds. Under normal conditions, C3b is rapidly degraded in plasma by FI and FH (FI cofactor), or on the cell surface by FI and glycosoaminoglycan-bound FH, membrane protein CD46 (or MCP), or CD35. Degradation of C3b to an inactive product (iC3b) on the cell membrane by membrane-bound FH, MCP, or CD35 requires FI, and is known as cofactor activity. IC3b cannot participate in any further complement activation. If not inactivated, C3b is able to bind to an activation product of FB (Bb) produced by FD-mediated cleavage of FB, and generate C3bBb (C3 convertase). C3 convertase is the engine of the complement pathways and deposit additional C3b molecules on membrane, which in turn amplify complement activation. C3 convertase is an important target for complement regulatory proteins. FH prevents the formation of C3 convertase and dissociate C3 convertase by competing with FBb for binding to C3b. This negative regulatory activity of FH is known as decay accelerating activity. If C3bBb remains intact, the complex is stabilized by FP (properdin) and binds to additional C3b to generate C3bBbC3b, which is also known as C5 convertase. C5 convertase activates C5 to generate C5b, which in turn binds to C6, C7, and C8, forming the C5b-8 complex that stably inserts into the lipid bilayer of the cell. Next, the C5b-8 complex binds and polymerizes multiple molecules of C9 (C5b-9n), forming cytolytic MAC of complement on the cell surface. CFB, factor B; CFD, factor D; CFH, factor H; CFI, factor I; GAG, glycosoaminoglycans; MAC, membrane attack complex; P, properdin. Reprinted from The University of Texas MD Anderson Cancer Center with permission.
Regulatory proteins of the alternative complement pathway involved in the pathogenesis of aHUS
Mutations can be detected in ∼50% to 60% of aHUS patients, with a majority of these mutations involving complement regulatory proteins (FH, FI, and MCP) and a small percentage of gain-of-function mutations in complement proteins C3 and FB.6,11 Lack of identifiable mutations in complement proteins does not rule out the possibility of aHUS, as became clear after the detection of mutations in coagulation proteins and metabolic enzymes in aHUS patients.
FH protein family
About half of detected mutations in aHUS are located in CFH (25% of all aHUS patients).6,11 FH is the main regulatory protein of the alternative complement pathway and is functional both in plasma and on the cell surface.16,17 FH is composed of 20 homologous structural domains, known as short consensus repeats (SCR). Despite the structural similarities, the SCRs have distinct functional roles. The N-terminal SCRs are necessary and sufficient for the regulation of C3 in the fluid phase.18 The C-terminal SCRs 19 and 20 are necessary for FH binding to glycosoaminoglycans on membrane, and hence complement regulation on cell surfaces (Figure 1). FH prevents autolysis of C3 in plasma, and together with FI, degrades C3b deposited on the cell surfaces to iC3b. Most aHUS mutations in FH are heterozygous, located in the region of the gene encoding SCRs 19 and 20, and do not change the quantity of FH in plasma, but reduce its activity on the cell membrane. Mutant FH molecules are unable to adhere to the cell surface and cannot regulate complement activation, resulting in complement-induced damage and microthrombi, especially in the renal microvasculature.
