Abstract
Graft-versus-host disease (GVHD) is a significant clinical problem after allogenic hematopoietic cell transplantation (HCT) associated with substantial morbidity and mortality that limits the potential utility of transplantation. Associated with GVHD is the well-recognized phenomenon of the graft-versus-leukemia (GVL) effect that results in reduced risk of disease relapse. GVL effects have been observed after treatment for a broad range of hematological malignancies. Both GVHD and GVL are the results of T cell–effector functions that frames a major question in the field of how linked are these two phenomena. A major goal of basic science and translational research has been to develop strategies to reduce the risk of GVHD while maintaining or enhancing GVL. In this review, a number of different strategies developed from preclinical animal models will be explored with a focus on those approaches that have been extended to the clinic in an attempt to achieve this goal. Needless to say, there is no proven strategy; however, with the use of modern technology and clinical translation, there has been substantial progress toward this goal of reducing the risks of GVHD while promoting and enhancing GVL responses.
Learning Objectives
To understand the biology of graft-versus-host disease (GVHD) and graft-versus-leukemia (GVL) responses in preclinical models
To learn about current and future strategies of suppressing GVHD while maintaining or enhancing GVL responses in the treatment of patients undergoing allogenic hematopoietic cell transplantation
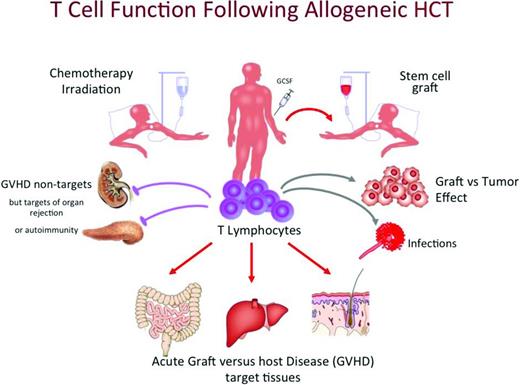
Allogenic hematopoietic cell transplantation (HCT) is an established therapy for a broad range of hematological malignancies, bone marrow failure states, and genetic diseases. After transplantation, donor-derived T cells provide many functions, such as enhancement of engraftment, protection from opportunistic infections, and, in the setting of malignancies, rejection of the underlying disease. However, these same T cells also result in graft-versus-host disease (GVHD) that can range from a mild skin rash to a life-threatening and in some instances life-ending complication (Figure 1). GVHD and the risk of opportunistic infections have limited the potential utility of allogenic HCT that could also be an effective therapy for the treatment of patients with severe autoimmune disorders, induction of organ transplantation tolerance, and perhaps other clinical settings if these problems could be addressed effectively. It has been recognized for several decades that a major benefit of allogenic HCT has been the graft-versus-leukemia (GVL) effect that is a result of donor T cells capable of recognizing residual tumor cells and rejecting these cells, resulting in dramatically reduced risk of relapse. The GVL concept was developed from many lines of evidence, including the increased risk of relapse after syngenic transplantation, the finding that T-cell depletion resulted in reduced risk of GVHD but also increased relapse rates, a decreased relapse rate associated with chronic GVHD, and finally the utility of donor leukocyte infusions (DLIs) for the treatment of patients who suffer a relapse after allogenic HCT in which some patients respond, often with durable remissions. Therefore, the concept that the graft can exert antitumor effects is well established in the field and is a mainstay of the mechanism of how allogenic HCT can potentially cure patients with complex, often refractory, hematological malignancies. Despite the acceptance of the concept of GVL, there is no consensus on the cells responsible for this effect other than T cells are clearly involved or of the target structures recognized on the tumor cells. Furthermore, it is recognized clinically that patients with some diseases, such as chronic myeloid leukemia (CML), chronic lymphocytic leukemia, and low-grade lymphomas, are better targets for GVL effects than diseases such as Hodgkin's disease and acute lymphoblastic leukemia, with other diseases such as non-Hodgkin's lymphoma, acute myelogenous leukemia, and multiple myeloma being intermediate. A biological explanation for this clinical observation has not been elucidated.
