Abstract
Bleeding manifestations in patients with immune thrombocytopenia (ITP) range from mild skin bruises to life-threatening intracranial hemorrhage (ICH). Severe bleeding is distinctly uncommon when the platelet count is >30 × 109/L and usually only occurs when the platelet count falls <10 × 109/L. Based on estimates from clinical studies, ITP registries and administrative databases, the frequency of ICH in patients with ITP is ∼0.5% in children and 1.5% in adults. Estimates of severe (non-ICH) bleeding are difficult to obtain because of the lack of standardized case definitions; the lack of a universally accepted, ITP-specific bleeding assessment tool; and the omission of reporting bleeding outcomes in many clinical studies. In practice, the presence of bleeding should dictate whether or not treatment is needed because many patients, especially children, can be safely managed with observation alone. Guiding principles for the management of ITP, based on the bleeding risk are: (1) Decide when treatment is needed and when it can safely be withheld; (2) for patients with chronic ITP, use the least toxic treatment at the lowest dose; (3) emergency treatment of severe thrombocytopenia-associated bleeding requires combination therapy; and (4) early aggressive therapy may result in durable platelet count responses.
Learning Objectives
Recognize the types of severe bleeding occurrences in patients with ITP and their frequency
Describe the methodologic challenges associated with bleeding outcomes in clinical studies
Develop a rational approach to treatment of ITP including emergency management of bleeding
Immune thrombocytopenia (ITP) is a common autoimmune disease characterized by low platelet counts and an increased risk of bleeding. The pathophysiology of ITP is not well understood and several mechanisms have been proposed including autoantibodies and cytotoxic T cells that target platelets and/or megakaryocytes. Not only are the underlying mechanisms of ITP heterogeneous, the clinical manifestations are also highly variable. Even at the same platelet count level, patients may have bleeding manifestations that range from none to severe. Given this heterogeneity, it seems likely that primary ITP is not one disease, but rather a group of disorders with different underlying mechanisms. In addition, there is no diagnostic test for ITP since only 40%-60% of patients have detectable antiplatelet autoantibodies on their platelet surface1 ; thus, the diagnosis rests on excluding other causes of thrombocytopenia. As a result, patients with other thrombocytopenic disorders are often mislabeled as ITP, which perpetuates the use of empiric treatments and the lack of reliable risk stratification models. A better understanding of the pathophysiology will improve the precision of diagnosis and allow for more personalized treatments. Until then, a management approach that considers the patient's bleeding risk is needed. This review focuses on the spectrum of bleeding complications that can occur in patients with ITP and provides a rational approach to treatment based on evidence and current practice.
Association between thrombocytopenia and bleeding
Platelets are critical for maintaining vascular integrity. They provide the surface for coagulation proteins and adhere to the vessel wall at sites of endothelial injury. Based on radio-labelled platelet survival studies in normal volunteers and in thrombocytopenic patients, it is estimated that approximately 7-8 × 109/L platelets are required to maintain vascular hemostasis.2 This minimum threshold is consistent with clinical studies in patients with chemotherapy-induced thrombocytopenia in whom severe bleeding tends to occur only when platelet counts are <10 × 109/L.3 One of the most important studies to describe the relationship between severe thrombocytopenia and bleeding was from 1977, which described a number of thrombocytopenic patients with a variety of disorders.4 Significant bleeding rarely occurred with platelet count levels >30 × 109/L, and major bleeding only occurred when platelet count levels were <10 × 109/L. More recently, a similar relationship was observed in children with chronic ITP.5 These platelet count thresholds have been widely applied; however, significant variability in bleeding risk is present across patients. A long-term prospective study would be needed to establish the correlation between very low platelet counts and bleeding within and between patients.