Several complement regulatory genes including genes encoding FH and FH-related proteins (FHRP1-5) are located in proximity to one another on chromosome 1, in a region known as the region of complement activation.19 The proximity of CFH and FHRP1-5 provides the opportunity for genetic recombination, resulting in deletions or hybrid genes. Heterozygous FHRP1-3 deletion is a relatively common polymorphism with a frequency of 2% to 9% (Europeans) to 16% (Africans) in the general population.20 Homozygous FHRP1-3 deletion is detected in patients with aHUS, most commonly associated with anti-FH antibodies. About 80% of aHUS patients with anti-FH antibodies have concomitant FHRP-1 deletion. Anti-FH antibodies result in acquired FH deficiency and are detectable in 3% to 8% of aHUS patients, mostly in children <16 years of age.6,11,21
FI
FI is a plasma serine protease that prevents activation of the alternative and classic complement pathways by degrading C3b to iC3b, and through additional cleavages of iC3b, to C3d and C3dg. C3b degradation products (ic3b, C3d, and C3dg) cannot bind to FB and cannot generate C3 convertase. FI protease activity depends on the concurrent binding to C3b of a cofactor, either FH, CD35, or CD46. FH binds to C3b (on cell surface or in plasma) and FI, and provides the platform on which FI cleaves C3b. Mutations in FI are heterozygous and detected in 4% to 8% of patients with aHUS. Approximately half of these mutations result in dysfunctional FI with normal plasma quantities.6,11
CD46 or MCP
MCP is a membrane protein expressed on nucleated cells and regulates both the alternative and classical complement pathways by serving as a cofactor for FI for cleavage of C3b and C4b. Gene encoding MCP is also located in the region of complement activation of chromosome 1q3.2. Most MCP mutations detected in aHUS are located in 4 extracellular SCRs, and the majority of these mutations decrease the expression of MCP that can be detected by flow cytometry on white blood cells in the peripheral blood. MCP mutations are detected in ∼5% to 9% of aHUS patients and are associated with frequent relapses, but overall, a mild clinical course. Majority of aHUS patients with MCP mutations have a complete recovery from their initial aHUS attack, and among them only 6% to 19% progress to develop end-stage renal disease (ESRD) and very few develop relapse after kidney transplant.6,11
C3 and FB
In addition to mutations in complement regulatory proteins, mutations in C3 and FB are detected in 2% to 8% and 1% to 2% of patients with aHUS, respectively. C3 mutations reduce binding of C3b to FH, and FB mutations increases the affinity of FB to C3b, resulting in a more stable C3 convertase and hyperactivation of the alternative complement pathway. Patients with aHUS associated with C3 or FB mutations have low plasma C3 levels, and have an aggressive clinical course with >88% developing ESRD, and ∼80% relapse in the transplanted kidney grafts.6,22,23
Mutations in noncomplement genes in aHUS patients
Thrombomodulin (THBD)
THBD is a membrane protein on endothelial cells that enhances thrombin-mediated activation of protein C and activation of carboxypeptidase B (also known as thrombin activatable fibrinolysis inhibitor). Carboxypeptidase B inactivates anaphylatoxins (C3a and C5a) by removing an arginine residue. In addition to its role in coagulation, THBD may participate in complement regulation, THBD enhances FI-mediated inactivation of C3b, and promotes the generation of thrombin activatable fibrinolysis inhibitor that in turn inactivates anaphylatoxins.24 Mutations in THBD can be detected in ∼5% of patients with aHUS.24
Diacylglycerol kinase ε (DGKE)
DGKE is present in endothelial cells, podocytes, and platelets, and terminates arachidonic acid-containing diacylglycerol-mediated signaling. Mutations in DGKE were identified in infantile recessive aHUS, with multiple relapses and proteinuria, and are usually unresponsive to anticomplement therapy.25,26
Plasminogen (PLG)
PLG is a component of the fibrinolytic system, and upon conversion to plasmin can degrade fibrinogen and fibrin. PLG also cleaves several complement proteins, and might have a role in the regulation of the complement system. Mutations in PLG have been reported in ∼5% of white American aHUS patients.13
C cobalamin (Cbl C) deficiency
Cbl C deficiency is a rare condition that is caused by the deficiency of methylmalonic aciduria and homocysteinuria type C, and inherited as an autosomal recessive disorder.6,27 Cbl C deficiency results in hyperhomocysteinemia, reduced methionine concentration in blood, and methylmalonicaciduria. Cbl C deficiency is usually detected in the first year of life in association with neurologic findings, such as hypotonia and seizure. Adult onset of symptoms is rare and can manifest as psychosis, ataxia, and cognitive impairment. In all age groups, hemolysis and kidney failure can be detected. Treatment of Cbl C deficiency includes the administration of pharmacologic doses of vitamins B12, B2, and folate, despite the presence of normal plasma levels of these vitamins in most of the patients.28
Clinical presentation and clinical course
