Abstract
Langerhans cell histiocytosis (LCH), juvenile xanthogranuloma (JXG), and Erdheim–Chester disease (ECD) represent histiocytic disorders with a wide range of clinical manifestations. Until recently, mechanisms of pathogenesis have been speculative and debate has focused on classification of these conditions as reactive versus neoplastic. Genomic studies have been challenged by scarce tissue specimens, as well as heterogeneous nature of the lesions with variable infiltration of pathologic histiocytes. Whole-exome sequencing recently revealed a very low frequency of somatic mutations in LCH, JXG, and ECD compared to other neoplastic disorders. However, at least in the cases of LCH and ECD, there is a very high frequency of activating mutations in MAPK pathway genes, most notably BRAF-V600E, as well as MAP2K1, in LCH and NRAS in ECD. In ECD, recurrent mutations in the PI3K pathway gene PIK3CA have also been described. The heterogeneous clinical manifestations of these disorders may therefore be the cumulative result of activation of MAPK mutations (along with modifying signals from other pathways) at distinct stages of myeloid differentiation. Implications of this model include redefinition of LCH, JXG, and ECD as a group of clinically diverse myeloid neoplastic disorders with a common mechanism of pathogenesis. This model supports refocusing therapeutic strategies for these diseases on a personalized approach based on specific mutations and the cell(s) of origin.
Learning Objectives
To understand the biological significance of recurrent somatic mutations in LCH, JXG, and ECD
To evaluate models of pathogenesis of LCH, JXG, and ECD based on emerging genomic and biological data
To develop therapeutic approaches for patients with LCH, JXG, and ECD in light of new models of pathogenesis
Histiocytic disorders encompass a broad range of disease with the common feature of cells of the mononuclear-phagocyte lineage with abnormal differentiation, proliferation and/or function. “Histiocyte” is an archaic term literally meaning “tissue cell” that is not particularly informative nor accurate, as cells in these diseases may reside in bone marrow, circulation, or tissue. Regardless, the nomenclature persists. In this review, we discuss recent advances in understanding of the cellular origins and molecular bases for pathogenesis of Langerhans cell histiocytosis (LCH), Erdheim–Chester disease (ECD), and juvenile xanthogranuloma (JXG). A unifying model is emerging in which clinical manifestations of these clinically distinct diseases are defined by pathologic MAPK pathway activation at specific stages in myeloid differentiation.
Langerhans cell histiocytosis
Clinical features of LCH
LCH is the most common histiocytic disorder, arising with similar frequency in children as Hodgkin lymphoma at ∼5 cases per million.1-3 Although the median age of presentation is 30 months, LCH may also present in adults de novo or as persistent or recurrent pediatric disease.4 Clinical manifestations of LCH vary widely from self-limited lesions to aggressive and potentially lethal disseminated disease that requires aggressive systemic chemotherapy or even hematopoietic stem cell transplant for cure. Lesions may arise in virtually any organ system, with higher risk of mortality in patients with disease involving bone marrow, liver, or spleen (“high-risk” LCH). Patients with “low-risk” LCH are at much lower risk of mortality, though >50% suffer long-term morbidity from their disease.5
The LCH identity crisis: reactive versus neoplastic
Histology of LCH lesions is relatively uniform across the spectrum of clinical presentations. LCH is defined by CD1a+/CD207+ histiocytes (median infiltration 8%; range, <1%-75%) among an inflammatory background including variable numbers of lymphocytes, eosinophils, and macrophages (Table 1; Figure 1).6,7 In the 1950s, the common histologic appearance of “eosinophilic granuloma,” Hand–Christian–Schüller disease (multifocal “low-risk” LCH), and Letterer–Siwe disease (multifocal “high-risk” LCH) led to the hypothesis that this collection of clinically diverse conditions represent a common disease arising from a common cell of origin. These conditions were collectively branded as “Histiocytosis X.” Decades later, the Birbeck granule, an organelle now associated with langerin (CD207), was identified by electron microscopy in both LCH histiocytes and epidermal Langerhans cells (LCs).8 At the time, Birbeck granules were thought to be exclusive to epidermal LCs, leading to the hypothesis that LCH arises from epidermal LCs and to the current nomenclature, “Langerhans cell” histiocytosis.
