Learning Objective
To understand the role of sickle cell screening tests in identifying sickle cell trait and sickle cell disease
Clinical vignette
An 18-year-old National Collegiate Athletic Association (NCAA) soccer player is tested for sickle cell trait (SCT) as a condition for participation. His sickle solubility test is negative. After college, he is shocked when his newborn is diagnosed with hemoglobin SC disease. His wife is known to have SCT. He presents for consultation to understand how his child inherited sickle cell disease (SCD) when his “sickle test” was negative. What are the sensitivities and specificities of screening tests for hemoglobin S and other variants?
Introduction
Infants with sickle cell trait (SCT), or other hemoglobin variants, are identified at birth in the US through universal newborn screening for hemoglobinopathies. In contrast to infants with sickle cell disease (SCD), who are referred for specialty care, the approach to those with SCT is not uniform.1 Parents may not be educated about their infant's screening result and adults may be unaware that they have SCT.2 There is increased recognition that SCT carries unique health risks, including increased risk for exertional heat-related injuries (EHI).3 This potential for adverse outcomes, along with the fact that adults may not know their trait status, has led some organizations to screen for SCT.
The United States Department of Defense (DoD) and the NCAA have released policy statements that address screening tests for SCT.4-6 Reports of EHI-related deaths among military recruits prompted the DoD to adopt a policy of universal screening for hemoglobin S in 1981. In an examination of military recruits from this era, the risk of sudden death was 27-fold higher among African American recruits with SCT compared to those without hemoglobin S.7 The DoD's 1981 policy recommended screening to identify hemoglobin S, confirmation of positive screening tests with a method able to quantify the percentage of hemoglobin S and define the hemoglobin phenotype, and lastly, consultation with medical personnel about the implications of the result.5 In 1996, the requirement for SCT screening was removed, when the DoD instead adopted universal practices to prevent EHI.6 Under circumstances similar to the DoD, the NCAA mandated screens in student athletes for SCT after EHI-related deaths.8 In contrast to the DoD policy, the NCAA statement recommends a sickle solubility test to screen for SCT without mention of confirmatory tests or post-test education.4
This mini-review examines the appropriate use and limitations of methodologies to screen for hemoglobin S. Because the NCAA specifically names the sickle solubility test as a method to screen student athletes for SCT, the solubility test will be a focus of this discussion.
Methods
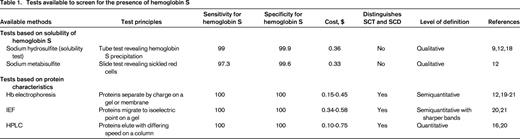
To examine tests used for sickle cell screening, we conducted PubMed and MeSH searches for the terms “sickle cell trait screening” and “sickle cell trait diagnosis.” Keywords describing known testing methodologies were added to the search terms. An English language filter was applied to the search. The search yielded 819 unique references, which were evaluated by title and keyword review for relevance. Following abstract review, commentaries, case reports, case series, and epidemiologic studies were eliminated. The remaining 55 articles determined to be relevant were reviewed for content. Additional articles were selected from the commonly cited references therein. The 21 references chosen for this review, including the table, were seminal articles about SCT and widely used tests to identify hemoglobin S [solubility tests, hemoglobin electrophoresis, isoelectric focusing (IEF), and high performance liquid chromatography (HPLC)]. We contacted the College of American Pathologists directly for current data on solubility tests.
Tests to evaluate for hemoglobin S
Two methodologies form the basis for tests to screen for hemoglobin S: (1) sickling tests that rely on the polymerization of hemoglobin S by reduction or deoxygenation, and (2) those that identify hemoglobin S, as well as other hemoglobin variants, by the biochemical properties of the hemoglobin's proteins.
The most widely used sickling test today, the sickle solubility test, reduces hemoglobin S with sodium hydrosulfite (Table 1).9 When exposed to sodium hydrosulfite, hemoglobin S from lysed erythrocytes precipitates, causing the solution to become turbid. The interpretation is based on a visual inspection of the test tube, although automated readers have been developed.10 Commercially available sodium hydrosulfite-based kits are quick and easy to use and have largely replaced the sodium metabisulfite test in the US. The sodium metabisulfite test remains widely used in resource-limited settings despite being limited by the readout requiring a peripheral blood smear.11 Sickle solubility tests identify hemoglobin S with high sensitivity and specificity. False-negatives are seen in patients with severe anemia, in those with a hemoglobin S fraction <10%, or in patients with high levels of hemoglobin F.12 Coinheritance of alpha-thalassemia trait or hereditary persistence of fetal hemoglobin may lead to false-negative results. False-positives may be observed in conditions associated with increased serum viscosity (eg, polycythemia, hyperlipidemia, elevated serum proteins) and with some hemoglobin variants (eg, hemoglobin I, hemoglobin Bart's, hemoglobin Jamaica-Plain).
