Abstract
It is an unprecedented time for the treatment of patients with chronic lymphocytic leukemia (CLL) with the recent approval of several targeted agents for use in frontline, relapsed, refractory, and high-risk disease. Traditionally, frontline management of CLL has been a combination of chemotherapy (fludarabine, cyclophosphamide, bendamustine, or chlorambucil) with an anti-CD20 monoclonal antibody (rituximab, ofatumumab, obinutuzumab). The current landscape is rapidly evolving with the advent of therapies that demonstrate selective inhibition of important pathways necessary for CLL proliferation and survival. Despite considerable progress, much is still unknown and optimal treatment selection and sequence is still debatable. None of the new agents have been compared against each other and the impact of adding an additional agent to monotherapy is not yet fully elucidated. In routine clinical practice, the choice of therapy is based on nonrandomized comparisons, presence of comorbidities, and toxicity considerations. These recently approved drugs (ibrutinib, idelalisib, and venetoclax) are reporting excellent outcomes, including patients with high-risk disease such as 17p deletion (17p−) or TP53 mutations (TP53mut). Ibrutinib and venetoclax have been approved for use in 17p− patients (frontline and relapsed, respectively). Ibrutinib is currently moving into the frontline space given recent regulatory approvals. This review will summarize and interpret the limited therapeutic sequencing data available, highlighting the need for additional studies to optimize combination strategies and treatments after failure or discontinuation of these novel agents.
Learning Objectives
Review current treatment strategies in the era of novel targeted agents based on fitness, comorbidity burden, and high-risk disease (presence of 17p deletion/TP53 mutation, short relapse after fludarabine-based therapy)
Discuss optimal treatment strategies and preferred salvage therapy after chemoimmunotherapy relapse or after discontinuation of novel targeted agents
Introduction
Chronic lymphocytic leukemia (CLL) is the most common form of adult leukemia in the Western hemisphere.1 In 2016, it is anticipated that 18 960 cases of CLL will be diagnosed in the United States.2 Optimal treatment strategies for CLL are evolving rapidly, with multiple approvals in recent years providing highly active and well-tolerated treatment options leading to improvements in overall survival (OS). Following diagnosis, most patients with early-stage disease are observed clinically. Therapy typically is not initiated until symptoms from the disease develop, including B symptoms, lymphadenopathy, organomegaly, or cytopenias.3 The understanding of the impact of chromosomal aberrations and genetic mutations on the disease has allowed us to refine the prognostic subgroups.4 Additionally, the discovery of the role of the microenvironment and of the signaling factors that play a key role in CLL pathogenesis has led to the development of agents that specifically target dysregulated pathways.5-7 The OS period is improving with the newly approved therapies, particularly in patients with high-risk disease. In spite of all of the recent advances, CLL remains an incurable disease without an allogeneic transplant.8-10 The goal of treatment is to optimize outcomes by achieving the best possible response while maintaining the patients’ quality of life. In this article, we discuss current recommendations for chemoimmunotherapy use and how the emerging therapies for CLL are changing the clinical landscape. The optimal treatment sequence is as heterogeneous as the disease itself and depends on several variables including prior therapies, the best response obtained, the length of the response, and the patient’s characteristics at the time of relapse.
Principles of CLL treatment
Prognostic factors
Given that the clinical course of CLL is widely heterogeneous, staging and prognostic assessment at the time of diagnosis are critical to anticipate the disease course and to allow for proper monitoring. The prognosis of CLL is affected by clinical staging, the patient’s cytogenetic and molecular profile, and the functional ability to tolerate the proposed therapy.11 There is currently no evidence that initiation of therapy for asymptomatic early-stage disease (Rai 0-II or Binet A) improves OS, including in patients with high-risk disease.12,13 Outside of clinical trials, treatment of early disease is recommended only if a patient develops B symptoms (fever, night sweats, unintentional weight loss) or shows rapid disease progression (eg, worsening lymphadenopathy or bone marrow failure).3 The majority of patients ultimately necessitate treatment during their lifetime. Unfavorable genomic and molecular features include the presence of the unmutated immunoglobulin heavy chain variable (IGHV) gene, CD38 overexpression, ζ-chain-associated protein kinase-70 (ZAP-70), and specific chromosomal aberrations found by karyotype, fluorescent in situ hybridization techniques, or by molecular testing, including 11q deletion, 17p deletion (17p−), and the presence of a TP53 mutation (TP53mut).14-16 A patient’s molecular profile influences treatment decisions, particularly in patients with evidence of 17p−/TP53mut. It is important to recognize that the mere presence of a high-risk prognostic marker is not synonymous with active disease requiring therapy. Even patients with high-risk disease harboring 17p− exhibit clinical heterogeneity, with some patients experiencing an indolent course. Factors reportedly associated with longer time to first treatment in 17p− patients include mutated IGHV, Rai stage 0 disease, β2-microglobulin <2 times the upper limit of normal, and ZAP-70 negativity.17,18 The presence of complex karyotype (CKT), defined as 3 or more karyotype aberrations, has been recognized as an emerging poor prognostic factor with significantly shorter OS in untreated patients.19 In a recent study of patients treated with the targeted agent ibrutinib, CKT was a stronger predictor of poor outcomes compared with patients with 17p−.20 These data suggest that the accumulation of several negative parameters may worsen the prognosis of isolated findings, especially because many of these mutations are not found at the time of initial diagnosis. These genetic lesions are acquired over time with clonal selection occurring preferentially in patients with unmutated IGHV, further contributing to worse outcomes.21,22 Ongoing trials are currently evaluating the role of early intervention with novel agents such as ibrutinib in high-risk disease to determine whether the natural course of the disease could be improved compared with the current recommendation to “watch and wait”. The study CLL12 (EudraCT number 2013-003211-22) is the first prospective, placebo-controlled, double-blind, phase 3 study in asymptomatic high-risk disease that attempts to address the question of the role of observation vs active intervention in the era of novel targeted agents.
