Abstract
Obesity and inflammatory bowel disease (IBD) are systemic inflammatory disorders that predispose to arterial and venous thrombosis through similar prothrombotic mechanisms. Obesity and IBD are chronic risk factors that lead to a persistently elevated risk of thrombosis, although the thrombotic risk with IBD appears to wax and wane with disease severity. Because of the lack of high-quality evidence to guide management decisions, approaches to the prevention and treatment of thrombosis in patients with obesity or IBD are based on extrapolation from general guidelines for antithrombotic therapy. Obesity alters the pharmacokinetics of some anticoagulant drugs, and IBD patients present the added management challenge of having a high risk of gastrointestinal bleeding while taking anticoagulants. An extended duration of anticoagulant therapy is often recommended for obese or IBD patients with unprovoked venous thromboembolism unless there is a high risk of bleeding, although more data and better biomarkers are needed to determine whether anticoagulation can be safely stopped in a subset of IBD patients during remission of active disease. Most patients with obesity or IBD require thromboprophylaxis in conjunction with hospitalization or surgery, with adjustment of anticoagulant dosing in patients with severe obesity.
Learning Objectives
To understand how the chronic inflammatory conditions of obesity and inflammatory bowel disease predispose to venous thrombosis through similar prothrombotic mechanisms
To describe how obesity alters the pharmacokinetics of some anticoagulant drugs
To explain why patients with IBD have a high risk of gastrointestinal bleeding while taking anticoagulants, especially during flares of disease activity
To understand that because of a lack of high-quality evidence to guide management decisions, approaches to the prevention and treatment of thrombosis in patients with obesity or IBD are based on extrapolation from general guidelines for antithrombotic therapy
Inflammation as a trigger of thrombosis
It has long been recognized that inflammation can activate coagulation systems, leading to consumptive coagulopathy, thrombosis, and tissue injury. The classic paradigm of inflammation-triggered coagulopathy is bacterial sepsis, in which the acute systemic inflammatory response to infection leads to endothelial injury and expression of tissue factor, a potent initiator of coagulation.1 The resultant activation and consumption of coagulation factors and platelets, along with impaired fibrinolysis, disruption of endothelial barrier function, and loss of physiological antithrombotic factors such as thrombomodulin2 create a clinical situation in which the patient may be at risk not only for hemorrhage but also for arterial, venous, or microvascular thrombosis. Acute, fulminant coagulopathy can also be triggered by inflammatory processes driven by noninfectious conditions such as cancer, trauma, severe burns, or complications of pregnancy.
Thrombosis is also a relatively common complication of conditions associated with chronic inflammation, such as obesity, inflammatory bowel disease (IBD), and other autoimmune disorders.3,4 Hematology consultants are often asked to provide recommendations regarding the prevention and management of thrombosis in such patients for whom evidence-based guidelines may not be available. This chapter will update the management of adult patients at risk for thrombosis as a result of obesity or IBD as exemplars of chronic prothrombotic inflammatory conditions.