The risk of bleeding also depends on the underlying condition. For example, intracranial hemorrhage (ICH) is more common in patients with allo-immune thrombocytopenia (eg, neonatal alloimmune thrombocytopenia)6 than in patients with primary ITP.7 The rapidity of the platelet count fall is also important, which can explain why bleeding is more common in patients with drug-induced ITP or cyclical thrombocytopenia where platelet count levels tend to fall quickly.8
Bleeding events in ITP
The first description of ITP from the mid-1500s was of a boy “with dark macules, resembling flea bites, with no fever and for several days had bloody discharges, eventually recovering”.9 Two centuries later, the German physician Paul Gottlieb Werlhof named the disease Morbus Maculosus Hemorrhagicus to indicate the bleeding tendency; however, it was soon renamed M. Maculosus Werlhofii and the reference to bleeding was removed. More recently, the modern day label for the disorder underwent a similar name change whereby “purpura” was removed from “idiopathic thrombocytopenic purpura” in favor of “immune thrombocytopenia” recognizing that many patients with ITP do not have bleeding symptoms.10 Even though severe bleeding is relatively rare, it is the most important clinical endpoint: it is what motivates physicians to institute treatment11 ; provokes physician, patient, and parental anxiety; and is an important cause of morbidity and mortality.12
Frequency of severe bleeding in ITP
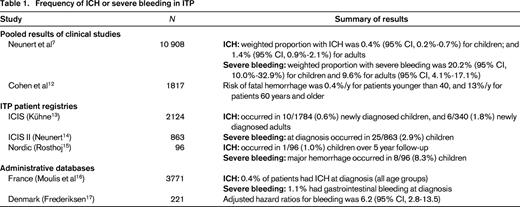
Platelet-type bleeding in ITP manifests as spontaneous skin bruises or petechiae (“dry purpura”); mucous membrane purpura, typically visible in the mouth (“wet purpura”); and frank bleeding from mucosal surfaces causing vaginal, gastrointestinal, or intracranial hemorrhage. Accurate estimates of the frequency of ICH and severe bleeding are required to inform treatment decisions. These estimates derive from pooled analyses of clinical studies, patient registries, and administrative databases (Table 1).
Prospective clinical studies are the most reliable method of capturing bleeding data, together with other pertinent patient characteristics. They are limited by inconsistencies in definitions of severe bleeding, low sample sizes, and relatively short follow-up. In a recent systematic review of prospective ITP studies enrolling at least 20 patients, investigators estimated the frequency of ICH by pooling results from 51 studies (n = 4,782) in adults and children.7 The weighted proportion of ICH was 1.0% overall [95% confidence interval (CI), 0.7%-1.3%]; 1.4% (95% CI, 0.9%-2.1%) for adults, and 0.4% (95% CI, 0.2%-0.7%) for children. ICH was more frequent among patients with chronic (1.6%; 95% CI, 1.0%-2.2%) compared with newly-diagnosed ITP [0.4% (95% CI, 0.1%-0.8%)]. For severe (non-ICH) bleeding, pooled weighted proportions, derived from 29 studies (n = 2,225), were 9.6% for adults (95% CI, 4.1%-17.1%) and 20.2% (95% CI, 10.0%-32.9%) for children. Another pooled analysis of clinical studies reported that the risk of fatal hemorrhage was 0.4% per year for patients younger than 40 year, and 13% per year for patients 60 years and older.12
Registry data can capture a larger sample of patients who are followed for longer periods and may be more representative of the spectrum of patients with ITP than individual clinical studies; however, infrequent follow-up visits do not capture the burden of bleeding, and the breadth of the data is generally smaller. In the Intercontinental Cooperative ITP Study (ICIS) Registry, ICH was reported in 6/340 (1.8%) newly-diagnosed adults and 10/1784 (0.6%) newly-diagnosed children13 ; and severe bleeding occurred in 25/863 (2.9%) children at diagnosis.14 In the Nordic ITP Registry, rates of ICH in children were similar (1/96, 1.0%) and rates of severe bleeding were higher (8/96, 8.3%) over the 5-year follow-up period.15
Administrative databases including health insurance claims are an efficient method of capturing large numbers of patients with and without disease over prolonged periods of time. These data may deviate from the primary information of interest, and coding [typically using International Classification of Diseases (ICD) codes] may not capture all relevant exposures, outcomes or patient characteristics. Thus, before these databases are used for research purposes, an adequate assessment of their reliability and validity is required. Using data from the French National Health Insurance database, investigators reported that 0.4% of ITP patients (all ages) had ICH at diagnosis, and 1.1% had gastrointestinal bleeding.16 In a similar population-based study from Denmark, the adjusted hazard ratio for bleeding was 6.2 (95% CI, 2.8 -13.5) among patients with ITP compared to the general population.17
Together, these data suggest that the frequency of ICH is approximately 0.5% in children and 1.5% in adults, and the risk increases with age. Estimates of severe (non-ICH) bleeding were variable ranging from 3% to 20% in children, and ∼10% in adults. Reported rates of severe bleeding may be lower in registries and administrative databases due to more stringent definitions that generally require the need for hospitalization or blood transfusion.