aHUS is rare but has a high mortality rate, high risk of progressing to ESRD, and high risk of relapse after kidney transplant. aHUS results in intravascular hemolysis, thrombocytopenia, and renal failure in a majority of patients. Although commonly assumed to be a pediatric disorder, ∼40% to 60% of aHUS cases occur in individuals >18 years of age.29 Thrombocytopenia can be moderate with 42% of children and 27% of adults having platelet counts <50 000/μL. Fifteen percent of aHUS patients have normal platelet counts.6 On the other hand, renal failure is severe and the majority of patients require hemodialysis (59% of children and 81% of adults). In addition to an elevated creatinine, hematuria, proteinuria, edema, and hypertension are other manifestations of renal damage in aHUS. Extra-renal findings are seen in 20% to 25% of aHUS patients involving the cardiovascular system, central nervous system (seizure, focal neurologic finding, and altered mental status), and the gastrointestinal tract (diarrhea, pancreatitis, and hepatitis).6 Although it was suggested that the presence of a platelet count >30 000/μL and a serum creatinine level >2.25 mg/mL eliminates the possibility of a severely deficient ADAMTS13 activity as the cause of TMA, additional studies provide a more complicated clinical picture. For example, aHUS associated with MCP mutations can mimic TTP,30 and there are anecdotal reports of aHUS patients with normal kidney functions with mainly neurologic findings.31
An additional factor that complicates the distinction between aHUS and other TMAs are the triggering events that often precedes an episode of aHUS and are also frequently seen in other TMAs, such as an infection or a pregnancy. Preceding or concurrent infections, as a result of various pathogens, are common in children with aHUS.6 Differential diagnosis of TMA in pregnancy is particularly challenging. The clinical and laboratory findings initially consistent with a diagnosis of HELLP syndrome (hemolysis, elevated liver enzymes, and thrombocytopenia) can evolve into TTP or aHUS.32 To make matters more complicated, mutations in complement regulatory proteins were reported in patients with a diagnosis of HELLP syndrome.32 Although TTP can occur in the second or third trimester of pregnancy, aHUS most commonly occurs in the postpartum period,34 with the highest frequency after the second pregnancy.
Finally, the interactions between the complement system and hemostatic factors,35-38 evident by the presence of dual-function proteins in the complement system and hemostasis, provide the real possibility that, in some patients, the pathogenesis of TMA involves abnormalities in both systems (2-hit hypothesis) such as concurrent mutations in both complement proteins and hemostatic proteins (coagulation proteins or ADAMTS13),39,40 or complement dysregulation in patients with a low ADAMTS13 activity level.41,42
Genotype and phenotype correlations in aHUS
Mutations in FH, MCP, FI, C3, FB, PLG, THBD, and DGKE, deletion of FHRP1-3 (usually concurrent with the presence of anti-FH antibodies), and Cbl C deficiency are detected in aHUS patients. It is important to mention that most of the detected mutations in aHUS are heterozygous. Penetrance of mutations in familial aHUS is not complete (∼50%) and only about half of the individuals that carry an abnormal mutation would develop the clinical manifestations of aHUS.6 One possible explanation is that the presence of high-risk haplotypes in FH (CFHtgtggt) or MCP (MCPggac) genes increases the risk of disease in patients carrying aHUS mutations.19,43
Genetic studies in aHUS patients have diagnostic and prognostic values, and might affect the natural course of disease and the long-term management of the patients. One important shortcoming of genetic studies is that a specific mutation can be identified in only about half of aHUS patients. Prognosis of aHUS patients depends on the presence of ESRD, extra-renal involvement, frequency of recurrence after kidney transplant, and the time lag between the onset of symptoms and initiation of therapy. About 50% of aHUS patients develop ESRD, with an overall mortality rate of 25%.6 However, the death or ESRD rates vary significantly among aHUS patients according to their mutations; with FH, FB, C3, and FI mutations being associated with the worst rates (60% to 80% death or ESRD) and MCP mutations with the best rates (30% to 40% death or ESRD)11 (Table 1). Prior to the availability and prophylactic use of anticomplement reagent, the rate of recurrence in transplanted kidneys in patients with FH and FI mutations were as high 80% to 90%. This rate was lowest in patients with MCP mutations and CHFR1-3 deletion (20%), and intermediate for the other mutations.
Diagnosis
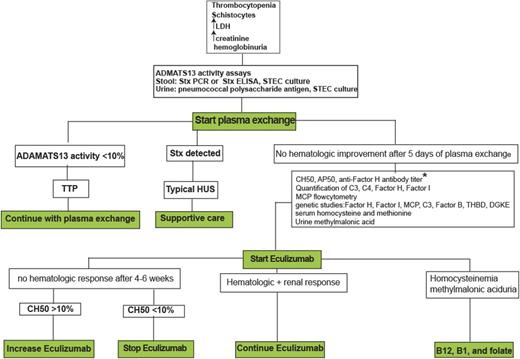
At the time of presentation, the etiology of TMA in most patients is not clear, and the clinicians need to consider several different diagnostic possibilities, and initiate laboratory studies that can identify the diagnosis and guide the long-term management. Unfortunately, several important laboratory results will not be available immediately, and may take from a few days (ADAMTS13 activity level, CH50, C3, C4, FH and FI levels, anti-FH antibody level) to a few weeks or months (genetic studies on complement genes) to be completed. Clinicians need to start treatment based on clinical and available laboratory clues, and modify the treatment according to the therapeutic response and laboratory results as they become available (Figure 2).