Obesity
The World Health Organization estimates that the global prevalence of obesity has more than doubled since 1980.5 In 2008, more than 1.4 billion adults were overweight (body mass index [BMI] ≥25 kg/m2) and nearly 500 million were obese (BMI ≥30 kg/m2).5 In 2010, the prevalence of obesity in the United States had reached 35% among adults and 20% among children 6 to 11 years of age.6
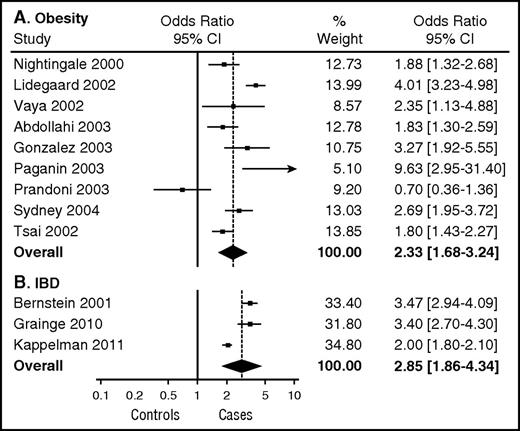
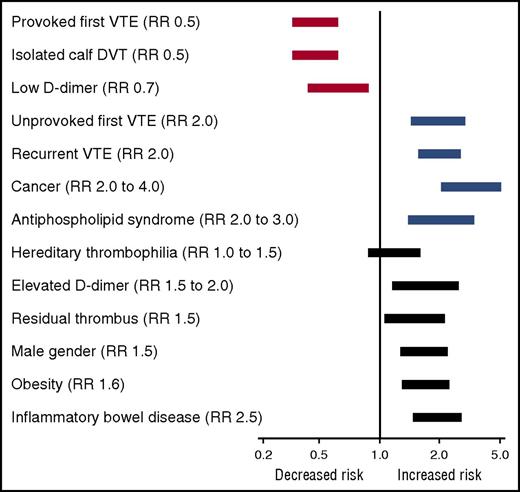
An abundance of clinical and epidemiological evidence has established that obesity is a risk factor for arterial and venous thrombosis. Obesity is a predictor of incident myocardial infarction independent of sex, age, or ethnicity.7 Obesity is also a risk factor for ischemic stroke, deep vein thrombosis (DVT), and pulmonary embolism (PE) in both men and women.8,9 In a meta-analysis of the influence of obesity on the risk of a first episode of venous thromboembolism (VTE), Ageno et al10 estimated an overall odds ratio for VTE of 2.3 (95% confidence interval, 1.7-3.2) (Figure 1A), which is similar to other established risk factors for first VTE such as heterozygous factor V Leiden and estrogen therapy but lower than major risk factors such as surgery, hospitalization, cancer, or major trauma.4 Analysis of data from the Atherosclerosis Risk in Communities and the Cardiovascular Health Study generated a similar hazard ratio of 2.2 for VTE among patients with obesity (BMI ≥30 kg/m2) and a slightly higher hazard ratio of 2.7 for patients with severe (class III) obesity (BMI ≥40 kg/m2).11 Obesity is also a risk factor for recurrent VTE. Eichinger et al12 found that the probability of recurrent VTE was significantly higher among patients with obesity compared with patients of normal BMI who had an estimated hazard ratio of 1.6. This degree of risk is strikingly similar to that of other risk factors for recurrent VTE13 (Figure 2). The impact of obesity on risk of recurrent VTE may be even higher in women than men. Rodger et al14 estimated a relative risk of 2.3 for recurrent VTE in women with obesity (BMI ≥30 kg/m2) after a first episode of unprovoked VTE.
Influence of obesity and IBD on the relative risk of a first episode of VTE. (A) Meta-analysis of the influence of obesity (BMI >30 mg/m2) on VTE risk. Reproduced from Ageno et al.10 (B) Meta-analysis estimating the risk of VTE in patients with IBD. Reproduced from Nguyen et al.42 CI, confidence interval.
Influence of obesity and IBD on the relative risk of a first episode of VTE. (A) Meta-analysis of the influence of obesity (BMI >30 mg/m2) on VTE risk. Reproduced from Ageno et al.10 (B) Meta-analysis estimating the risk of VTE in patients with IBD. Reproduced from Nguyen et al.42 CI, confidence interval.
Influence of clinical factors, including obesity and IBD, on the relative risk (RR) of recurrent VTE. Risk estimates are taken from the World Health Organization5 , Ageno et al,10 and Kearon and Akl.13 The lengths of the bars represent rough approximations of the variance in relative risk.