Role of T cells in different aspects of allogenic HCT (courtesy of Andreas Beilhack, University of Wurtzburg, Wurtzburg, Germany).
Role of T cells in different aspects of allogenic HCT (courtesy of Andreas Beilhack, University of Wurtzburg, Wurtzburg, Germany).
Lessons from preclinical models
Animal model systems have been essential in developing our understanding of the concepts related to GVHD and GVL effects. Both canine and murine model systems have been critically important to understand the requirement of T cells, activation events, the role of different cytokines, and the trafficking and homing of cell populations resulting in a GVHD response. Canine models have also been instructive in developing many of the GVHD prevention treatment strategies that are commonly used in the clinic. Murine models have been more helpful in dissecting the underlying biology of GVHD and GVL responses. However, both suffer from the limitations of animal modeling and the tumor systems that are used in murine models that are primarily either transplantable tumors or develop in a relatively short period of time in the context of the animal's life. Therefore, although these studies have been extremely important development concepts, they have not always resulted in accurately depicting the clinical situation, especially in the context of understanding GVL responses.
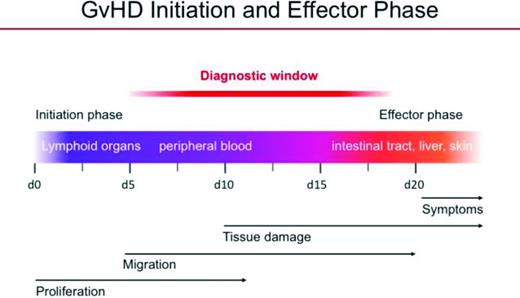
After the adoptive transfer of donor-derived T cells across either major or minor histocompatibility barriers, T cells home very rapidly within 3-5 days because of secondary nodal sites, including lymph nodes and spleen in which they become activated and upregulate the expression of other molecules that allow entry into GVHD target structures, such as the skin, gastrointestinal tract (GIT), and liver.1,2 After a somewhat delayed course of 5-7 days across major histocompatibility barriers and 2-3 weeks across minor histocompatibility barriers, T cells traffic to these GVHD target structures and subsequently induce damage resulting in the manifestations of GVHD, such as diarrhea, weight loss, fur loss, and ultimately animal mortality (Figure 2). Key to these observations is that, once the clinical symptoms of GVHD develop, many events have already occurred resulting in a challenging immunological cascade that is difficult to control. One can speculate that a similar series of biological events are occurring in patients destined to develop GVHD, although the tools to visualize these processes are not yet available. These murine studies have highlighted critical early events in T-cell activation and trafficking and are in concert with some of the clinical approaches being applied currently, such as the use of post-transplant methotrexate (MTX) and cyclophosphamide in these very early time periods of active T-cell proliferation, as well as the use of agents that block trafficking of cell populations to major sites of GVHD pathophysiology, namely, the GIT.
Migration of T cells in the initiation and development of GVHD demonstrating early migration to lymphoid organs, activation, migration, and ultimately infiltration into GVHD target organs, resulting in clinically evident disease (adapted with permission from Bauerlein et al1 ).
Migration of T cells in the initiation and development of GVHD demonstrating early migration to lymphoid organs, activation, migration, and ultimately infiltration into GVHD target organs, resulting in clinically evident disease (adapted with permission from Bauerlein et al1 ).
These studies have also highlighted the underlying biology of GVHD as a dysregulated, uncontrolled cascade of immunological events caused by excessive T-cell proliferation and lack of proper immune regulation. Several groups have focused on immune regulatory mechanisms in controlling GVHD. In these studies, the adoptive transfer of donor or third-party–derived immune regulatory cells, such as CD4+CD25+FoxP3+ regulatory T (Treg) and natural killer T (NKT) cells are capable of suppressing GVHD and importantly do not appear to interfere with or augment GVL responses (for review, see Schneidawind et al3 ) The underlying mechanism of the separation of GVHD from GVL is thought to be related to the control of T-cell proliferation by Treg cells that block the excessive T-cell proliferation characteristic of GVHD responses but allow for maintenance of GVL responses especially in the setting in which the T-cell frequency of tumor antigens is relatively high and in which the burden of disease is relatively low, a situation typical of allogenic HCT. Other populations of Treg cells, including expanded TR1 cells, myeloid-derived suppressor cells, and as B Treg cells, also have biological activity in animal model systems, and translation to the clinic has either been performed or is under active investigation (for review, see Schneidawind et al3 ).