Diagnostic and therapeutic algorithm in TMAs. Role of eculizumab in the treatment of secondary aHUS (cancer-, chemotherapy-, solid-organ transplant- or HSCT-, pregnancy-, or autoimmune disorder-associated) have not been established and is out of the scope of this study. *In patients with anti-FH antibody, immunosuppressive reagents (steroid, rituximab, cyclophosphamide, and azathioprine) should be added to eculizumab. ELISA, enzyme-linked immunosorbent assay; LDH, lactate dehydrogenase; PCR, polymerase chain reaction.
Diagnostic and therapeutic algorithm in TMAs. Role of eculizumab in the treatment of secondary aHUS (cancer-, chemotherapy-, solid-organ transplant- or HSCT-, pregnancy-, or autoimmune disorder-associated) have not been established and is out of the scope of this study. *In patients with anti-FH antibody, immunosuppressive reagents (steroid, rituximab, cyclophosphamide, and azathioprine) should be added to eculizumab. ELISA, enzyme-linked immunosorbent assay; LDH, lactate dehydrogenase; PCR, polymerase chain reaction.
The clinical manifestations of aHUS,8,9,44 similar to other TMAs, are caused by anemia and acute kidney failure, and include pallor, fatigue, failure to thrive, edema, and drowsiness. Hypertension, either new-onset or worsening of a previously well-controlled disease, is an important finding that should be considered as a diagnostic red flag. Extra-renal manifestations, including cardiac events, seizure, diffuse or focal neurologic findings, abdominal pain, and vomiting occur in ∼20% of aHUS patients.6 Concurrent infections are not rare, and might also affect the clinical presentation. Laboratory findings are consistent with intravascular hemolysis (anemia, elevated reticulocyte counts, reduced haptoglobin, elevated lactate dehydrogenase, hemoglobinuria, negative Coombs tests [except in the case of pneumococcal HUS]) and microangiopathy (thrombocytopenia and evidence of microangiopathic hemolytic anemia with fragmented red blood cells in the peripheral blood smear). Abnormal kidney function tests are one of the main laboratory abnormalities, and include elevated creatinine, microscopic hematuria, and proteinuria that can be in the range of nephrotic syndrome.
Although the presence of comorbidities causing secondary TMA (such as cancer, HIV infection, pregnancy, solid organ transplant, or HSCT) complicates the initial manifestation of TMA, the most important diagnostic step is to consider the possibility of aHUS in the differential diagnosis. The initial attack of aHUS may occur at any age and in both genders.45 In infants, the possibility of hereditary causes including mutations in genes encoding complement proteins, ADAMTS13, DGKE, and methylmalonic aciduria and homocysteinuria type C, should be considered. However, the development of TMA in siblings within a short time period also raises the possibility of exposure to Stx and STEC-HUS. Pregnancy is an important trigger for TMA, and the initial presentation of TMA in the postpartum period should raise the possibility of aHUS.