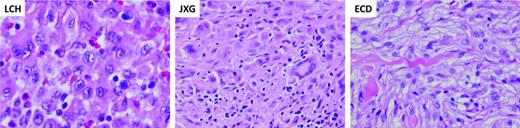
Characteristic histology of histiocytic lesions. Diagnostic biopsy specimens of LCH, JXD, and ECD lesions stained with hematoxylin and eosin. The LCH biopsy demonstrates characteristic clusters of histiocytes with reniform nuclei. The background inflammatory infiltrate includes lymphocytes, eosinophils, and macrophages. The JXG biopsy demonstrates characteristic xanthomatous histiocytes along with multinucleated Touton giant cells. The ECD biopsy is similar to JXG, also wth xanthomatous histiocytes and inflammatory infiltrate. (Images courtesy of Dr M. John Hicks, Baylor College of Medicine.)
Characteristic histology of histiocytic lesions. Diagnostic biopsy specimens of LCH, JXD, and ECD lesions stained with hematoxylin and eosin. The LCH biopsy demonstrates characteristic clusters of histiocytes with reniform nuclei. The background inflammatory infiltrate includes lymphocytes, eosinophils, and macrophages. The JXG biopsy demonstrates characteristic xanthomatous histiocytes along with multinucleated Touton giant cells. The ECD biopsy is similar to JXG, also wth xanthomatous histiocytes and inflammatory infiltrate. (Images courtesy of Dr M. John Hicks, Baylor College of Medicine.)
Over the following decades, models of LCH focused on neoplastic transformation versus aberrant activation of an otherwise normal epidermal LC. The granulomatous appearance of the LCH lesion with “benign” appearing histiocytes supported the possibility of LCH as a reactive or autoimmune disorder. Many hypotheses were presented suggesting that LCH might arise from LCs or precursors in a state of arrested development, misguided to sites of disease by pathologic cytokines/chemokines (for a review, see Laman et al9 ). A neoplastic basis for LCH was supported by the major breakthrough proving LCH histiocytes are clonal.10,11 However, clonality in immune cells does not necessarily imply malignancy, and failure to identify genetic abnormalities in systematic analyses of LCH lesions cautioned against classification of LCH as a neoplastic or malignant disorder.12
Molecular landscape of LCH: MAPK activation
A major breakthrough in the LCH puzzle came with discovery of recurrent somatic BRAF-V600E mutations in histiocytes in >50% of LCH lesions,13 which has been validated in multiple subsequent studies (for a review, see Berres et al14 ). BRAF is a central kinase of the MAPK pathway (RAS/RAF/MEK/ERK) that transduces extracellular signals and regulates critical cellular functions (Figure 2).15 The V600E mutation results in RAS-independent constitutive activation of downstream MEK and ERK.16 Besides the V600E mutation, single case reports have described additional somatic mutations within the BRAF gene locus (BRAF V600D, BRAF 600DLAT), as well as the germline mutation/polymorphism BRAF T599A with potential functional consequences.17,18
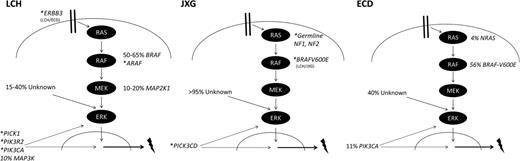
Genomic landscape of histiocytic neoplasias. The diagrams highlight specific mutations associated with LCH, JXG, and ECD and their potential relationship to ERK activation. Estimated frequencies of reported recurrent mutations are indicated next to the gene name. Asterisk (*) indicates genetic lesions with case reports. The lightning bolt represents the downstream functional consequences of histiocytic mutations that drive pathogenesis and influence clinical outcomes.
Genomic landscape of histiocytic neoplasias. The diagrams highlight specific mutations associated with LCH, JXG, and ECD and their potential relationship to ERK activation. Estimated frequencies of reported recurrent mutations are indicated next to the gene name. Asterisk (*) indicates genetic lesions with case reports. The lightning bolt represents the downstream functional consequences of histiocytic mutations that drive pathogenesis and influence clinical outcomes.