Predictors of severe bleeding
Identifying which patients with ITP are at highest risk of bleeding would help determine who should receive more aggressive treatments and who can be safely observed. These risk factors have not been well characterized, due to challenges in the measurement of bleeding, the low frequency of significant bleeding events in ITP and ethical and feasibility concerns that preclude a natural-history study in untreated patients. Nevertheless, predictors of severe bleeding have been identified in clinical studies, including the presence of severe thrombocytopenia (platelet count <10-20 × 109/L),15 previous minor bleeding18 and advanced age.19 In several trials of the thrombopoietin (TPO) receptor agonists, response to treatment was associated with an improvement in bleeding outcomes20,21 ; however, when the results of 6 TPO randomized trials were pooled (n = 808 patients), no significant difference in severe bleeding was demonstrated despite an improvement in platelet count levels.22 Further studies are needed to develop reliable risk-stratification models, which will help inform treatment decisions.
Bleeding measurement tools in ITP
Rates of severe bleeding are highly variable across studies not only because of differences in study design mentioned above, but also because of the lack of a universal, standardized bleeding measurement tool. In a recent systematic review of bleeding in ITP, the quality of bleeding assessments was found to be low in 77 studies (65.8%); moderate in 12 studies (10.3%) and high in 28 studies (23.9%).7 Low-quality bleeding assessments were those that reported bleeding as “present or absent” or by anatomical site without a definition of severity. Moderate quality bleeding assessments used generic measurement tools to capture bleeding events such as the World Health Organization bleeding score23 or the Common Terminology Criteria for Adverse Events.24 High-quality bleeding assessments used measurement tools that were specific for ITP patients. Ten such tools were identified; of those, the Buchanan tool for children25 and the Page score for adults,26 have been used widely and have demonstrated adequate reliability in independent studies. The Buchanan tool provides an overall bleeding grade from 1 to 5, which includes assessments at 3 anatomical sites (oral, epistaxis, and skin) graded from 0 (no bleeding) to 5 (life-threatening or fatal bleeding).25 The Page score, also known as the ITP Bleeding Scale (IBLS) assigns a bleeding severity score from 0 (no bleeding) to 2 (marked bleeding) at 9 anatomical sites by history (skin, oral, epistaxis, gastrointestinal, urinary, gynecologic, pulmonary, intracranial, and subconjunctival), and from 2 anatomical sites by physical examination (skin and oral).26
Recently, a new ITP-specific bleeding tool has been developed that quantifies bleeding from skin (s), visible mucosa (m), and internal organs (o; “SMOG”) using a cumulative severity scale from 0 (no bleeding) to 5 (fatal bleeding).27 The performance of this tool has not yet been evaluated.
Bleeding as an outcome in ITP clinical trials
Virtually all clinical studies in ITP use platelet count endpoints to estimate treatment effects, and clinical endpoints such as bleeding or quality of life are either secondary endpoints or omitted altogether. Among the 51 randomized trials in ITP published between 1970 and 2014, only 30 (58.8%) reported bleeding outcomes.7 Whether the platelet count by itself is a suitable endpoint is a matter of debate28 : The use of platelet count as a “surrogate” outcome may be justified since thrombocytopenia represents disease activity, and an improvement in the platelet count is an established benefit of treatment. However, thrombocytopenia does not capture the full burden of ITP for many patients who have reduced quality-of-life despite improvements in platelet count5 and the correlation between bleeding and severe thrombocytopenia has not been established.29 Conversely, the use of severe bleeding as the primary outcome for clinical trials is limited by the low event rate and measurement bias. Investigators have estimated that a trial powered to reduce the risk of severe bleeding in children with ITP from 3% to 1% would require 1730 patients, which would not be feasible.14 In one study of 60 consecutive patients with acute ITP, bleeding, rather than platelet count was used to guide management.30 Even though all patients had a platelet count <20 × 109/L, intravenous immune globulin (IVIG) was administered only to those with a high bleeding score; otherwise, patients received corticosteroids. This treatment strategy reduced the use of IVIG and the need for hospitalization with no reported increase in life-threatening bleeding.