Mechanisms of thrombosis in obesity
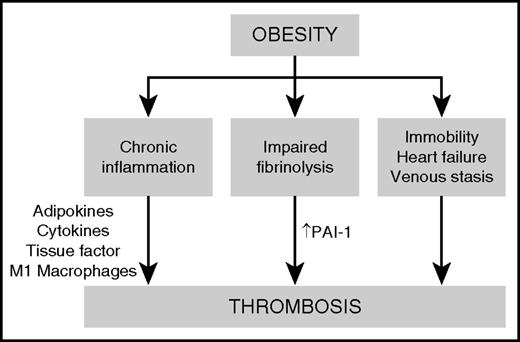
The major mechanisms proposed to be responsible for obesity-associated thrombosis are chronic inflammation, impaired fibrinolysis, and clinical factors such as immobility, obstructive sleep apnea, heart failure, and venous stasis (Figure 3). Obesity is a chronic inflammatory condition that is associated with dysregulation of metabolic homeostasis, resulting in insulin resistance, dyslipidemia, altered regulation of blood pressure, and increased risk of diabetes, cardiovascular disease, chronic kidney disease, cancer, and thrombosis.15 Chronic, low-grade inflammation is triggered by inflammatory cytokines secreted by adipocytes, leading to the recruitment of macrophages to adipose tissue. Adipose-resident macrophages progressively accumulate as the fat mass increases, especially in visceral white adipose tissue. The inflamed adipose microenvironment promotes the polarization of anti-inflammatory M2 macrophages to proinflammatory M1 macrophages, which further contribute to the secretion of cytokines such as tumor necrosis factor α, interleukin-6, and interleukin-1β. These proinflammatory cytokines have autocrine and paracrine effects within adipose tissue and also promote systemic inflammatory responses in other target tissues such as the liver.16
Proposed mechanisms of thrombosis in obesity. Obesity causes a prothrombotic state driven by chronic inflammation, impaired fibrinolysis, and clinical factors such as immobility, obstructive sleep apnea, and heart failure. Adipokines and proinflammatory cytokines secreted by M1 macrophages within adipose tissue contribute to the upregulation of procoagulant factors such as tissue factor and plasminogen activator inhibitor-1 (PAI-1), leading to increased thrombin generation, enhanced platelet activation, and an increased risk of thrombosis.
Proposed mechanisms of thrombosis in obesity. Obesity causes a prothrombotic state driven by chronic inflammation, impaired fibrinolysis, and clinical factors such as immobility, obstructive sleep apnea, and heart failure. Adipokines and proinflammatory cytokines secreted by M1 macrophages within adipose tissue contribute to the upregulation of procoagulant factors such as tissue factor and plasminogen activator inhibitor-1 (PAI-1), leading to increased thrombin generation, enhanced platelet activation, and an increased risk of thrombosis.
One of the major consequences of the chronic inflammatory state of obesity is the activation of prothrombotic signaling pathways in vascular cells. Stimulation of vascular endothelium, platelets, and other circulating vascular cells by proinflammatory cytokines, as well as adipokines such as leptin, leads to upregulation of procoagulant factors such as tissue factor and adhesion molecules, downregulation of anticoagulant regulatory proteins, increased thrombin generation, and enhanced platelet activation.16 Finally, inflammation in obesity is associated with impairment of fibrinolysis because of a marked increase in the expression of plasminogen activator inhibitor-1 (PAI-1).
Prevention and management of thrombosis in obese patients
Other than weight loss, which reverses many of the prothrombotic metabolic effects of obesity,17 no obesity-specific therapeutic approaches to prevent or treat thrombosis are available. Treatment decisions in obese patients are complicated by the fact that relatively few patients with severe obesity were included in the major clinical trials that investigated antithrombotic drugs. Because of a lack of high-grade evidence to support specific recommendations for obese patients, general recommendations for the prevention and treatment of thrombosis in obese patients are very similar to those in lean patients. Although obese individuals have an elevated relative risk for VTE, the absolute risk is not high enough to justify the routine use of long-term anticoagulant therapy for primary prevention. Patients should be advised to avoid lifestyle factors that might compound thrombotic risk, such as cigarette smoking and inactivity. Acutely ill obese patients at high risk for thrombosis (eg, Padua prediction score ≥4)18 should receive anticoagulant thromboprophylaxis in conjunction with hospitalization unless they are at high risk of bleeding.19 Most obese patients undergoing major surgery have at least a moderate risk (modified Caprini score ≥3) for VTE20 and should receive anticoagulant and/or mechanical thromboprophylaxis, depending on the risk of bleeding.21 For obese patients with provoked VTE, the recommended duration of anticoagulant therapy is similar to that in patients without obesity (typically, anticoagulant therapy for a minimum of 3 months).22 For obese patients with unprovoked VTE, anticoagulant therapy is often extended indefinitely unless there is a high risk of bleeding. Obese patients may also have an elevated risk of postthrombotic syndrome, but the degree to which they might benefit from catheter-directed thrombolytic therapy is not known.
Pharmacology and dosing of anticoagulants in obese patients
The dosing of anticoagulants is inherently challenging in obese patients, especially for anticoagulants administered in fixed doses, such as low-molecular weight heparins (LMWHs) and fondaparinux when used for thromboprophylaxis and the direct oral anticoagulants (DOACs).