Unfortunately, sickle solubility tests do not distinguish SCT from SCD, nor do they identify non-S hemoglobin variants.9 For many healthy adults and even some health care providers, a positive result on a solubility test may cause confusion about the diagnosis of SCT or SCD. A negative sickle solubility test may dissuade an adult from a pre-conception evaluation because adults with hemoglobin C trait, β-thalassemia trait, or other hemoglobin variants may believe that they are not at risk to have a child with SCD. The amount of education necessary to inform a patient about the interpretation of a negative sickle solubility test is extensive, and probably rarely occurs without a mandate.
Tests to evaluate for SCD (and hemoglobin S)
Unlike solubility tests, other strategies to detect the presence of hemoglobin S distinguish trait from disease and identify hemoglobin variants (Table 1). Because hemoglobin electrophoresis, IEF, and HPLC do not rely on a behavior unique to the hemoglobin S protein, they are able to detect many variant hemoglobins. The specifics of each technique has been reviewed recently.13 These tools differ in their methodology, but all use the biochemical characteristics of the hemoglobin protein for identification. All of these methods quantify hemoglobin S and variants, albeit with varied accuracy, allowing their use to diagnose SCD.
Although these tests are highly sensitive, under the conditions of a particular test, the behavior of 2 hemoglobins may be similar enough to prevent differentiation. For example, hemoglobin S co-migrates with less common hemoglobins D, G, and Lepore on alkaline electrophoresis; hemoglobin E co-elutes with A2 on HPLC. Therefore, even though hemoglobin electrophoresis, IEF, and HPLC are highly sensitive for the diagnosis of SCD, confirmation is still required with another methodology.
These methods are limited by the need for equipment and expertise, which add complexity and cost. The cost per test estimated in Table 1 does not account for equipment or personnel costs. IEF requires minimal equipment, and minimal training to perform and interpret, but incurs higher consumable costs than solubility testing. Hemoglobin electrophoresis requires similar equipment and technical expertise to IEF to perform, but can be more challenging to interpret than IEF. HPLC systems cost tens of thousands of dollars for equipment. Once equipment and technical expertise are acquired, however, the costs of consumables and technician time are lower than IEF.14 Public health laboratories often use IEF or HPLC for newborn screening for hemoglobinopathies and for confirmatory testing. Many hospital laboratories and reference laboratories also use these techniques for diagnosis of SCT, SCD, and hemoglobin variants. Sickle solubility tests are used to confirm the presence of hemoglobin S. Other methodologies with similar diagnostic capacity, such as ELISA or mass spectroscopy, have been evaluated but are not broadly used.15,16
Summary
The former soccer player's hemoglobin profile is repeated with HPLC: hemoglobin C trait is confirmed.
The sickle solubility test may seem like the ideal methodology to screen for hemoglobin S: simple, inexpensive, and readily available. When used specifically for the detection of hemoglobin S, the sickle solubility test is highly sensitive and specific. Because the NCAA's goal is to identify student athletes with SCT in order to risk-stratify for EHI, the sickle solubility test is adequate. However, the presence of hemoglobin S, without the additional information provided by other testing modalities, does not denote a specific diagnosis. In the absence of systematic confirmation and education, as mandated by the DoD, positive tests may be misconstrued as disease and negative tests may be misinterpreted as normal (AA) hemoglobin. Furthermore, prevention of EHI can be achieved without screening for SCT. Techniques that identify and quantify hemoglobin S and other variants are readily available through reference laboratories and are not cost-prohibitive. Although no studies directly compare screening tests for SCT, the potential to cause confusion along with the availability of alternative tests that diagnose SCD as well as screen for SCT make the net benefit of sickle solubility tests to screen for SCT not optimal (grade 2C).17 The recent NCAA policies about SCT testing may have brought these issues into the limelight, but they are not specific to student athletes. The appropriate use of tests for the identification of abnormal hemoglobin, along with the necessary education about the implications of those results is both a weakness and an opportunity for our entire healthcare system.
Correspondence
Venée N. Tubman, 450 Brookline Ave, SW 330-A, Boston, MA 02215; Phone: 617-632-4771; Fax: 617-730-0641; e-mail: Venee.Tubman@childrens.harvard.edu.
References
Competing Interests
Conflict-of-interest disclosures: V.N.T. declares no competing financial interests. J.J.F. has received research funding from NKT Therapeutics and Astellas, and has consulted for NKT Therapeutics.
Author notes
Off-label drug use: None disclosed.