Principles of ITP treatment
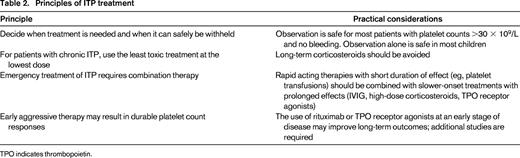
The following guiding principles provide a rational approach for the treatment of ITP considering disease severity and risk of bleeding: (1) decide when treatment is needed and when it can safely be withheld; (2) for patients with chronic ITP, use the least toxic treatment at the lowest dose; (3) emergency treatment of ITP requires combined modality treatment; and (4) early aggressive therapy may result in durable platelet count responses (Table 2).
Decide when treatment is needed and when it can safely be withheld
One of the ASH 2014 Choosing Wisely recommendations is that patients with ITP should not be treated unless they are bleeding or have very low platelet counts.31 The ASH evidence-based practice guideline for ITP recommends that most adult patients with a platelet count >30 × 109/L and no bleeding do not require treatment and can be managed with observation alone; and that most children, even those with mild bleeding, can be managed with observation alone regardless of platelet count.32 The safety of this approach has been mainly studied in children, who often have transient episodes of acute thrombocytopenia. In a 5 year, retrospective study in a large, urban, pediatric tertiary care hospital (n = 311), 34.9% of children who received care between 2007 and 2010 were managed with observation alone. This proportion increased to 71.1% in 201233 following the publication of the ASH guidelines. In that study, observation did not result in an increase in later treatments or bleeding symptoms. Preliminary results of a multicenter randomized trial of IVIG versus observation in 172 children with newly-diagnosed ITP (platelets <20 × 109/L) and mild to moderate bleeding were presented at the 2014 ASH annual meeting.34 At 1 month, 80.2% (69/86) of children in the IVIG group achieved an overall platelet count response (platelet count ≥30 × 109/L and doubling from baseline) compared with 60.5% (52/86) in the observation group. Complete responses (platelet count ≥100 × 109/L) were achieved by 61.6% (53/86) and 39.5% (34/86), respectively. Five severe bleeds were reported in the observation group compared with none in the IVIG group. Thus, the safety of this approach requires further study. In summary, treatment can be withheld for most patients with platelets >30 × 109/L; however, additional research is needed to establish which patients with more severe thrombocytopenia can be safely managed without pharmacologic treatment.
For chronic ITP, use the least toxic treatment at the lowest dose
In general, ITP treatment is largely empiric and typically proceeds in a stepwise fashion starting from the least toxic.35 First-line therapy is corticosteroids with or without IVIG. Rhesus immune globulin may be as effective as IVIG for nonsplenectomized, rh-positive patients; however, hemolysis is a limiting toxicity36 and fatal cases of disseminated intravascular coagulation have been reported leading to a black box warning.37 Several courses of first-line therapy may be required during the first 6-12 months after initial presentation, when spontaneous remissions are still possible. If there is no platelet count response after 4-6 weeks, or if a relapse occurs, I consider adding an immunosuppressant agent such as mycophenolate or a TPO receptor agonist medication. If no sustained improvement in platelet count is achieved after 6-12 months, splenectomy or rituximab should be considered as second-line therapies because they are both associated with durable remissions. My preference is to use splenectomy first if the patient can tolerate surgery, because of longer durations of platelet count responses with splenectomy which is often sustained for many years,38 whereas the effect of rituximab is less durable. In one report, only 20% of patients who achieved an initial response to rituximab remained in remission 5 years later.39 A recent randomized trial comparing corticosteroids plus either rituximab or placebo found no difference in the cumulative incidence of overall response at 78 weeks (81% vs 73%, p = 0.15).40 Vaccinations against S. pneumoniae, N. meningitides, and H. influenza type B should be administered in advance of either splenectomy or rituximab.41 Rituximab may be appropriate for patients who are not candidates for, or who are unwilling to undergo, splenectomy.
TPO receptor agonists are useful for patients with persistent severe thrombocytopenia after splenectomy and/or rituximab. In extension studies, serious adverse events were infrequent even with prolonged exposure to romiplostim (n = 292)42 or eltrombopag (n = 299).43 Thromboembolic events occurred in 6.5% of patients on romiplostim and 4% of patients on eltrombopag. Combination immunosuppressant therapy should be reserved for patients with refractory ITP44 and long-term corticosteroids should be avoided.