Heparin
When used for therapeutic indications, unfractionated heparin dosing is initially weight-based (eg, an initial bolus dose of 80 U/kg followed by 18 U/kg per hour as a continuous infusion), and the dose is adjusted by using the activated partial thromboplastin time. Heparin has unpredictable bioavailability in obese patients, however, and patients with severe obesity may have altered weight-based heparin requirements. One recent retrospective cohort study found that critically ill patients who weighed more than 165 kg required lower doses of heparin per kg of total body weight, whereas patients with true body weights from 105 to 164 kg had weight-based heparin requirements similar to those of nonobese patients.23 When subcutaneously administered unfractionated heparin was used for thromboprophylaxis, a dosage of 7500 units 3 times per day was found to be more effective than standard dosing (5000 units 2 or 3 times per day) in preventing VTE and did not increase bleeding in patients with BMI ≥40 kg/m2.24
LMWH and fondaparinux
LMWHs and fondaparinux have more predictable bioavailability than unfractioned heparin, even in patients with obesity. In general, anticoagulant activity, as measured by anti-Xa activity, is increased to appropriate levels when enoxaparin, dalteparin, or tinzaparin are administered to obese patients without an excess of major bleeding in doses based on total body weight up to a weight of approximately 150 kg.25 For therapeutic dosing of LMWHs, dosing based on total body weight is reliable for patients into the obese body weight range (BMI 30 to 39 kg/m2 or weight 100 to 150 kg), but data are sparse for heavier patients (BMI ≥40 kg/m2 or body weight >150 kg). Although there is some disagreement regarding whether or not to cap the dose of LMWHs for patients with a body weight above 150 kg, most guidelines recommend LMWH dosing based on total body weight without capping for the treatment of severely obese patients.26
As might be expected, for thromboprophylaxis with fixed-dose (not weight-adjusted) LMWHs, there is an inverse correlation between total body weight and anti-Xa levels in patients weighing up to 150 kg, which suggests that obese or severely obese patients may require higher fixed doses of LMWHs than lean patients. Most guidelines recommend increasing the prophylactic dose of LMWHs by 30% to 50% for patients with BMI ≥40 kg/m2, but these are low-grade recommendations largely based on expert opinion. Table 1 summarizes the University of Iowa institutional recommendations for dosing of enoxaparin when used for VTE prophylaxis in obese and severely obese patients. Anti-Xa monitoring and dosing based on anti-Xa levels might be expected to offer benefit for adult patients with severe obesity. However, validated anti-Xa target ranges for thromboprophylaxis are lacking, and current American College of Chest Physicians guidelines do not recommend routine coagulation monitoring for prophylactic dosing, even in obese patients.25 This recommendation is supported by a recent systematic review of the Cochrane database, which concluded that routinely determining anti-Xa concentrations in obese patients to monitor the clinical effectiveness of LMWH is not warranted on the basis of the current evidence.27
University of Iowa enoxaparin prophylaxis dosing guidelines for adult patients
| Indication . | Nonobese (BMI <30 kg/m2) . | Obese (BMI 30 to 39 kg/m2) . | Morbidly obese (BMI ≥40 kg/m2) . | |||
|---|---|---|---|---|---|---|
| Dose (mg) . | Dose frequency . | Dose (mg) . | Dose frequency . | Dose (mg) . | Dose frequency . | |
| Medical patients | ||||||
| Acute medical | 40 | Once per day | 30 or 40 | Once every 12 h | 30 or 40 | Once every 12 h |
| Medical ICU | 30 | Once every 12 h | 30 or 40 | Once every 12 h | 30 or 40 | Once every 12 h |
| Trauma or surgical patients | ||||||
| Moderate risk | 40 | Once per day | 30 or 40 | Once every 12 h | 30 or 40 | Once every 12 h |
| Laparoscopic, vascular, or minor surgery | ||||||