Upon the initial suspicion of TMA, additional laboratory studies should be ordered (Figure 2). The goal of laboratory evaluation in the short-term is to identify TTP and STEC-HUS patients. The next step is to identify complement dysregulation and specially the presence of anti-FH antibody. The last step is to detect aHUS mutations. Severely reduced ADAMTS13 activity level (<10%) is the first result that determines the nature of TMA by identifying TTP patients. The presence of Stx in stool samples (detected by either enzyme-linked immunosorbent assay or polymerase chain reaction) and positive Sorbitol-MacConkey stool culture (detecting E coli O157:H7, which is the most common STEC), confirms the diagnosis of STEC-HUS. Although rare, a urine culture should be obtained to rule out STEC infection in the urine. In TMA patients with invasive pneumococcal infections (pneumonia, empyema, or meningitis) and positive direct Coombs test, the possibility of pneumococcal HUS should be considered. The second group of results that will be available are the titer of anti-FH antibody, functional complement assays, and quantifications of individual complement proteins. The presence of anti-FH antibody alters the management of aHUS and mandates the addition of immunosuppressive therapy. The titer of anti-FH antibody can be monitored to determine the length of therapy. Functional complement assays include CH50, AP50, and the FH functional assay.46 CH50 (antibody-coated sheep erythrocyte lysis assay) determines the total complement activity (classic and alternative pathways) and is normal in aHUS patients. AP50 (rabbit erythrocyte lysis assay) mainly determines the alternative complement activity and is reduced in aHUS patients due to the consumption of alternative pathway proteins. In aHUS patients, one would expect normal CH50 and low AP50 values. In the FH functional assay (uncoated sheep erythrocyte lysis assay), FH present in normal serum binds to uncoated sheep erythrocytes and prevents lysis. In aHUS caused by FH mutations or anti-FH antibodies, uncoated sheep erythrocytes lyse in the presence of patient’s serum. More recently, a modified Ham test has been proposed as a functional assay of the alternative complement pathway to distinguish aHUS from TTP, STEC-HUS, and disseminated intravascular coagulation.47 If proven by additional studies to be superior to traditional functional complement assays, a modified Ham assay can be an important step forward in the diagnostic evaluation of TMA. Quantification of C3, C4, FH, and FI by enzyme-linked immunosorbent assay or western blotting, and MCP by flow cytometry on mononuclear cells are usually conducted in the reference laboratories together with the genetic studies. A low C3 and normal C4 are consistent with the activation of the alternative complement pathway in aHUS. One should consider that ∼35% of patients with proven diagnosis of aHUS have normal C3 levels.6 Most patients with aHUS caused by FB or C3 mutations have low serum C3 levels. Majority of patients with FH mutations have a normal quantity of FH in the serum, and ∼40% of aHUS patients with FI mutations have lower levels of FI.11
The results of genetic studies in aHUS patients are the last results to become available. These results will confirm the diagnosis of aHUS and impact the long-term management of patients, such as the length of anticomplement therapy and decisions about kidney transplant. Genetic studies are conducted in the specialized reference laboratories that should be contacted to obtain detailed information about the processing of the collected blood samples. Collection of 10 mL EDTA-anticoagulated whole blood (2 purple-top tubes) and 10 mL of coagulated blood (red-top tubes) would be enough to complete genetic and functional complement studies.48
Treatment
Plasma therapy
Infusion of plasma or plasma exchange has been reported to be effective in improving hematologic parameters in patients with aHUS, and as a prophylaxis against relapse after kidney transplant.11 Despite improvement in the hematologic parameters in 50% of aHUS patients with plasma therapy, ∼48% of children and 67% of adult aHUS patients die or develop ESRD despite plasma therapy.14 Guidelines for the management of pediatric49 and adult patients50 with aHUS recommended initiation of plasma therapy soon after the diagnosis of aHUS is suspected (daily exchange of 1.5 to 2× of plasma volume or daily plasma infusion of 20 to 30 mL/kg of body weight). These guidelines were developed prior to the availability of anticomplement therapy, and one should consider treatment with an anticomplement reagent as the first-line therapy in patients with a certain diagnosis of aHUS, such as in patients with relapsed aHUS or in a sibling of a patient with a known diagnosis of aHUS. The therapeutic response to plasma therapy depends on the aHUS-causing mutations.9,11,44 Patients with C3 or THBD mutations and anti-FH antibodies are more responsive to plasma therapy, and patients with FH or FI mutations are less responsive. In patients with anti-FH antibody, immunosuppressive reagents (steroid, rituximab, cyclophosphamide, and azathioprine) should be added to plasma exchange.14 MCP is a membrane protein, and plasma therapy is not effective in aHUS patients with MCP mutations. In fact, most of the aHUS patients with MCP mutations will have complete remission with or without plasma therapy.
In practice at the time of the initial presentation, every patient with a clinical diagnosis of TMA should be started on plasma exchange. Plasma exchange should be continued for 5 days or until ADAMTS13 activity assay and Stx assay results become available (Figure 2). If ADAMTS13 level is >10% or if Stx is detected, plasma exchange can be discontinued. In patients with ADAMTS13 activity >10% and negative results for Stx, if hemolysis and thrombocytopenia do not improve after 5 days of plasma therapy, anticomplement therapy should be considered.