ERK is universally activated (phosphorylated) regardless of BRAF genotype in LCH lesions.13,19 If approximately one-half of LCH lesions carry BRAF-V600E mutation, by what mechanisms is ERK activated in the rest? Whole-exome sequencing (WES) was performed on a cohort of matched LCH lesions and peripheral blood control samples to determine the overall frequency of somatic mutations in LCH, the presence of modifying mutations in cases with BRAF-V600E, and to search for alternative mechanisms of ERK activation. Surprisingly, the overall mutation frequency was extraordinarily low compared to other pediatric malignancies with 0.03 somatic mutations per Mb, or a median of 1 mutation per LCH sample exome (range, 0-5).19 The major finding from this study was observation of activating mutations in exons 2-3 of MAP2K1 in 33% of LCH lesions with wild-type BRAF. Targeted sequencing studies identified MAP2K1 mutations in 15%-50% of cases with wild-type BRAF (Figure 2).20,21 Another WES study of 3 LCH cases revealed 1 lesion with BRAF-V600E, 1 with a complex somatic activating mutation in ARAF, and 1 without MAPK gene mutations identified. As in our series, Nelson et al also described a very low overall rate of somatic mutations.22 In summary, ∼75% of LCH lesions have been shown to harbor mutually exclusive somatic MAPK pathway mutations resulting in ERK activation.
The remaining cases without identified MAPK mutations may be explained by the technical challenge of identifying somatic mutations in lesions with low penetrance by pathologic LCH cells, MAPK alterations that are undetectable by exome sequencing (eg, fusions, deletions, or duplications), activating mutations in other pathways that impact LCH pathogenesis, or other mechanisms of pathogenesis (eg, methylation or RNA processing). However, the fact that ERK activation is universal in LCH13,19 suggests that underlying MAPK alterations remain to be discovered. In primary cell culture models, response of cells to pharmacologic inhibition of BRAF-V600E or MEK function resulted in predictable, mutation-specific decreases in ERK activation. However, responses of cells without identified MAPK mutations were highly variable, suggesting there are heterogeneous mechanisms leading to ERK activation yet to be discovered.19 In our LCH WES study, few mutations were detected in PI3K pathway genes. However, mutations were identified in PICK1 and PIK3R2 (both in the same patient, also with a MAP2K1 mutation).19 A single mutation has also been reported in PIK3CA.23 Recurrent loss-of-function mutations in MAP3K1, encoding MEKK1 (which primarily impacts JNK signaling but also interacts with MAPK pathway) that are not exclusive to BRAF have also been described in a targeted sequencing approach in 3/30 LCH cases, but were not detected in our WES study of 41 LCH lesions.21 Larger series will be required to determine the frequency and functional significance of recurrent mutations in pathways outside of MAPK signaling. The role of acquired modifying mutations or development of subclonal populations also requires further investigation.
Functional consequences of activated ERK in LCH
The frequency of MAPK pathway mutations in LCH strongly supports a functional role for pathologic ERK activation in the pathogenesis of this disease. Interestingly, although BRAF-V600E is identified in ∼8% of all human cancers, it is infrequently associated with nonhistiocytic hematologic malignancies, with the notable exception of its near-universal occurrence in hairy cell leukemia.24 ERK signaling has also been implicated in myeloid cell differentiation and maturation under physiologic conditions that may be relevant to the functional alternations of histiocytes in LCH (for a review, see Berres et al14 ). Cellular context appears to influence the impact of the BRAF-V600E mutation, which is also observed in more benign conditions, including epidermal nevi and colon polyps. In metastatic melanoma, BRAF-V600E is one of a constellation of mutations that influence potential for secondary mutations and response to therapy25,26 In LCH, on the other hand, BRAF-V600E or other MAPK mutations arise in an otherwise “quiet” genome. The mechanisms of pathogenesis downstream of activated ERK remain undefined, and the relative impact of different MAPK mutations (or PI3K modifying mutations) also remains uncertain. In other diseases characterized by MAPK activation, such as juvenile myelomonocytic leukemia, mutations at specific levels of the pathway correlate with characteristic clinical features. In several LCH studies, BRAF-V600E status did not correlate with age, extent of disease, or survival. However, in our institutional series, BRAF-V600E was associated with a 2-fold increase in risk of relapse.6,13,18,27
Cell(s) of origin: LCH as a myeloproliferative disorder?