| General surgery | ||||||
| Gynecologic surgery | ||||||
| Urologic surgery | ||||||
| Burn injury | ||||||
| High risk | 30 or 40 | Once every 12 h | 30 or 40 | Once every 12 h | 40 or 60 | Once every 12 h |
| General surgery with risk factors* | ||||||
| Burn injury with risk factors† | ||||||
| Cancer surgery | ||||||
| Spinal cord injury | ||||||
| Bariatric surgery | ||||||
| Major trauma | ||||||
| Orthopedic surgical patients | ||||||
| Hip replacement | 30 | Once every 12 h | 30 or 40 | Once every 12 h | 40 or 60 | Once every 12 h |
| Knee replacement | ||||||
| Hip fracture surgery | ||||||
| Indication . | Nonobese (BMI <30 kg/m2) . | Obese (BMI 30 to 39 kg/m2) . | Morbidly obese (BMI ≥40 kg/m2) . | |||
|---|---|---|---|---|---|---|
| Dose (mg) . | Dose frequency . | Dose (mg) . | Dose frequency . | Dose (mg) . | Dose frequency . | |
| Medical patients | ||||||
| Acute medical | 40 | Once per day | 30 or 40 | Once every 12 h | 30 or 40 | Once every 12 h |
| Medical ICU | 30 | Once every 12 h | 30 or 40 | Once every 12 h | 30 or 40 | Once every 12 h |
| Trauma or surgical patients | ||||||
| Moderate risk | 40 | Once per day | 30 or 40 | Once every 12 h | 30 or 40 | Once every 12 h |
| Laparoscopic, vascular, or minor surgery | ||||||
| General surgery | ||||||
| Gynecologic surgery | ||||||
| Urologic surgery | ||||||
| Burn injury | ||||||
| High risk | 30 or 40 | Once every 12 h | 30 or 40 | Once every 12 h | 40 or 60 | Once every 12 h |
| General surgery with risk factors* | ||||||
| Burn injury with risk factors† | ||||||
| Cancer surgery | ||||||
| Spinal cord injury | ||||||
| Bariatric surgery | ||||||
| Major trauma | ||||||
| Orthopedic surgical patients | ||||||
| Hip replacement | 30 | Once every 12 h | 30 or 40 | Once every 12 h | 40 or 60 | Once every 12 h |
| Knee replacement | ||||||
| Hip fracture surgery | ||||||
ICU, intensive care unit.
Risk factors include cancer, previous venous thromboembolism, duration of surgery >3 h, and age >60 y.
Risk factors include extensive lower extremity burns, concomitant lower extremity trauma, use of a femoral vein catheter, and age >60 y.
DOACs
The anticoagulation landscape has changed dramatically in the last 5 years, driven by the approval and licensing of four DOACs: dabigatran, rivaroxaban, apixaban, and edoxaban. Dabigatran is a direct thrombin inhibitor, whereas rivaroxaban, apixaban, and edoxaban are direct factor Xa inhibitors.28 The DOACs represent a major advance in oral anticoagulant therapy because (at least in nonobese patients) they can be administered in fixed doses without the need for laboratory monitoring. The DOACs are rapidly replacing warfarin as the anticoagulant of choice for many indications, including stroke prevention in atrial fibrillation, treatment of venous thromboembolism, and postoperative thromboprophylaxis in patients undergoing orthopedic surgery. DOACs are currently not approved for prevention of thrombosis in patients with acute medical illness, heart failure, peripheral arterial disease, mechanical heart valves, or other medical devices. DOAC trials are ongoing for many of these indications. Rivaroxaban is approved in Europe but not in the United States for patients with acute coronary syndromes. The use of fixed-dose DOACs can be challenging in patients with very low or very high body weight.28 Because they are taken in fixed rather than weight-adjusted doses, there is a potential concern that DOACs may not reach adequate blood levels in patients with severe obesity. Studies in healthy volunteers suggest that obesity has only modest effects on the pharmacokinetic profile of dabigatran, rivaroxaban, or apixaban and that dose adjustments for obesity may not be necessary.29 For thromboprophylaxis after orthopedic surgery, a meta-analysis found similar efficacy and safety of DOACs in elderly obese and non-obese patients.30 A meta-analysis of the major DOAC trials for treatment of VTE found no difference in efficacy or major bleeding between subgroups of patients with body weight <100 kg vs >100 kg.31 It should be noted, however, that these trials had very few patients with body weight >120 kg. For this reason, a recent guideline from the International Society on Thrombosis and Haemostasis recommends against the use of DOACs in patients with a BMI >40 kg/m2 or a body weight >120 kg.32