Treatment options for frontline therapy
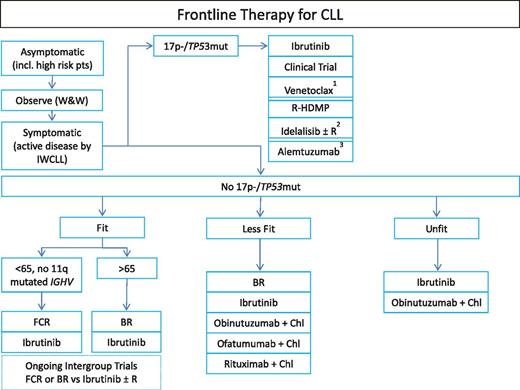
The lack of evidence that CLL can be cured or that survival can be improved with currently available modalities has resulted in a “watch-and-wait” approach with therapy indicated only at the time that patients become symptomatic. Except for allogeneic bone marrow transplants,8-10,23 which are seldom offered to the majority of individuals aged 70 years and older, current treatment approaches are not proven to be curative. At the time the disease becomes active and before any line of therapy has started, it is critical to determine the presence of 17p− and/or TP53mut as these patients are not expected to have a meaningful sustained response with conventional chemoimmunotherapy.24,25 Figure 1 lists existing options for frontline CLL therapy. Choosing the treatment of an individual patient remains a challenge.
Suggested algorithm for initial CLL therapy. 1Venetoclax is not yet approved for frontline therapy in 17p−, currently in clinical trials. 2Idelalisib is not approved for frontline therapy in 17p− in the United States. Given significant concerns for autoimmune complications or fatal infections reported, would only use if no other options available. 3Alemtuzumab is only available via patient-access programs. Chl, chlorambucil; IWCLL, International Workshop on Chronic Lymphocytic Leukaemia; R-HDMP, rituximab/high-dose methylprednisolone; W&W, watch and wait.
Suggested algorithm for initial CLL therapy. 1Venetoclax is not yet approved for frontline therapy in 17p−, currently in clinical trials. 2Idelalisib is not approved for frontline therapy in 17p− in the United States. Given significant concerns for autoimmune complications or fatal infections reported, would only use if no other options available. 3Alemtuzumab is only available via patient-access programs. Chl, chlorambucil; IWCLL, International Workshop on Chronic Lymphocytic Leukaemia; R-HDMP, rituximab/high-dose methylprednisolone; W&W, watch and wait.
Fludarabine chemoimmunotherapy.
Current recommended initial treatment of CLL includes a combination of cytotoxic chemotherapy in combination with a CD20 monoclonal antibody in young patients or elderly fit patients with no major comorbidities. The most common regimens are (1) fludarabine, cyclophosphamide (FC), and rituximab (FCR) and (2) bendamustine plus rituximab (BR). FCR became the standard of care for young fit patients based on the FCR300 study reporting an overall response rate (ORR) of 95% with 72% of complete responses (CRs) and a median progression-free survival (PFS) of 6 years.26 A larger phase 3 trial (CLL8 by the German CLL Study Group) confirmed these findings and solidified the role of FCR. In the study, 817 treatment-naive patients were randomized to 6 cycles of either FCR or FC.27 After 3 years of follow-up, improved ORR and PFS were seen with FCR vs FC with significantly longer OS. The OS rate at 3 years was 87% with FCR vs 83% with FC, and the rate of PFS was 65% with FCR vs 45% with FC. As had been observed in phase 2 trials, grade 3 to 4 neutropenia was more often reported in the FCR arm; however, this did not correlate with higher rates of severe infections. With a median follow-up of 47 months, a superior PFS was maintained in patients receiving FCR (57.9 vs 32.9 months).28 Early intervention with FCR has been studied in untreated patients with early-stage CLL at a high risk for progression of disease. This phase 3 trial of 824 patients compared the efficacy of early against delayed FCR treatment; after a median follow-up of 42 months the event-free survival but not OS was increased in patients receiving FCR early compared with the patients receiving therapy later.12 High-risk patients experienced a shorter survival irrespective of the timing of FCR treatment.