Analysis of GVHD and GVL responses
Key to developing an understanding of the biological differences of GVHD and GVL reactions is our ability to dissect the immune responses with rigor and clarity. A number of studies have focused on the immunophenotyping, cytokine production, and effector functions of cell populations, as well as the use of spectratyping to gain greater insights into the cells responsible for GVHD compared with those responsible for GVL. A major challenge has been that there is no accepted in vitro assay for GVL responses, and identifying those cells that are responsible for this biological effect have been challenging. With new approaches, such as the use of sequencing technologies, inroads into this vexing clinical problem are anticipated. T-cell receptor high-throughput sequencing (TCR HTS) is of particular interest given that this approach allows for the analysis of the entire repertoire of T cells involved in immune reactions such as GVHD and GVL responses. A number of studies have focused on GVHD and anti-cytomegalovirus responses to date, demonstrating the clinical utility of these types of analyses.4,5 For example, using TCR HTS clonal populations of T cells were found in patients who developed clinically significant GVHD in gastrointestinal biopsies and identified a population of patients responsive to corticosteroids in which these infiltrating clones receded compared with those patients who had steroid refractory GVHD in which the representative clones expanded dramatically in the peripheral blood of these patients such that up to 10%-20% of all T cells were of this particular TCR sequence. A similar analysis could be performed for GVT responses once available assays can be developed to assess how similar or different are the populations responsible for these diverse biological reactions. Therefore, it is hoped that using these and other sophisticated analytical approaches that will give greater insights into the repertoire of T cells responsible for GVHD and GVL and allow for greater understanding of the overlap of these different cell populations. Ultimately, it may be possible using TCR sequence analysis to develop strategies to identify targets of GVHD and GVL responses as has been done initially in the study of tumor-infiltrated T cells and certain viral infections.6
Strategies to control T-cell proliferation
As highlighted by these preclinical animal models, GVHD is characterized by robust T-cell activation and proliferation, followed by infiltration of GVHD target structures. It is less clear to what degree T-cell activation and proliferation is required for effective GVL responses, and, in animal model systems, control of T-cell proliferation has been an effective strategy to uncouple GVL from GVHD. This has led to the concept that it may be possible to identify patients at great risk for GVHD earlier in their clinical course. Major efforts in developing biomarkers to identify patients that are at higher risk and to predict GVHD severity are at the forefront of our current thinking.7-9 These studies have enormous potential to allow for earlier interventions in those patients at greatest risk and to also gain novel insights into the underlying pathophysiology of GVHD in the clinic
A number of approaches have been used to control GVHD responses in patients undergoing HCT using immunosuppressive drugs, such as calcineurin inhibitors (CNIs; cyclosporine A or tacrolimus), MTX, mycophenolate (MMF), and rapamycin, among others. It is of interest that these agents limit T-cell proliferation through a variety of different mechanisms in concert with the preclinical animal modeling studies. Currently, the standard approach is to use a CNI and MTX after myeloablative conditioning and a CNI and either MMF or MTX after reduced conditioning for GVHD prevention. A recent prospective randomized clinical trial by the Bone Marrow Transplant Clinical Trials Network (BMT CTN) compared FK506/MTX with FK506/rapamycin, showing similar outcomes but more challenges with the use of rapamycin.10 It is clear that these global immunosuppressive approaches have merit but also significant limitations, including increased risk of opportunistic infections and toxicities of the drugs that can affect different organ systems. T-cell depletion has been studied by a number of different