Warfarin
Despite rapid growth in the use of DOACs, warfarin and other vitamin K antagonists remain widely prescribed anticoagulant drugs. Warfarin dosing is highly variable and is influenced by many clinical and genetic factors, including BMI. The risk of bleeding has been reported to be both higher and lower in obese compared with nonobese individuals treated with warfarin.33,34
Prevention of thrombosis with bariatric surgery
Bariatric surgical procedures, such as open or laparoscopic Roux-en-Y gastric bypass, sleeve gastrectomy, duodenal switch surgery, or gastric banding, are effective for weight loss and improving obesity-associated health conditions. Bariatric surgery is commonly performed in patients with severe obesity (BMI ≥40 to 50 kg/m2) and is associated with an in-hospital postoperative risk of VTE of up to 2.2%.35 The risk is even higher if 30-day postdischarge rates are considered.36 In addition to obesity, risk factors for postoperative VTE in bariatric surgery patients include male sex, increased age, smoking, time for surgery longer than 3 hours, and previous history of VTE.37
Current guidelines from the American College of Chest Physicians, the American Association of Clinical Endocrinologists, the Obesity Society, the American Society for Metabolic and Bariatric Surgery, as well as the Interdisciplinary European Guidelines on Metabolic Surgery all provide similar recommendations for thromboprophylaxis in conjunction with bariatric surgery.36 Recommendations include early ambulation and lower extremity compression (elastic stockings, graduated compression stockings, and/or intermittent pneumatic compression) for all patients. In addition, pharmacological prophylaxis is recommended for most patients, unless the bleeding risk is judged to be excessive. LMWH is generally preferred over unfractionated heparin, although studies comparing LMWH to unfractionated heparin have not been conclusive.36 Warfarin is not recommended, and DOACs have not yet been studied for this indication. Optimal prophylactic dosing of LMWH for thromboprophylaxis for bariatric surgery is not known. Most published studies have used empiric LMWH dose adjustments, such as 150% of standard prophylactic doses, given in either fixed or weight-adjusted regimens. A meta-analysis did not demonstrate a significant difference in the efficacy or safety between adjusted-dose vs nonadjusted-dose LMWH.38 Because of the substantial risk of late (after discharge from the hospital) VTE, continuing prophylaxis with LMWH for at least 10 days after hospital discharge may be beneficial.
The use of inferior vena caval filters (VCFs) for prevention of PE in bariatric surgery has been evaluated in several studies, most of which were retrospective or registry studies with many differences between the VCF and non-VCF groups that likely confounded the findings.39 Some of these studies found higher rates of DVT but similar rates of PE in the VCF groups, which might suggest a possible benefit of VCFs in preventing PE in high-risk patients. Alternatively, the higher rates of DVT might indicate that VCFs increase the risk of postoperative lower extremity venous thrombosis. Two systematic reviews with meta-analysis concluded that VCFs do not appear to decrease the risk of PE with bariatric surgery.40,41 Complications of VCFs include strut fracture, filter misplacement or migration, inferior vena cava perforation, arteriovenous fistula formation, infection, and failure to retrieve the filter with subsequent increased risk of future DVT. In summary, the currently available data do not support the routine use of VCFs in bariatric surgery.