Long-term follow-up from patients treated with FCR demonstrates that a small cohort of patients can achieve remarkable remission durations despite the toxicities associated with the regimen. Rossi et al retrospectively studied a cohort of 404 Italian patients and reported that certain predictive biomarkers could be used to distinguish a subgroup of CLL patients with outstanding outcomes. About a third of the patients in this cohort with mutated IGHV without evidence of 17p or 11q deletions had excellent PFS (71% at 5 years with median PFS not reached) and OS (91% at 5 years). After 4 years, the risk of relapse decreased significantly and similar survival outcomes were found when this subgroup was age-matched to the general Italian population without a diagnosis of CLL.29 Furthermore, Thompson et al recently reported long-term outcomes from the FCR300 trial. The FCR regimen achieved sustained remissions in patients with mutated IGHV without observed relapses, particularly if they had achieved minimal residual disease (MRD) negativity in the bone marrow after therapy; indeed, half of the patients with a mutated IGHV achieved MRD negativity and demonstrated a PFS of 79.8% at 12.8 years. Overall, the 12.8-year PFS was 53.9% for patients with mutated IGHV and 8.7% for patients with unmutated IGHV.30 Similarly, Fischer et al described that after 5.9 years of follow-up of the CLL8 study, FCR remained superior to FC (median PFS was 56.8 and 32.9 months, respectively). Median OS was not reached for the FCR group and was 86.0 months for the FC group, with unmutated IGHV and 17p− being the strongest negative prognostic indicators. In mutated IGHV patients, the median PFS has not yet been reached with very few relapses appearing after 7 years.31
These studies introduce the concept of a nontransplant approach able to deliver a prolonged disease-free state in a select group of patients, comparable to achieving a “cure”. These data strongly support the initial therapy of young fit patients (in particular patients with mutated IGHV and no evidence of poor prognostic markers) with FCR if the patient is not a candidate for participation in a clinical trial.
Bendamustine chemoimmunotherapy.
In patients with comorbidities that significantly raise the likelihood of toxicities, the goal is to administer treatments to control symptoms and disease-related manifestations while preserving quality of life. Unfortunately, FCR therapy is not well tolerated in most CLL patients older than 65 years of age due to myelosuppression and risk of serious infections. The combination of BR has been widely adopted as an alternative regimen due to better tolerability. In order to determine how this regimen compares to FCR, the CLL10 study randomized 561 treatment-naive patients to FCR or BR. This study excluded participation of 17p− patients given their poor responses to chemoimmunotherapy. After a median observation time of 37.1 months, BR had a median PFS of 41.7 months compared with 55.2 months in the FCR arm. FCR was associated with increased rates of CR and MRD negativity. It is important to note that most patients were fit with little comorbidity burden and in spite of this, severe neutropenia and infections occurred. Infections were more frequently observed in the FCR arm, and it was more pronounced in patients older than 65 years of age.
Exploratory post hoc subgroup analyses for PFS and response rate were performed for age, staging, cytogenetics, IGHV mutational status, and sex. In patients with unmutated IGHV, the median PFS for the FCR cohort was 42.7 months against 33.6 months in the BR cohort (P = .017), whereas for patients with mutated IGHV the median PFS was not reached in the FCR arm vs 55.4 months in the BR arm (P = .089). It is important to acknowledge an imbalance in the distribution of patients based on mutational status and age with a higher proportion of unmutated IGHV and older ≥70 years in the BR arm. Given the presence of this IGHV status imbalance, a matched analysis was done to confirm the longer PFS for patients in the FCR arm. Although exploratory analyses ought to be interpreted with caution, it is important to mention that the difference in PFS was statistically not significant between both groups (FCR vs BR) in patients aged 65 years or older. Nonetheless, this analysis may not have been powered to detect a difference; more importantly, the PFS may change with a longer observation time. All of these data together may suggest that BR could be an appropriate alternative initial treatment regimen in elderly fit patients who are at high risk for the potential toxic effects of FCR therapy.32
Chlorambucil chemoimmunotherapy.
The FCR and BR trials excluded patients with serious comorbidities that could raise the likelihood of toxicities. These trials did not include the typical elderly CLL patient seen in routine clinical practice. The majority of patients with CLL are diagnosed at an advanced age and by the time patients need therapy, they have acquired additional medical comorbidities that limit their quality of life and performance status.33,34 The goal in these patients is to choose treatments that control disease-related manifestations of the disease in a manner that preserves their quality of life.