Eculizumab
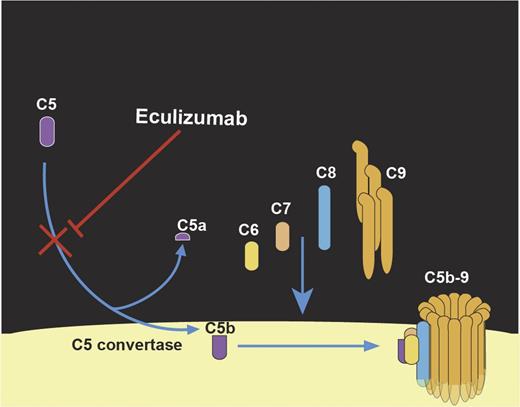
Eculizumab is a humanized immunoglobulin G2 monoclonal antibody against complement C5 (Figure 3) with a plasma t1/2 of 11 hours that blocks complement activation for 2 to 3 weeks after a single IV infusion.51,52 In 2011, eculizumab was approved by the US Food and Drug Administration for the treatment of aHUS, based on the results of two phase 2 clinical trials on a total of 37 patients.53 Administration of eculizumab in aHUS patients resulted in hematologic remission and improvement in kidney functions, as was evident by an increase in glomerular filtration rate, reduction in proteinuria, and reduction in dialysis-dependency. Improvement in kidney function was more pronounced if treatment with eculizumab was initiated earlier after the diagnosis of aHUS. After 2 years of treatment with eculizumab, TMA event-free status was maintained in the vast majority of these patients. Kidney functions remain stable in most patients, and continued to modestly improve in aHUS patients started on eculizumab earlier after the initial diagnosis.54 The key questions about eculizumab treatment are: who should receive it, when to start it, how to monitor it, and when to stop it. Patients with familial aHUS or relapsed aHUS with native or a transplanted kidney should receive eculizumab. Patients with TMA, normal ADAMTS13 activity level, and negative Stx assays, and patients with TMA and resistance to or dependency on plasma therapy should be candidates for receiving eculizumab. Patient with aHUS, who undergo kidney transplantation and have a high risk for recurrence based on their mutations, should receive prophylactic eculizumab.51 Despite the possibility of complement activation in TTP, there is not enough evidence to justify treatment with eculizumab simultaneously with plasma exchange in TTP patients, unless genetic studies show a concurrent mutation in complement proteins. Eculizumab should not be used in aHUS caused by Cbl C deficiency or DGKE mutation. Eculizumab is not shown to be effective in STEC-HUS except in anecdotal cases. Similarly, the role of complement activation in the pathogenesis of secondary aHUS (post-HSCT, cancer, and drug-induced aHUS) is not proven, and treatment with eculizumab in secondary aHUS should be considered mainly in the frame of research protocols. Patients with a suspicion of aHUS should receive eculizumab at a dose of 900 mg once weekly for 4 weeks (induction), and then 1200 mg once every other week (maintenance). Patients who continue to undergo plasma exchange should receive an additional dose of 600 mg of eculizumab after each plasma exchange.
Mechanism-of-action of eculizumab, a humanized monoclonal antibody that binds to C5 with a high affinity and inhibits cleavage of C5 by C5 convertase, and prevents generation of C5a and formation of C5b-9 MAC.
Mechanism-of-action of eculizumab, a humanized monoclonal antibody that binds to C5 with a high affinity and inhibits cleavage of C5 by C5 convertase, and prevents generation of C5a and formation of C5b-9 MAC.
Every aHUS patient should receive tetravalent vaccine (preferably conjugated) against Neisseria meningitides (A, C, Y, W135), at least 2 weeks prior to the initiation of eculizumab, and a booster dose at least 2 months after the first vaccination. If vaccination is performed <2 weeks prior to eculizumab, then patient should receive prophylactic antibiotics against N meningitides for 2 weeks after vaccination. Immunocompromised patients (ESRD or kidney transplant) should continue to receive prophylactic antibiotics as long as treatment with eculizumab continues.51 Patients from North America and Europe on eculizumab should also receive vaccination against N meningitides serotype B. Additionally, an annual influenza vaccination should be recommended.