IBD
Like obesity, IBD confers an increased risk of thrombosis. A meta-analysis of 3 large population-based retrospective studies revealed a 2.8-fold increased relative risk of a first episode of VTE in patients with IBD42 (Figure 1B). The thrombotic risk is higher during flares of active disease than during remission.43 The relative risk of VTE appears to be similar for Crohn’s disease and ulcerative colitis. IBD confers a 2.5-fold increased risk of recurrent VTE, which is similar to that of obesity and many other risk factors (Figure 2).44 IBD is also a risk factor for intra-abdominal (splanchnic) thrombosis of the portal, splenic, and mesenteric veins, especially in the perioperative setting,42 and seems to be associated with increased risk of arterial thromboembolic events such as myocardial infarction and stroke.45
Mechanisms of thrombosis in IBD
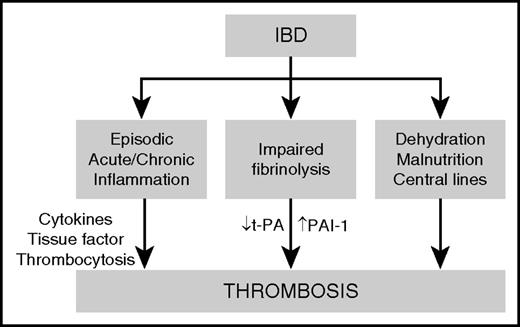
Proposed mechanisms by which IBD drives thrombosis are illustrated in Figure 4. Episodes of acute and chronic intestinal inflammation lead to the elaboration of inflammatory cytokines and the development of a systemic prothrombotic state, manifested by thrombocytosis, upregulation of tissue factor, and impaired fibrinolysis caused by decreased expression of tissue plasminogen activator (t-PA) and increased expression of PAI-1. The frequent use of corticosteroids in IBD patients may exacerbate the prothrombotic state by increasing fibrinogen and decreasing t-PA, leading to further impairment of fibrinolysis.46 In addition, several clinical risk factors may contribute to thrombotic risk in patients with IBD, including dehydration, malnutrition, and placement of central venous catheters.
Proposed mechanisms of thrombosis in IBD. IBD predisposes to thrombosis by inducing episodes of acute and chronic intestinal inflammation, leading to a systemic prothrombotic state characterized by upregulation of tissue factor, an elevated platelet count, and impaired fibrinolysis as a result of decreased expression of tissue plasminogen activator (t-PA) and decreased expression of plasminogen activator inhibitor-1 (PAI-1). Clinical risk factors, such as dehydration, malnutrition, and placement of central venous catheters also contribute to thrombotic risk in patients with IBD.
Proposed mechanisms of thrombosis in IBD. IBD predisposes to thrombosis by inducing episodes of acute and chronic intestinal inflammation, leading to a systemic prothrombotic state characterized by upregulation of tissue factor, an elevated platelet count, and impaired fibrinolysis as a result of decreased expression of tissue plasminogen activator (t-PA) and decreased expression of plasminogen activator inhibitor-1 (PAI-1). Clinical risk factors, such as dehydration, malnutrition, and placement of central venous catheters also contribute to thrombotic risk in patients with IBD.
Prevention and management of thrombosis in patients with IBD
The general approach to prevention and treatment of thrombosis in patients with IBD is similar to that in obese patients. The main differences are that strategies for the prevention of thrombosis in IBD patients are influenced by the episodic nature of Crohn’s disease and ulcerative colitis, the use of anticoagulant therapy requires consideration of the high risk of gastrointestinal bleeding in IBD patients with active disease, and anticoagulant dosing adjustments are generally not necessary in IBD patients.
A series of consensus recommendation statements was developed recently by a panel of authors from the Canadian Association of Gastroenterology who performed a systematic literature review of studies of VTE in IBD patients.42 The authors were able to reach a clear consensus (60% to 100% agreement) despite the fact that most of the statements were supported only by low-quality evidence. Their recommendations include the use of anticoagulant thromboprophylaxis with LMWH, unfractionated heparin, or fondaparinux for hospitalized medical or surgical patients with IBD in remission or with active flares of IBD without major gastrointestinal bleeding. For IBD patients undergoing major surgery, the risk of VTE in the absence of prophylaxis was estimated to be 3% to 6%, which corresponds to a moderate to high risk using the modified Caprini risk assessment model.20 It is noteworthy that the recommendation for anticoagulant thromboprophylaxis includes patients with active IBD and minor gastrointestinal bleeding, despite the fact that gastrointestinal bleeding of any severity is often considered to be an absolute contraindication to anticoagulant therapy. The safety of anticoagulant therapy in this setting is supported by a retrospective study of hospitalized IBD patients with minor rectal bleeding who received anticoagulant thromboprophylaxis without progression to major bleeding.47 For IBD patients hospitalized with major gastrointestinal bleeding, mechanical thromboprophylaxis (graduated compression stockings, and/or intermittent pneumatic compression) is suggested.42 Anticoagulant prophylaxis is not recommended for outpatients with flares of active IBD with no previous history of VTE, a recommendation that is similar to that for patients with obesity or other VTE risk factors. For outpatients with a previous episode of VTE, however, preventive anticoagulation is recommended during episodes of active IBD.