Although still used in clinical practice, chlorambucil monotherapy is no longer the treatment of choice in elderly and frail patients. A randomized phase 3 trial by the German CLL Group in elderly patients (older than 65 years of age) with CLL showed that there was no improvement in PFS or OS when compared against fludarabine monotherapy despite a higher response rate seen with fludarabine.35 Chlorambucil monotherapy use started diminishing after phase 2 trials demonstrated the favorable safety and efficacy of the combination of chlorambucil and rituximab.36,37 These trials formed the basis for the study of chlorambucil in combination with monoclonal antibodies as part of 2 large phase 3 trials in elderly or frail untreated CLL patients, the COMPLEMENT 1 and CLL11 trials.
In the phase 3 COMPLEMENT 1 study, 447 patients aged 65 years and older were randomized to chlorambucil alone against chlorambucil in combination with ofatumumab. Ofatumumab is a fully human type I monoclonal antibody that targets a novel CD20 epitope. Ofatumumab exhibits greater complement-dependent cytotoxicity activity and is less dependent on the cell-surface density of CD20 than rituximab.38 After a median of 28.9 months of follow-up, the median PFS was 13.1 months in patients receiving chlorambucil compared with 22.4 months in patients receiving chlorambucil plus ofatumumab. Superior ORRs and CR rates were seen in the combination arm (ORR, 82%/CR, 14% vs ORR, 69%/CR, 1%). Grade 3 to 4 infusion-related reactions and neutropenia occurred more frequently with the combination arm; however, thrombocytopenia and infections were more common with chlorambucil monotherapy.39
In the CLL11 trial, 781 untreated CLL patients were randomized 1:2:2 to receive chlorambucil alone, or in combination with rituximab or obinutuzumab. Obinutuzumab is a type II glycoengineered anti-CD20 antibody with an increased antibody-dependent cell-mediated cytotoxicity and antibody-dependent cellular phagocytosis.40 Rather than chronological age as single-inclusion criteria, this study also used a high Cumulative Illness Rating Scale score as a measure of functional comorbidity. Most participants were older than 70 years of age and had significant comorbidities. Obinutuzumab plus chlorambucil was superior to rituximab plus chlorambucil in terms of PFS (median, 26.7 vs 16.3 months), CR (20.7% vs 7.0%), and rate of negative testing for MRD, both in peripheral blood (37.7% vs 3.3%) and bone marrow (19.5% vs 2.6%).41 Although no statistically significant difference in OS benefit was observed between these 2 chemoimmunotherapy regimens (P = .08), the combination of obinutuzumab plus chlorambucil did demonstrate OS benefit when compared with chlorambucil monotherapy (P = .002). Negative testing for MRD in blood after obinutuzumab-chlorambucil treatment has been associated with a favorable disease course during follow-up. Adverse events of grade 3 or higher were equivalent among combination treatment groups and included infusion-related reactions, neutropenia, thrombocytopenia, infections, anemia, and leukopenia.
Ibrutinib monotherapy.
Ibrutinib is an orally bioavailable, small-molecule, irreversible inhibitor of Bruton tyrosine kinase that has been shown to induce rapid lymph node responses in patients with CLL.42 As with other kinase inhibitors developed to treat patients with CLL, ibrutinib inhibits several signaling pathways and interferes with the protective effect of stromal cells.5 Ibrutinib was recently approved for frontline use in CLL patients based on the randomized, multicenter, open-label phase 3 RESONATE-2 trial of ibrutinib vs chlorambucil. The trial randomized 269 treatment-naive patients aged 65 years or older to ibrutinib or chlorambucil. Ibrutinib achieved a 91% reduction in the risk for disease progression and an 84% reduction in the risk for death compared with chlorambucil.43 Median progression-free survival has not yet been reached in the ibrutinib group compared with 18.9 months for chlorambucil. Ibrutinib was also associated with a significant improvement in ORR compared with chlorambucil (82.4% vs 35.3%). Five patients randomized to ibrutinib achieved CR vs 2 in the chlorambucil arm. The most common adverse reactions of all grades in the ibrutinib arm (>20%) were diarrhea, musculoskeletal pain, cough, and rash. A major weakness of this study was the selection of chlorambucil monotherapy as an appropriate comparator given the significant OS benefit that has been shown with the combination of chlorambucil and obinutuzumab as compared with chlorambucil monotherapy. At the time of the protocol design, results from the chlorambucil combination trials were not available. Although ibrutinib is currently approved for frontline therapy, most clinicians are not recommending ibrutinib to young/fit patients yet as this choice commits the patient to lifelong therapy. Continuous therapy started in a young patient can pose many issues, including lack of compliance with a daily oral medication and the possibility of long-term toxicities from daily exposure to a targeted agent. Ibrutinib use is moving into the frontline space given recent regulatory approvals, but most clinicians are awaiting results from 2 large ongoing multicenter trials before changing their practice. The first trial, E1912, compares FCR against ibrutinib in combination with rituximab in young fit CLL patients (NCT02048813). The second trial, A041202, compares BR against ibrutinib ± rituximab in CLL patients aged 65 years or older (NCT01886872). At present, given the options, patient preference should be discussed based on quality of life, toxicities, and financial considerations.