groups in which earlier studies with complete T-cell depletion resulted in increased risk of relapse. However, more partial T-cell depletion may be an alternative strategy that could thread the needle between excessive T-cell activation, resulting in reduction in GVHD yet providing sufficient numbers of T cells to provide GVL responses.11 Ongoing clinical trials, such as the randomized BMT CTN study comparing conventional FK506/MTX immune prophylaxis with post-transplant cyclophosphamide or CD34+ cell selection, may provide answers to these questions. In vivo T-cell depletion, for example, through the use of anti-thymocyte globulin, has resulted in a decreased rate of acute (grade II-IV, 33% versus 51%) and especially chronic GVHD (extensive, 12% versus 42.6%) without an apparent increase in disease relapse, suggesting that GVL responses were maintained in a randomized clinical trial.12 A number of strategies have been developed to attempt to deplete alloreactive T cells either by in vitro or in vivo depletion methodologies. These studies have been particularly important in the setting of haploidentical transplantation in which T cells have resulted in severe GVHD and rigorous T-cell depletion has affected immune recovery, rendering patients at high risk for opportunistic infections. Studies involving in vitro depletion involve exposure of the donor cells to recipient antigen presenting cells, resulting in T-cell activation followed by strategies to deplete those activated T cells using either photoactive dyes or some other depletion technology. These strategies have shown promise in both animal models and early-phase clinical trials but are challenged by the somewhat cumbersome laboratory-based approaches that make broad applicability less likely. Other approaches, such as the use of anti-interleukin (IL)-6 antibodies in addition to standard prophylaxis, have resulted in an impressive reduction in acute GVHD rate grade II-IV to 12%.13 Modulation of T-cell function has also been explored through the use of vorinostat in preclinical models and now in phase 1/2 studies with an apparent reduction in GVHD risk without increase in relapse rates compared with historical controls.14 Ultimately, randomized clinical trials of these and other approaches will be required to prove efficacy of GVHD reduction without affecting relapse rates.
An alternative strategy that has gained significant clinical evidence has been the use of cyclophosphamide in the post-transplant setting that depletes alloreactive T cells that are activated at these early time points.15 It is striking that the timing of cyclophosphamide addition on days 3-5 after transplant is also the same timing that would be predicted by preclinical model systems in which T cells are activated and proliferating in nodal sites. At the current time, we do not have the capability of assessing these T-cell activation events in patients through sampling and/or imaging approaches, but it is likely that similar biological phenomena are occurring. These results have demonstrated that post-transplant cyclophosphamide is an effective approach for controlling GVHD after both haploidentical and human leukocyte antigen matched transplant settings.15,16 Mechanistically, it is felt that not only are alloreactive T cells depleted but Treg cells are relatively spared by cyclophosphamide because of increased aldehyde dehydrogenase expression.17 The use of post-transplant cyclophosphamide has opened the field of haploidentical transplantation as a feasible approach that is currently being compared head-to-head with double umbilical cord blood (UCB) transplantation in an important clinical trial sponsored by the BMT CTN. The concern of this approach is that post-transplant cyclophosphamide may also deplete those T cells responsible for GVL responses because, theoretically, these would also be the alloreactive T-cell population and relapse rates have been significant after transplantation.10 Nonetheless, the introduction of post-transplant cyclophosphamide has been a major addition to the strategies to control GVHD and is associated with low levels of both grade III-IV acute GVHD and chronic GVHD. Interestingly, several studies have reported relatively low rates of disease relapse after UCB transplantation through mechanisms that are not yet apparent.