For patients with clinically inactive IBD who present with VTE associated with a provoking factor other than IBD, the recommended duration of anticoagulant therapy is a minimum of 3 months, with continuing therapy until 1 month after resolution of the provoking factor.42 For IBD patients with unprovoked VTE presenting during clinical remission of IBD, indefinite anticoagulant therapy is recommended. Patients who present with a first episode of otherwise unprovoked VTE in the setting of an active flare of IBD, an extended duration of anticoagulant therapy is suggested, to be continued until the IBD has been in remission for at least 3 months. Whether or not to continue anticoagulation indefinitely after the IBD enters a remission phase is a challenging dilemma for clinicians, because insufficient data are available for determining thrombotic risk during remission, and reliable biomarkers of IBD activity are lacking. Flares of disease activity are typically diagnosed clinically and by endoscopic biopsy rather than by measurement of laboratory parameters. Currently available noninvasive biomarkers of disease activity such as leukocytosis, erythrocyte sedimentation rate, and C-reactive protein lack both sensitivity and specificity. Thus, there is a need for better biomarkers to assess IBD activity and the potential need for thromboprophylaxis. Calprotectin (S100A8/S100A9) is an inflammatory protein complex that is released into the feces by activated neutrophils, and it may help predict a flare of IBD,48 but more data are needed to establish its sensitivity and specificity as a predictive biomarker of thrombotic risk in IBD.
Routine laboratory testing for thrombophilic factors is not recommended for IBD patients presenting with thrombosis, with the exception of JAK2 V617F testing (which may identify patients with an underlying myeloproliferative disorder) in patients with splanchnic vein thrombosis.42 Recommendations for anticoagulant therapy for IBD patients with splanchnic vein thrombosis are identical to those for IBD patients presenting with VTE.42
Summary and perspectives
Obesity and IBD are systemic inflammatory disorders that predispose to arterial and venous thrombosis through similar prothrombotic mechanisms. The relative risks of first and recurrent VTE associated with obesity and IBD are comparable to those for other established VTE risk factors such as hereditary thrombophilia, estrogen therapy, and male sex but lower than major risk factors such as surgery, hospitalization, cancer, or major trauma. Both conditions are also associated with an increased risk of arterial thrombotic events such as stroke. Because of a lack of high-quality evidence to guide management decisions, approaches to the prevention and treatment of thrombosis in patients with obesity or IBD are often based on extrapolation from general guidelines for antithrombotic therapy. Although patients with obesity or IBD have an increased risk of first thrombosis, the absolute risk is usually not high enough to support the use of long-term anticoagulant therapy for primary prevention. Similarly, the presence of obesity or IBD alone usually does not justify the use of indefinite anticoagulation after a first episode of provoked VTE. Obese or IBD patients with unprovoked VTE generally should receive indefinite anticoagulant therapy unless there is a high risk of bleeding, although more data and better biomarkers are needed to determine whether anticoagulation can be safely stopped in a subset of IBD patients during remission of active disease. In the absence of such data, the decision on whether or not to discontinue anticoagulant therapy during remission of IBD is challenging; this situation is reminiscent of patients with cancer-associated VTE in complete remission. Most patients with obesity or IBD require thromboprophylaxis in conjunction with hospitalization or surgery, with adjustment of anticoagulant dosing in patients with severe obesity. The following are important considerations for future research: the potential role of DOACs, and perhaps low-dose DOACs,28 as anticoagulant drugs in patients with obesity or IBD; the identification and validation of better noninvasive biomarkers to predict flares and remissions of IBD activity; and the potential utility of anti-inflammatory therapies to prevent thrombosis in obesity and IBD, as well as other prothrombotic inflammatory disorders such as rheumatoid arthritis and antiphospholipid syndrome.
Correspondence
Steven R. Lentz, University of Iowa Carver College of Medicine, Department of Internal Medicine, C21 GH, 200 Hawkins Dr, Iowa City, IA 52242; e-mail: steven-lentz@uiowa.edu.
References
Author notes
This article was selected by the Blood and Hematology 2016 American Society of Hematology Education Program editors for concurrent submission to Blood and Hematology 2016. It is reprinted with permission from Blood 2016, Volume 128.
Competing Interests
Conflict-of-interest disclosure: The author has received research funding from and has consulted for Novo Nordisk.
Off-label drug use: None disclosed.