Initial therapy for patients with 17p− or TP53 mutation.
None of the frontline chemoimmunotherapies described thus far has shown evidence of sustained clinical activity in patients with a 17p deletion or the presence of a TP53 mutation. Initial treatment of these patients should include ibrutinib. The approval for use in frontline 17p− patients was based on the report from Byrd et al of outcomes of 17p− patients with relapsed or refractory CLL treated with ibrutinib.42,44 The trial included high-risk patients with a median age of 64 years (34% had 17p− and 78% had unmutated IGHV). This was a heavily pretreated group of patients with a median number of 4 prior therapies. With long-term follow-up, ORR was 90%, including those with high-risk disease. The PFS at 30 months was 69%. For patients with 17p− and 11q−, the median PFS was 28 months and 38.7 months, respectively.44 Though PFS in patients with 17p− appears inferior to that observed in patients without 17p−, these results are significantly better than ever achieved with chemoimmunotherapy, hence the decision to approve the drug for frontline use in patients with 17p−. In this phase 1b/2 trial for relapsed/refractory CLL (including patients with high-risk disease such as 17p−), OS was an exploratory end point. The 26-month estimated rate of OS was reported as 83% for all patients and was 70% in the 28 patients with a 17p−. These promising findings paved the way for the RESONATE-17 trial, one of the largest dedicated studies conducted in relapsed or refractory CLL with 17p−. The study evaluated 144 previously treated patients with 17p− who received ibrutinib until progression or unacceptable toxicity. A total of 116 patients were determined to have baseline genetic characteristics with the potential to influence treatment outcomes; these genomic variants included mutations, rearrangements, insertions, deletions, and copy-number variants affecting coding regions. The primary end point was ORR as measured by an independent review committee. At the time of the most recent data assessment, the median treatment duration was 11.1 months with 70% of patients continuing therapy. After a median follow-up of 11.5 months, the investigator-assessed ORR including partial response with lymphocytosis for all treated patients was 83% (17% partial response with lymphocytosis) and 65% by independent review committee. Although median PFS and OS have not yet been reached, the 12-month PFS and OS rates were 79% and 84%, respectively. Overall, results were consistent across subgroups, as the addition of other known poor prognostic markers such as NOTCH1, BIRC3, and SF3B1 to the deletion 17p did not increase the risk of relapse. There appears to be a difference in outcomes related to ATM mutational status; however, given the small numbers, a definitive conclusion is not possible at this time. Bulky lymphadenopathy (defined as >5 cm in diameter at baseline) also appears to result in a difference in patient outcomes with worse PFS, with the difference larger than observed for the genetic variants.45
Additional studies have confirmed the activity of 17p− in the frontline setting without increased toxicities from those noted in the relapsed setting. O’Brien et al reported on the outcomes of 31 patients with CLL or small lymphocytic lymphoma (SLL) who received ibrutinib frontline, 6% of whom had 17p−.46 Overall, the toxicity profile was mild (grade 1-2), with the most common adverse events being diarrhea, nausea, and fatigue. Three patients (10%) developed grade 3 infections. One patient developed grade 3 neutropenia, and 1 developed grade 4 thrombocytopenia. Similarly, Farooqui et al reported the results of the phase 2 study of ibrutinib in 51 high-risk CLL patients (47 had 17p−, 4 had TP53mut without 17p−) including 35 patients (69%) who received ibrutinib as frontline therapy.47 The ORR in the treatment-naive patients was 97% and the estimated 2-year OS was 84%. Burger et al reported a single-arm phase 2 study of ibrutinib in combination with rituximab in high-risk disease as well. This study included 4 treatment-naive 17p− patients who also demonstrated excellent outcomes without an increase in toxicity by the addition of the monoclonal antibody.48 This combination strategy reported a shorter duration of ibrutinib-associated redistribution lymphocytosis presumably abrogated by the addition of rituximab. Whether the addition of a monoclonal antibody to ibrutinib monotherapy significantly changes the long-term prognosis is still up for debate. Combination trials of ibrutinib with monoclonal antibodies or chemoimmunotherapy are currently ongoing.
If the patient is unable to receive ibrutinib due to comorbidities (eg, history of recent intracranial bleed), an option is to offer participation in a clinical trial with a promising novel targeted agent. Off-label options of therapy include venetoclax, idelalisib, a combination of steroids with rituximab, or alemtuzumab.
Venetoclax is a selective, potent BCL2 inhibitor.49 Venetoclax monotherapy induces rapid reduction in CLL disease burden and a high overall response rate of ∼80% and complete response rates of 6% to 20% in patients with relapsed or refractory CLL, including 17p−.50,51 It was approved for use in relapsed 17p− CLL based on a phase 2 multicenter study of 107 relapsed patients harboring 17p−. At a median follow-up of 12.1 months, ORR was 79.4%. The most common grade 3 to 4 adverse events were neutropenia (40%), infection (20%), anemia (18%), and thrombocytopenia (15%). Given the risk for tumor lysis syndrome, the drug is administered via a weekly dose ramp-up schedule over 4 to 5 weeks followed by daily 400 mg continuous dosing until disease progression. Venetoclax is currently undergoing testing for use in treatment-naive patients.