Emergency treatment of ITP requires combination therapy
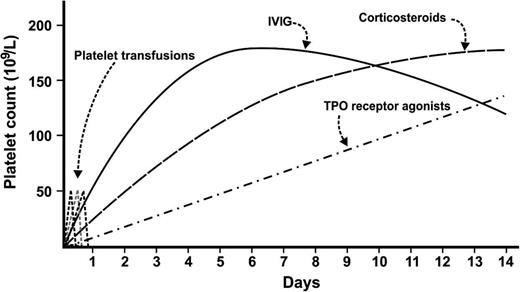
Patients with severe thrombocytopenia (<20 × 109/L) and significant bleeding should be hospitalized. As with nonemergency presentations, a thorough search for secondary causes of thrombocytopenia is required including examination of the peripheral blood film to exclude thrombotic microangiopathy and a careful history to identify recent exposure to new drugs. Treatment should be administered urgently with the goal of achieving a rapid platelet count rise. High-dose corticosteroids (eg, high-dose dexamethasone or intravenous methylprednisolone) results in a platelet count improvement by 2-5 days, and often works faster than standard dose prednisone.45 IVIG (1-2 g/kg) should be considered for patients with, or at risk for severe bleeding because it can produce a platelet count rise even faster, within 12-48 hours. Platelet transfusions, especially when administered in multiple doses or as a continuous infusion, can increase the effective circulating platelet mass instantaneously, but the effects are very short-lived lasting only minutes to hours; thus, platelet transfusions should be reserved for patients with severe or life-threatening bleeding. The durability of the platelet count response is another important consideration. The duration of the effect of corticosteroids is variable, the effects of IVIG typically last several weeks, and TPO receptor agonists can cause a steady rise in platelet count 1-2 weeks after administration.
Thus, for emergency management of patients with severe ITP and severe bleeding, I recommend high-dose corticosteroids; add IVIG for patients with, or at risk for severe bleeding; add platelet transfusions for severe or life threatening bleeding; and add a TPO receptor agonist to avoid rapid recurrences of severe thrombocytopenia (Figure 1). Emergency splenectomy should be considered for patients with severe refractory thrombocytopenia and life-threatening bleeding.
Approach to emergency management of severe bleeding in patients with immune thrombocytopenia using combination therapy.
Approach to emergency management of severe bleeding in patients with immune thrombocytopenia using combination therapy.
Early aggressive therapy may result in durable platelet count responses
For some patients, aggressive treatment for ITP administered early in the disease process can produce durable platelet count responses. For example, investigators have evaluated the impact of using rituximab early, even at initial presentation. A meta-analysis of 5 randomized trials showed that complete platelet count responses, as assessed at least 6 months after treatment, were more common when 4 weekly doses of rituximab were added to standard of care compared with standard of care alone (relative risk 1.4, 95% CI, 1.1-1.8).46 A small single-arm study recently evaluated the use of a single course of high-dose dexamethasone (40 mg orally per day on days 1 to 4) immediately followed by eltrombopag (50 mg daily from days 5 to 32) in 12 adults with newly-diagnosed ITP.47 At 6 months, rates of complete (platelets >100 × 109/L) and any response (>30 × 109/L) were 50% and 75%, respectively. Controlled trials are needed to confirm that early aggressive therapy can cause long-term remissions.
Conclusion
Based on estimates from clinical studies, ITP registries, and administrative databases, the frequency of ICH in patients with ITP is ∼0.5% in children and 1.5% in adults. The frequency of severe bleeding is variable because of the lack of a standardized bleeding assessment tool and the emphasis on platelet count levels rather than bleeding as the primary outcome in clinical trials. The presence of bleeding should dictate whether or not treatment is needed because many patients, especially children, can be safely managed with observation alone. Emergency management of thrombocytopenia-associated bleeding in ITP requires a combination of treatments to improve the platelet count quickly and maintain safe platelet levels at least until the bleeding subsides. The benefit of early aggressive treatment for patients with newly-diagnosed ITP requires further study.
Acknowledgment
D.M.A. holds the John G. Kelton Chair in Translational Research (McMaster University).
Correspondence
Donald M. Arnold, Department of Medicine, McMaster University, 1280 Main St West, Rm 3V50, Hamilton, ON, L8S 4K1 Canada; e-mail: arnold@mcmaster.ca.
References
Competing Interests
Conflict-of-interest disclosure: The author has received research funding from Amgen and GlaxoSmithKline; and has consulted for Amgen and Bristol Myers Squibb.
Author notes
Off-label drug use: Rituximab is not licensed for the treatment of ITP.