As discussed above, preclinical animal modeling has demonstrated that the infusion of Treg cells in addition to conventional T cells results in control of GVHD with maintenance of GVL responses. Several groups have explored the adoptive transfer of purified populations of CD4+CD25+ Treg cells after both UCB and haploidentical transplantation.18,19 These studies have been remarkable in that they have demonstrated the translatability of the preclinical animal models and clearly demonstrated that Treg cells have biological function. The most illustrious study from the University of Perugia has used myeloablative conditioning regimens, rigorous T-cell depletion, and no post-transplant immunosuppression in the setting of haploidentical transplantation for patients with advanced hematological malignancies. Their previous studies demonstrated that as few as 5 × 104 conventional T cells/kg were associated with significant GVHD risk. In their studies, patients first receive purified (∼80% FoxP3+) Treg cells at a dose of ∼2 × 106 highly purified Treg cells, followed 4 days later by the administration of conventional CD4 and CD8 cells at 106 T cells/kg and CD34+ cells with no post-transplant immunosuppression, resulting in a remarkably low risk of GVHD and improved immune reconstitution compared with their historical cohorts.18 These cell doses are remarkable because the administration of this dose of conventional T cells would clearly be above the acceptable threshold after haploidentical transplantation without post-transplant immunosuppression and demonstrate that the Treg cells have biological function. Although there still is a significant risk of infection, an extremely low risk of relapse has been observed following this approach, demonstrating that GVL responses have been retained even in those patients receiving grafts from NK non-alloreactive donors.20
Another approach of exploiting Treg cell biology includes alternative strategies for preparation of patients, for example, using total lymphoid irradiation and antithymocyte globulin (TLI/ATG). TLI/ATG conditioning is based on murine modeling that demonstrated that the administration of TLI and ATG was associated with an alteration in the ratio of conventional T cells to invariant NKT (iNKT) cells such that 1000 times the number of T cells could be administered without significant GVHD.21 GVHD protection in TLI/ATG prepared animals was attributable to host iNKT cells that were more radioresistant and produced IL-4. This concept has been translated to the clinic in the setting of treating patients with hemolytic malignancies demonstrating the feasibility of the approach and low risk of both acute and chronic GVHD with achievement of long-term remissions, indicating that GVL responses are preserved because there is little anti-leukemia efficacy of this preparative regimen.22,23 TLI/ATG has been directly compared with fludarabine and TBI in a randomized clinical trial performed by investigators in Belgium. Patients prepared with TLI/ATG had outcomes similar to those prepared with fludarabine/TBI associated with lower transplant–related mortality, although with a higher relapse risk.24 Therefore, TLI/ATG conditioning serves as a useful platform that hopefully can be augmented by other immunotherapeutic interventions. These and other strategies demonstrates that using our understanding of the biology of Treg cells holds promise for controlling GVHD and perhaps maintaining GVL responses in diverse clinical settings. Other pharmacological approaches for activating and expanding Treg cells are needed as the adoptive transfer studies are important for proof of concept but are cumbersome to perform on a wide-scale basis. Other strategies, such as the use of drugs that may activate T cells, are also of great interest. One such drug, ibrutinib, has been associated with T-cell activation and suppression of B-cell proliferation and holds promise for potential expansion of GVL responses in the post-transplant setting, which is currently under active investigation. Janus kinase 1/2 inhibition has emerged recently as an interesting target for treatment of both acute and chronic GVHD. A recent observational study by Zeiser et al25 evaluated 54 patients with steroid refractory acute GVHD and 41 patients with steroid refractory chronic GVHD. The overall response rate after treatment with ruxolitinib of >80% in these two challenging subsets of patients is highly encouraging. Clearly, additional studies are warranted with this agent.