If all LCHs were to arise from a common precursor, one might envision a typical cancer model in which disease severity may be determined by the accumulation of somatic mutations. However, based on the genomic data generated to date, it is not possible to differentiate a patient with a single LCH skull lesion potentially cured by simple curettage from one with lethal high-risk disease. Furthermore, exome mutation results were identical from all serial LCH samples tested, suggesting that generation of novel mutations is unlikely a significant driver of tumor progression.19 Although the discovery of recurrent MAPK mutations in histiocytic neoplastic disorders was a major breakthrough, the common presence of BRAF-V600E in a patient with a single LCH lesion and a patient with fatal disseminated LCH, and the absence of other mutations suggests that additional factors influence the clinical significance of pathologic MAPK activation.
One possible explanation is that the impact of pathologically activated MAPK may depend on the cell in which the mutation arises. The concept that LCH arises from aberrant epidermal LCs has been challenged by observations that langerin (CD207) and Birbeck granules arise in a wider spectrum of lineages and subpopulations than initially appreciated.7,28-31 Furthermore, the transcriptional profile of LCH lesion CD207+ cells has many features of immature myeloid cells compared with the epidermal LC,32 and LCH lesions include cells with BRAF-V600E at variable stages of differentiation.27 These observations make a model in which LCH arises from transformation or activation of the epidermal LC less likely.
The BRAF-V600E mutation provided a molecular “bar code” that made it possible to evaluate peripheral blood and bone marrow aspirate from patients with BRAF-V600E LCH lesions for precursor cells. In peripheral blood, BRAF-V600E was identified in circulating CD11c+ myeloid DC precursors and CD14+ monocytes of patients with high-risk LCH, implying that the mutation arose in a myeloid precursor. Further investigation in bone marrow aspirates identified BRAF-V600E in CD34+ hematopoietic stem cells of some high-risk LCH patients. The state of differentiation of the myeloid precursors in which BRAF-V600E was found was associated with clinical manifestations of LCH: BRAF-V600E was primarily detected in circulating cells in patients with high-risk disease and was not detectable in peripheral blood from most subjects with low-risk LCH. The functional significance of MAPK activation in early myeloid cells in LCH was supported by the finding that enforced BRAF-V600E expression in CD11c+ cells in a mouse model recapitulated a high-risk LCH-like phenotype. Interestingly, enforced expression of BRAF-V600E in langerin+ cells resulted in a more attenuated phenotype without detectable circulating cells with BRAF-V600E, similar to human low-risk LCH. Together, these findings support the misguided myeloid DC model of LCH pathogenesis in which pathologic ERK activation drives proliferation, survival, differentiation, and activation of myeloid DC precursors (Figure 3).6 In this model, the potential distribution of pathologic myeloid precursors that give rise to the LCH lesion DCs is defined by the differentiation of the cell in which the BRAF-V600E (or other activating MAPK mutation) arises.
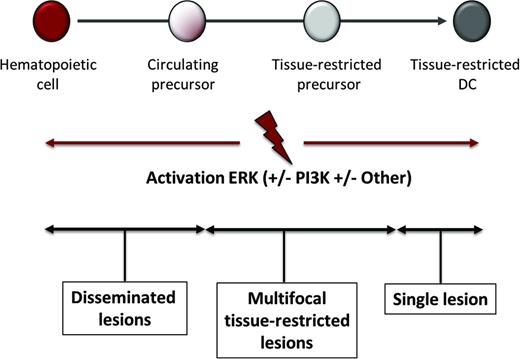
Misguided myeloid DC model of pathogenesis. According to this model, the stage of differentiation in which pathologic ERK activation arises determines the clinical manifestations of LCH, JXG, and ECD. Activating mutations in hematopoietic stem cells or undifferentiated myeloid DC precursors result in multifocal disseminated disease, whereas mutations in tissue-restricted precursors results in multifocal disease with lesions in limited tissues, and mutations in more differentiated tissue restricted precursor cells results in a single lesion.
Misguided myeloid DC model of pathogenesis. According to this model, the stage of differentiation in which pathologic ERK activation arises determines the clinical manifestations of LCH, JXG, and ECD. Activating mutations in hematopoietic stem cells or undifferentiated myeloid DC precursors result in multifocal disseminated disease, whereas mutations in tissue-restricted precursors results in multifocal disease with lesions in limited tissues, and mutations in more differentiated tissue restricted precursor cells results in a single lesion.