Idelalisib is a selective first-in-class oral δ selective phosphatidylinositol-3-kinase inhibitor.52 The drug has been reported to have clinical activity in 17p−, though it has not been approved for that indication. It is currently only approved for relapsed, refractory CLL in combination with rituximab based on the phase 3 registration trial comparing the combination against rituximab monotherapy.53 At the data cutoff for the second interim analysis,54 median PFS was not reached in the idelalisib and rituximab group and was 5.5 months in the placebo and rituximab group; 12-month PFS was 66% and 13%, respectively. Median PFS also favored idelalisib and rituximab in all risk subgroups, including patients with 17p− and TP53mut. In the frontline setting, idelalisib and rituximab was evaluated in 64 patients, 9 of whom had either 17p− or TP53mut. The ORR was 100% in the 9 patients with 17p−/TP53mut.55 Frontline use of idelalisib is not recommended given significantly increased rates of toxicity. This trial reported that 89% of patients receiving idelalisib and rituximab experienced a toxicity grade 3 or higher, most commonly colitis (42%) and pneumonia (19%).55 A second frontline trial with the combination of idelalisib and ofatumumab was associated with a high rate of grade ≥3 toxicity, including transaminitis (15%), colitis (13%), and pneumonitis (10%).56 The mechanisms underlying these toxicities and their increased commonality in the frontline setting are still being investigated but, based on the finding of immune cell infiltrates in affected organs and responsiveness to immunosuppressive therapy, they are thought to be immune-mediated. Our recommendation is to avoid the use of idelalisib in the frontline setting altogether.
A small phase 2 study combined high-dose methylprednisolone with rituximab and showed significant response rates and complete remissions in frontline patients, including 4 patients with either 11q− or 17p−, reporting 100% ORR.57 Another option for frontline 17p− therapy is alemtuzumab, a humanized chimeric monoclonal antibody targeting CD52, now available only via patient-access programs. In the CAM300 study, 11 patients with 17p− treated with alemtuzumab reported an ORR of 64% and a median PFS of 10.7 months.58 Both of these regimens may be suitable in patients who cannot tolerate other drugs or are unable to obtain any of the available targeted agents. Severe infections are a major concern during and following completion of both regimens.
Sequence of therapies
Treatment options for relapsed or refractory CLL
Similar to the selection of frontline therapy, patient- and disease-specific characteristics should be considered prior to initiation of therapy at the time of symptomatic relapse. The patients’ response to previous treatment is a critical consideration. If the patient previously received FCR and had a PFS of longer than 3 years, repeating FCR or using BR may be a reasonable option. Thus, appropriate therapy at the time of relapse is based on the following considerations: time of relapse (within 24-36 months), prognostic findings at restaging (presence of 17p−/TP53mut or CKT), prior therapies, and patient’s comorbidities.
Early relapse after fludarabine-based therapy (within 24-36 months) or presence of 17p−/TP53mut.
Poor response to intensive fludarabine-based therapy (eg, FCR) is associated with short OS and is considered “ultra-high risk” (“highest-risk CLL”24 ) with a median life expectancy below 2 to 3 years with standard regimens.59 Given the concern for a lack of sustained response with chemoimmunotherapy approaches, choosing a targeted agent such as ibrutinib, idelalisib, or venetoclax is recommended. The choice is based on the immediate prior therapy received and the patients’ comorbidities. Given that ibrutinib is generally well tolerated, it is the drug of choice for most patients in second line. O’Brien et al reported that among previously treated patients, those receiving ibrutinib in an earlier (1 prior) line of therapy experienced better survival outcomes compared with patients who received ibrutinib in later (≥2 prior) lines of therapy.60 These results may be due to clonal selection from treatment resulting in chromosomal aberrations that shift the disease to more aggressive clones including TP53, NOTCH1, SF3B1, or BIRC3 mutations. Most investigators hypothesize that genetically unstable clones carrying these high-risk genomic variants, often accompanied by CKT, may be responsible for evolving resistance patterns. Switching from FCR to second-line ibrutinib therapy may interrupt the selection for some of these aggressive clones as suggested by the better outcomes observed with the use of ibrutinib earlier in the management of the disease. However, patients with 17p− and CKT continue to show the shortest remission duration on ibrutinib and are the patients at higher risk for earlier relapse. Woyach et al reported the emergence of Bruton tyrosine kinase or phospholipase Cγ mutations as resistance mechanisms leading to progression of disease and ibrutinib discontinuation.61 Considerable efforts are currently geared toward better understanding of the molecular mechanisms of resistance underlying progression of disease while on ibrutinib, idelalisib, and venetoclax.