Role of B cells
A growing body of evidence has demonstrated a role for B cells in GVHD pathophysiology, especially chronic GVHD. Strategies to deplete B cells with, for example, B cell–depleting antibodies have been encouraging.26,27 Randomized studies are anticipated. B-cell signaling inhibition with, for example, ibrutinib is another strategy well supported by preclinical data.28
Strategies to enhance GVL responses
A number of strategies have been explored to enhance GVL in the post-transplant setting. Earliest among these is the use of DLIs in which a number of studies have shown that patients who suffer a relapse can be rendered back into complete response, sometimes even complete molecular response, by the infusion of leukocytes from the original donor.29 DLI has been most effective in the treatment of CML, but other malignancies have also been responsive although at a significantly lower frequency. However, DLI has been associated with a significant risk of GVHD that has limited enthusiasm for this approach. However, the use of DLI has highlighted that post-transplant infusion of lymphoid populations could be a strategy that might hold promise for controlling eventual relapse. Another population of cells that has generated significant interest are NK cells. For reasons that are not completely understood, NK cells do not appear to cause GVHD, which may be related to the more limited proliferative capacity of this cell population and the somewhat lower trafficking to GVHD target sites.30 NK cells are known to recognize major histocompatibility complex Class I molecules that prevents their activation, and transplantation is an excellent model system in which to exploit NK cell biology. Clinical studies have demonstrated the potential of NK cells that are activated after haploidentical transplantation because of the lack of inhibitory engagement with Class I molecules of the recipient leukemic cells.31 This has led to the concept of exploring the adoptive transfer of NK cells after allogenic transplantation either from haploidentical or potentially from third-party donors to attempt to exploit this underlying biology. To date, these studies have shown feasibility and limited GVHD risk except in some situations in which NK cells have been associated with significant GVHD activity when activated before infusion.32,33 Therefore, this approach holds promise but also some caution in translation.
Among T cells, naive T cells expressing CD45RA are more likely to be associated with GVHD responses. Several different laboratories have shown that the depletion of naive T cells is associated with reduced risk of GVHD and retention of GVL in animal modeling systems.34,35 This has raised the possibility of using memory T cells, either total T cells or CD8 memory cells for maintaining GVL responses after allogenic transplantation.36 Ongoing clinical trials are exploring this possibility.
Activated T cells have been associated with reduced capacity for GVHD after adoptive transfer. Several different approaches using either anti-CD3/CD28 or anti-CD3 and IL-2 with interferon-γ termed cytokine induced killer (CIK) cells have resulted in expansion of T cells with antitumor properties yet have limited GVHD capacity.37,38 After expansion, CIK cells have antitumor capabilities primarily through NKG2D-mediated mechanisms, as well as other cytolytic functional receptor ligand interactions. These cells home to the tumor site39 that has allowed for the concept of using CIK cells to deliver other payloads, for example, oncolytic viruses.40 Certainly, another approach to provide a GVL response that has enormous potential is the use of CAR-T cells that are the subject of intense investigation as a strategy of a bridge to transplant or instead of transplantation.41 These cells have been used in a small number of patients from bone marrow donors and shown to have limited capacity for GVHD induction but exert an antitumor effect. Therefore, the use of adoptive T-cell strategies holds great promise for enhancing GVL responses yet limiting GVHD.
Targets of GVL
A key question in the study of GVL is, what are the targets recognized by the immune system? A number of potential targets have been identified using either antibody or T cell–based assays systems, including NuSAP1,42 WT1,43 and minor histocompatibility antigens.44 Adoptive transfer of T cells have been attempted with responses noted in some patients receiving T cells directed against minor histocompatibility antigens,45 leukemia reactive T-cell clones,46 and WT1.47 With the development of more effective engineering of T cells, additional refinements can be anticipated.
Conclusions
A major goal in the setting of HCT has been to suppress GVHD while promoting GVL responses. A number of different strategies have demonstrated that this is feasible in well-defined preclinical model systems. However, decisive strategies in the clinic have been elusive. Significant progress is being made in a number of different areas to better define the T-cell populations associated with these different biological responses and in developing strategies to enhance GVL responses while suppressing GVHD. Participation of patients in well-designed clinical trials with this focus remains an area of active and ongoing need, and through these biological explorations comes the hope that strategies will be developed to accomplish this long sought after clinical goal.
Correspondence
Dr Robert S. Negrin, Department of Medicine-Blood and Marrow Transplantation, Stanford University, CCSR Building, Room 2205, 269 W Campus Dr, Stanford, CA 94305; Phone: 650-723-0822; e-mail: negrs@stanford.edu.
References
Competing Interests
Conflict-of-interest disclosure: The author has consulted for Sanofi, Amgen, and UpToDate and has equity ownership in ConcentRx.
Author notes
Off-label drug use: None disclosed.