Juvenile xanthogranuloma
Clinical features of JXG
Juvenile xanthogranuloma is a primarily pediatric histiocytic disorder characterized by xanthomatous histiocytic lesions that has been assumed to arise from dermal dendrocytes, based on histology including fascin+ and CD1a−, CD207−, and absent Birbeck granules. The JXG cells also share features with macrophages, including CD14+, CD68+, CD163+, and factor XIIIa+(Table 1; Figure 1).33 Lesions with JXG histology may also arise in patients with other LCH lesions, or as mixed phenotype within a single lesion. Incidence is estimated to be ∼1 case per million children.34 The majority of cases are clinically trivial skin-limited skin lesions, but disease may also be disseminated and life-threatening. Disseminated JXG has been reported in soft tissue, central nervous system, bone, lung, liver, spleen, pancreas, adrenal glands, intestines, kidneys, lymph nodes, bone marrow, orbit, and heart.
Molecular landscape of JXG
Interestingly, before BRAF-V600E and MAPK pathway activation were implicated in the pathogenesis of LCH, several cases of JXG were reported in children with germline mutations in NF1 or NF2 as well as with coincident JMML.35,36 Several studies have performed direct sequencing for BRAF-V600E, and most failed to identify any cases of JXG with the mutation. In our institutional series, we did identify 2 patients with disseminated histiocytic disease with some lesions with characteristics of LCH, as well as JXG with the BRAF-V600E mutation. Additionally, one patient with “pure” JXG was found to have a germline mutation in NF1. Whole-exome sequencing of 4 JXG lesions identified a slightly higher mutation frequency than LCH (median of 4 exomic mutations per case; range, 0-9 mutations), though this series is too small to generalize conclusions about relative mutation frequency. None of the JXG patients with mixed JXG/LCH lesions had somatic mutations in MAPK genes other than BRAF. One JXG patient had PI3KCD mutations in serial biopsy specimens.19 Emerging information about the role of MAPK and potentially PI3K pathways in histiocytic diseases and the data from a handful of JXG cases sequenced to data supports a plausibility of pathologic ERK activation playing a role in pathogenesis of JXG, and also supports the likely utility of further genotype-phenotype studies in these patients.
Erdheim–Chester disease
Clinical features of ECD
ECD is an extremely rare and fascinating disorder of adults (median age 53) that is histologically similar to JXG (CD14+, CD68+, fascin+, factorXIII+, CD1a−, CD207−; Figure 1).33 ECD is generally systemic with lesions that may involve skin, bones, and brain/pituitary, similar to LCH. Retroperitoneal, renal, and cardiac involvement are unique features of ECD. Poor overall survival (median 3 years) is also an unfortunate feature of this disease.
Molecular landscape of ECD
Very similar to LCH, the BRAF-V600E mutation is identified in ECD in approximately one-half of reported cases.37 Targeted sequencing of a large cohort identified mutually exclusive activating mutations in BRAF-V600E in 56% and in NRAS in 4%. Interestingly, PIK3CA mutations were identified in 11% and were not related to BRAF status. In 1 patient in which peripheral blood mononuclear cells were analyzed, CD14+ cells carried the same NRAS mutation as the ECD lesion cells, supporting the hypothesis that ECD, like LCH, may arise from activated precursor cells.38 Furthermore, the identification of recurrent PIK3CA mutations supports a potential role for the PI3K pathway in influencing the clinical manifestations of ECD. Timing of PIK3CA mutation relative to BRAF-V600E acquisition in patients with both mutations is not clear. BRAF-V600E, however, does appear to be critical to pathogenesis as patients with ECD with the BRAF-V600E mutation treated with vemurafenib have reproducible and sustained clinical responses.39
Mixed histiocytic disorders
Mixed histiocytic disorders defined as lesions with histopathology consistent with more than one phenotype (eg, LCH/JXG or LCH/ECD) are probably more common than we currently recognize. In some cases, 1 “disease” precedes another. In other cases, lesions with distinct phenotypes may arise simultaneously. Additionally, mixed phenotypes may be recognized in the same lesion. In the few sample pairs analyzed to date, the genotype appears to be fixed across time, anatomic location, and lesion phenotype.6,40 One possible explanation may be to apply the misguided myeloid DC model to JXG and ECD, as well as LCH, in which the range of potential phenotypes is defined by the state of differentiation in which MAPK activation arises, with terminal differentiation of the pathologic histiocytes influenced by stochastic or environmental factors. An additional possibility is that additional genetic and/or epigenetic events may arise in subclones that influence terminal differentiation of the pathologic histiocytes (Figure 3).