Post-TKI failure.
Over time, patients may develop resistance to targeted agents and relapse. After tyrosine kinase inhibitor (TKI; ibrutinib or idelalisib) failures or discontinuations, clinicians may have little time to initiate the next-line therapy as some patients experience rebound lymphadenopathy and symptoms upon discontinuation. Median survival after discontinuing ibrutinib has been reported to be as short as 3 months without immediate salvage therapy,62,63 although it is important to recognize that patients treated in the early trials had few therapeutic options for salvage available at the time of the original studies. The current available data suggest that the best outcomes postibrutinib discontinuation are observed in patients who receive ibrutinib as the first or second line of therapy.60 With longer follow-up, results of outcomes to subsequent anticancer regimens will allow for better understanding of salvage therapy. Mato et al presented a multicenter chart review evaluating outcomes in 178 patients with CLL who had discontinued kinase inhibitor–based therapy with ibrutinib (n = 143) or idelalisib (n = 35).64 In this pooled analysis, the median PFS from the start of the first kinase inhibitor was 10.5 months. The median PFS outcomes were substantially affected by the reason for discontinuation, being shorter in patients who developed a Richter transformation (6 months) and longer in patients who discontinued due to intolerance (10 months). The median OS from the initiation of the first kinase inhibitor was 29 months. The most common reason for discontinuation was toxicity, which accounted for more than half of ibrutinib and idelalisib discontinuations. Disease progression accounted for a third of discontinuations in both groups. Looking at the toxicity of individual agents, the most common toxicities leading to ibrutinib discontinuation were atrial fibrillation, infection, hematologic toxicity, and bleeding. The most common toxicities leading to idelalisib discontinuation were pneumonitis and colitis. In total, 40 patients were transitioned to an alternate kinase inhibitor (ie, from ibrutinib to idelalisib or vice versa). The median PFS from the start of the alternative kinase inhibitor was almost 12 months. In addition, switching to the alternate kinase inhibitor exhibited clear activity (ORR, 50%), whereas those patients salvaged with chemoimmunotherapy had a much lower response (ORR, 25%). Interestingly, patients who switched to BCL2 inhibitors showed an ORR of 76%, although this drug was not commercially available at the time of the chart review. Based on these data, either idelalisib or venetoclax are reasonable choices for patients who relapse and progress after ibrutinib or who discontinue due to drug intolerance. In summary, data suggest that switching between kinase inhibitors or to the BCL2 inhibitor is a very reasonable strategy to consider. In practice, some patients may develop rapid disease progression or rebound lymphadenopathy upon discontinuation of a targeted agent, hence the recommendation that they be prepared to initiate subsequent therapy immediately if this rapid progression is observed.
Recently, interim data from the phase 2, open-label, 2-arm study evaluating venetoclax monotherapy for CLL patients after ibrutinib or idelalisib were presented. Eligibility criteria included patients with refractory disease while on a TKI treatment or the development of recurrence/progression after discontinuation of either ibrutinib or idelalisib. Responses were observed as early as 8 weeks, which included the 5-week dose ramp-up and 3 weeks at the target 400 mg daily dose. With a median time on venetoclax of 36 weeks (40 weeks for ibrutinib and 24 weeks for idelalisib), the 43 ibrutinib-treated patients had an ORR of 61% and the 21 idelalisib-treated patients had an ORR of 33%. The lower ORR in the idelalisib arm may be due to the shorter time on study for these patients, as 29% of these patients had not yet reached the 24-week assessment; this reported ORR may continue increase as the data mature. MRD assessments in peripheral blood were performed in 34 patients at week 24, of which 8 achieved MRD negativity. Median PFS and OS have not yet been reached. Safety was consistent with prior reports.65 Venetoclax is the first agent to demonstrate robust activity in patients with CLL who were previously treated with a B-cell receptor inhibitor, including patients with ibrutinib- or idelalisib-refractory disease.
Until the recent approval of venetoclax for 17p− patients, ibrutinib progressors had only idelalisib with or without rituximab as a reasonable salvage therapy. Clinicians must weigh the benefits and risks of each treatment with their patients and select the optimal treatment strategy as venetoclax could now also be used for salvage (off-label).
Late relapse after fludarabine-based therapy (≥36 months) and lack of 17p−/TP53mut.