The therapeutic response to eculizumab can be assessed by monitoring disease activity markers, such as platelet counts, lactate dehydrogenase, haptoglobin, and creatinine. If eculizumab fails to improve aHUS activity markers, the complement activity markers should be measured. There are no standard ways of monitoring complement activity on eculizumab. However, a few studies have shown that CH50, Weislab complement activity assays,52 or C5b-9 deposition on endothelial cells in vitro55 can be used to confirm the blockade of complement activity by eculizumab. Among these assays, CH50 is the most readily available one. Effective therapy of aHUS with eculizumab is expected to decrease CH50 from normal values to <10%.14 If disease activity markers and CH50 show a suboptimal response to eculizumab, one can consider increasing the maintenance dose of eculizumab by 300 mg.55,56 The concentration of eculizumab in blood has not been used as a monitoring tool, but a concentration of ≥100 μg/mL is considered to be a therapeutic level.51
Currently, there are no clear guidelines available about the discontinuation of eculizumab therapy. However, a few facts can be helpful in making a decision about the length of therapy with eculizumab. There are aHUS genotypes that are associated with a higher risk of relapse and progression to ESRD (FH, C3, FB, and FI mutations). More than 50% of aHUS patients relapse during the first year after the initial presentation. Long-term eculizumab therapy may be considered in patients with kidney transplant and high-risk genotypes, and in aHUS patients with initial severe extra-renal findings. In patients with anti-FH antibody, when the titer of antibodies is ≤2.5× the upper limit of normal, and in patients with stable ESRD, discontinuation of maintenance eculizumab therapy can be considered.57 All of the aHUS patients who stop eculizumab should be closely monitored for the evidence of relapse. One group reported a successful eculizumab discontinuation strategy in aHUS patients by regular self-monitoring of hemoglobinuria, using urine dipsticks.58
Kidney transplantation
Many aHUS patients develop ESRD, and require hemodialysis and kidney transplantation. Prior to the availability of eculizumab, 50% of aHUS patients relapsed after kidney transplant resulting in a graft failure rate of 80% to 90% after relapses. Living donor transplant (particularly from members of the same family) was contraindicated in aHUS patients due to the concerns about the possibility of aHUS in the transplanted kidney. Currently, aHUS patients who undergo kidney transplant require rigorous risk assessment and preparation,51 including vaccinations and administration of eculizumab to be started prophylactically prior to and continued after transplant. The length of prophylactic therapy with eculizumab after kidney transplant depends on the risk of recurrence in the recipients.51 Patients with a high risk of recurrence (FH, C3, and FB mutations) need life-long prophylactic eculizumab therapy. In recipients with a moderate risk of recurrence (isolated FI mutation, persistent low titer of anti-FH antibody, or no detectable complement mutations), prophylactic eculizumab can be discontinued after a 12-month relapse-free posttransplant period. MCP expression in the donor kidney might protect against aHUS recurrence, and patients with MCP mutations only rarely develop graft failure as a result of aHUS relapse.11 Recipients with an isolated MCP mutation or no detectable anti-FH antibody might not need prophylaxis with eculizumab after transplant. As a result, it is important to conduct a through genetic evaluation in aHUS patients, who are candidates for kidney transplant. However, because no genetic abnormalities can be detected in about half of aHUS patients, unrelated kidney donors are preferred. Several complement proteins including FH, FI, FB, and C3 are produced in the liver, and there are few cases of liver-kidney transplant performed in aHUS patients resulting in complete remission.59 One would expect that the availability of effective anticomplement therapies will make this aggressive therapeutic option less desirable in the future.
Conclusion
Studies on the pathogenesis of aHUS provide important information about the overlap between complement biology, hemostasis and thrombosis, and vascular biology. Despite diagnostic challenges, the availability of anticomplement reagents have revolutionized the management of aHUS, and changed the natural course of this disorder. More robust genetic tests and functional complement assays, with quicker turnaround, are needed to facilitate the diagnosis and monitoring of the therapeutic response of aHUS patients.
Correspondence
Vahid Afshar-Kharghan, Section of Benign Hematology, MD Anderson Cancer Center, 1515 Holcombe Blvd, Houston, TX 77030; e-mail: vakharghan@mdanderson.org.
References
Competing Interests
Conflict-of-interest disclosure: V.A.-K. is on the Board of Directors or on an advisory committee for Alexion.
Author notes
Off-label drug use: None disclosed.