LCH, JXG, and ECD as myeloproliferative disorders
As the lines between different histiocytic diagnoses become increasingly blurred, mounting evidence supports a shared model of pathogenesis. Although many details of this proposed misguided myeloid DC model remain speculative, the concept accommodates current and emerging data. The presence of recurrent MAPK activating mutations in myeloid progenitor cells, the ability of enforced expression of BRAF-V600E to drive pathogenesis in a mouse model, and clear responses to targeted MAPK inhibition, support reclassification of LCH, JXG, and ECD as MAPK-driven myeloproliferative neoplastic disorders (MPNs). The lack of mitoses and unremarkable Ki67 expression suggests that the proliferation occurs upstream of the lesion in progenitor cells. Precursors presumably migrate to sites of lesions where they differentiate into characteristic histiocytes that in turn recruit and activate inflammatory cells (Figure 3).
Clinical implications
Rebranding LCH, JXG, and ECD as inflammatory MPNs has important practical and philosophical implications. From a practical standpoint, this concept moves the therapeutic target from the terminally differentiated lesion histiocyte to the myeloid precursor. In our opinion, “wait-and-watch” or localized therapy for patients with clinically significant systemic disease is therefore not logical. As with any MPN, ideal therapy would focus on eradication of the stem cell.
The current standard of care for multifocal LCH, vinblastine/prednisone for 12 months, has arisen empirically from heroic efforts of the Histiocyte Society to conduct serial international clinical trials over the past decades. However, improved outcomes for patients with LCH treated with chemotherapy remain suboptimal, with more than one-half of patients treated for multifocal disease failing to be cured with vinblastine/prednisone.41 If LCH is a MPN, agents with efficacy against other MPNs/AML/MDS may be preferred in LCH to agents with more of a track record treating ALL or inflammation. This hypothesis is supported by small phase II series and institutional trials demonstrating responses of patients with relapsed and refractory LCH to the nucleoside analogs cladribine, cytarabine, and clofarabine (for a review, see Allen et al42 ). Finally, deactivating the “stem cell” with MAPK inhibition has shown early promise in adults with ECD and LCH. Determining the pathway target(s), duration of therapy, and combination with chemotherapy for each patient will require a personalized approach to optimize efficacy and minimize toxicity. From a philosophical standpoint, rebranding LCH, JXG, and ECD as MPNs may catalyze opportunities to test and refine evidence-based approaches to therapy by including patients with these diseases in cooperative cancer group initiatives.
Summary
LCH is a fascinating disease with a complex history of histologic insights driving classification from Letterer–Siwe/Hand–Christian–Schüller /eosinophilic granuloma to histiocytosis X to LCH. Advances in technology once again offer insight into the spectrum of conditions now recognized as “LCH.” The “X” in histiocytosis X was meant to indicate that the cell of origin was uncertain. It may therefore be more appropriate to envision this group of conditions as “histiocytosis X, Y, Z….” where every patient has their own personal version of disease defined by the cell of origin and the specific activating signal(s) (Figure 3). This model may account for the various presentations of JXG, ECD, and mixed histiocytic disorders as well. The snapshots we have from institutional series supports efforts to more comprehensively define the mutation landscape of these disorders by treating patients on cooperative prospective trials that include lesion genotyping, with the ultimate goal to optimize therapy with personalized approaches for patients with histiocytic MPNs.
Correspondence
Carl E. Allen, Texas Children's Cancer Center, 1102 Bates Avenue, Suite 1025.22, Houston, TX 77030; Phone: 832-824-4312; Fax: 832-825-1206; e-mail: ceallen@txch.org; or D. Williams Parsons, Texas Children's Hospital, 1102 Bates Avenue, Suite 1030.15, Houston, TX; Phone: 832-824-4643; Fax: 832-825-4038; e-mail: dwparson@txch.org.
References
Competing Interests
Conflict-of-interest disclosures: The authors declare no competing financial interests.
Author notes
Off-label drug use: None disclosed.