Treatment guidelines include recommendations for various therapeutic agents for the management of patients with relapsed or refractory CLL. Several factors have implications in the choice of therapy, including IGHV mutational status, previous treatment regimens, and length of response to the last known therapy. The treatment of late relapses should take into consideration the patients’ comorbidities and preference. Any of the frontline regimens previously discussed could be used at the time of relapse as there is clinical activity in relapsed disease even if previously exposed to same regimen, especially if the initial response was long-lasting. Although not yet approved for use in all patients with relapsed or refractory CLL, it is important to discuss the possible future role of obinutuzumab and venetoclax. Obinutuzumab is currently approved in combination with chlorambucil for the treatment of patients with previously untreated CLL only. The GREEN study is an ongoing multicenter phase 3b trial investigating obinutuzumab in 460 patients previously untreated or after relapse (NCT01905943). In the study, obinutuzumab is administered as monotherapy or in combination with chemotherapy (FC or chlorambucil or bendamustine). Preliminary safety reports from this study are in line with the known profile of obinutuzumab in similar populations.66 There are currently several ongoing trials studying the role of obinutuzumab in combination with the novel targeted agents. It will be of interest if novel combination strategies with obinutuzumab could augment the rates of CR and MRD negativity seen in the original combination trial with chlorambucil.
Nevertheless, the most recent update on the combination of venetoclax with rituximab is extremely promising as a group of patients were able to achieve CR or MRD− status allowing them to successfully interrupt therapy without immediate evidence of progression of disease. This phase 1b, dose-escalation study assessed safety, pharmacokinetics, and efficacy of venetoclax in combination with rituximab in 49 patients with relapsed CLL. Venetoclax was dosed daily using the ramp-up schema, and monthly rituximab was administered for 6 cycles when target dosing was achieved. Protocol-guided drug cessation was allowed for patients who achieved a CR or bone marrow MRD negativity. Half of the patients achieved CR after combination treatment and 57% of all patients achieved marrow MRD negativity. These rates are unprecedented in the context of treatment of relapsed/refractory CLL with other targeted agents such as ibrutinib and idelalisib. The trial reported data on 13 responders who were able to discontinue all therapy. Eleven MRD− responders remain progression-free after several months. Two patients with MRD+ CR who progressed after 24 months off therapy responded after reinitiation of venetoclax.67 These data support a possible future time-limited targeted strategy (similar to chemoimmunotherapeutic regimens) for select patients who achieve deep responses. MRD could be an end point of future trials with cessation of therapy as a possibility. This change from continuous therapy to limited drug exposure could decrease the emergence of resistant clones that may appear from selective pressure. In January 2016, venetoclax in combination with rituximab was granted Breakthrough Therapy Designation by the US Food and Drug Administration for the treatment of patients with relapsed or refractory CLL.
Role of allogeneic SCT.
The role of allogeneic stem cell transplant (SCT) is changing rapidly based on the recent advances in care of high-risk disease patients. In the past, young fit patients with 17p− were counseled to consider upfront transplant due to poor OS. In the era of novel targeted agents, it is reasonable to delay SCT consideration to later stages of the disease. Allogeneic SCT is still recommended to patients with evidence of poor response to the B-cell receptor inhibitors or venetoclax and for patients who develop a Richter transformation.
Conclusions.
The recent advances obtained over the last few years, that is, the development of inhibitors of B-cell signal transduction, immunomodulators of the CLL microenvironment, and glycoengineered monoclonal antibodies, are revolutionizing the management of CLL. The era of targeted therapy in CLL has arrived and we anticipate that progress will continue as second-generation agents (with possible less toxicity) are currently in development. Despite of all the recent advances in therapy, challenges still remain. The early identification of those patients at highest risk for drug-resistance development is of utmost importance as these are the patients who will relapse sooner and who may not respond to subsequent therapy. Treatment strategies to salvage patients when resistance occurs need to be readily available to avoid clinical decline. Future success is critically dependent on the development of rational treatment combination strategies that could improve the current success rates without increasing the toxicities. Venetoclax, in the right combination regimen, may allow the eradication of MRD with the possibility of achieving a “drug holiday”. Several combinations and sequencing strategies are currently in clinical evaluation to optimize the management of CLL patients. It is conceivable that the incorporation of the novel targeted agents into chemotherapy or immunotherapy regimens may generate deeper responses and lead to a “cure-like” state similar to the subset of good prognostic CLL patients who, more than a decade later, remain in remission after FCR therapy.
Acknowledgments
The author thanks all members of the CLL Research & Treatment Program as well as all patients participating in their study protocols.
This work was supported in part by National Institutes of Health/National Center for Advancing Translational Sciences (grant UL1TR00457), the 2015 American Society of Hematology–Harold Amos Medical Faculty Development Program fellowship, and philanthropic contributions from the Karches Foundation, Jerome Levy Foundation, Leon Levy Foundation, and the Feinberg Family Foundation (J.C.B.).
Correspondence
Jacqueline C. Barrientos, CLL Research and Treatment Program, Department of Internal Medicine, Hofstra Northwell School of Medicine, 410 Lakeville Rd Suite 212, New Hyde Park, NY 11042; e-mail: jbarrientos@northwell.edu.
References
Competing Interests
Conflict-of-interest disclosure: J.C.B. is on the Board of Directors or an advisory committee for Janssen, Pharmacyclics/Abbvie, and Gilead and has received research funding from Pharmacyclics/Abbvie and Gilead.
Author notes
Off-label drug use: None disclosed.