Abstract
For patients without a human leukocyte antigen (HLA)-matched sibling or unrelated donor, options include transplantation from HLA-mismatched related donors, HLA-mismatched unrelated donors, or unrelated cord blood units. Graft failure remains a problem in 10%-20% of cord blood transplants that contain a limited number of hematopoietic cells. Many approaches are tested in clinical trials to offset the risk of graft failure after cord blood transplantation. GVHD remains a hurdle with any HLA mismatched graft. The use of post-transplant cyclophosphamide holds the promise to overcome the HLA barrier and prevent GVHD despite donor mismatch for a full HLA haplotype. Priority should be given to enrolling patients onto transplant protocols addressing the fundamental problems of engraftment, GVHD, relapse or treatment-related mortality tested with one or more of the alternative stem cell sources. Principles for prioritization of alternative stem cell sources are discussed separately for children and adults who cannot be enrolled on clinical trials. It is difficult ranking currently available sources in the face of multiple factors affecting outcomes, rapidly changing transplant technology and without results from comparative trials.
Learning Objectives
To present successes and challenges with transplantation from alternative donors, including HLA mismatched related, HLA mismatched unrelated, and cord blood sources
To review current results of alternative donor transplantation
The human major histocompatibility complex (MHC)
The discovery of the MHC system on human chromosome 6 has allowed successful transplantation of hematopoietic stem cells by selecting human leukocyte antigen (HLA)-matched sibling donors for the treatment of hematologic malignancies and nonmalignant disorders. The probability of HLA matching is only 25% per sibling and therefore an HLA-identical sibling donor is only available to ∼30% of patients in the developed world. Providing a healthy allogeneic donor for all patients who need a transplant remains an unmet need in the field.
Development of HLA-typed volunteer donor and cord blood registries
Based on the observation that donor HLA matching is paramount to engraftment, control of graft-versus-host disease (GVHD), and immunological tolerance, a worldwide network of HLA-typed volunteer donor and cord blood registries has been developed that nowadays contains >26 million donors and >680 000 cord units. DNA sequencing of the HLA complex has defined 15 functional genes that are relevant to transplantation, and molecular testing has permitted the identification of closely matched unrelated donors or cords.
Unrelated volunteer donor matching
At this time, matching for the 4 pairs of HLA-A, -B, -C, and -DRB1 genes is considered standard for the selection of unrelated donors.1 Mismatching for 1 of the 8 alleles is associated with increased risk of graft loss, GVHD, and death. Certain mismatches in the HLA sequence that encode for peptide-binding sites at the bottom of HLA groove are associated with higher risk of GVHD.2 Matching for loci that are expressed at low level, HLA-DRB3/4/5, DQB1, and DPB1, minimizes the risk of GVHD and death after 7/8 HLA-A, -B, -C, -DRB1-matched grafts.3 Mismatching for HLA alleles appears well tolerated in some cases without dire consequences, like HLA-C*0303 versus *0304, and DPB1 disparities that elicit low or no T-cell immunity.1,4,5 Polymorphisms for the 3′ UTR of DPB1 exon 6 sequences can regulate HLA gene expression and affect both the levels of HLA protein expression and immunogenicity.6 With more precise matching, survival after HLA-matched unrelated donor grafts approximates survival after HLA-identical sibling donor grafts.7
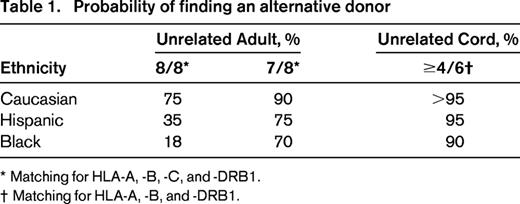
Probability of finding an HLA compatible unrelated donor or cord blood unit
HLA inheritance is associated with an individual's ethnicity. With a US registry size >10 million donors, the probability of finding a 8/8 HLA-A, -B, -C, and -DRB1 match is 75% for an individual of western European descent, but as low as 18% for a African American (Table 1).8 The variability in matching probabilities depends on two major factors: the composition of the registry is predominantly western European, and the degree of genetic disparity within each ethnic group that is the largest in blacks. The probability of finding a donor increases to at least 65% for everyone, if a 7/8 match is allowed. As the pool of cord blood units is substantially smaller, the probability of finding a matched cord is low, and for most adult patients unrelated cord blood is used for patients without a HLA-matched sibling or a HLA-matched volunteer donor. At least in part because of the small T-cell dose contained in cord units, the probability of acute and chronic GVHD is less for cords than adult marrows or mobilized blood stem cells, and in general a cord is considered suitable if at least matched for 4/6 HLA-A, -B, and -DRB1 loci. Adopting such criteria, almost all children can find a suitable volunteer donor or cord.8 Adults require a larger total cell dose than children, therefore reducing the available cord blood pool and decrease the probability of finding a suitable unit.8 Recent data demonstrated that allele disparities for HLA-A, -B, -C, and -DRB1 worsen cord blood engraftment and increase transplant-related mortality.9 If more stringent minimal matching criteria are adopted for cord blood, fewer patients will have access to transplantation with this graft source.
Related donors identical for one HLA haplotype (haploidentical or “haplo”)
A haplo donor is available to virtually everyone who has an available first degree relative. A limited degree of donor HLA disparity on the unshared HLA haplotype is tolerated when using conventional post-grafting immune suppression regimens. However, transplants from donors with greater than one HLA-A, -B, -C or -DRB1 locus disparity require modified protocols to overcome the otherwise higher risk of graft failure, GVHD, and death. Effective novel approaches have been developed, making haplos an additional alternative source of hematopoietic cell for patients without a matched donor (see “Post-transplant cyclophosphamide for GVHD prevention”).
A donor for everyone
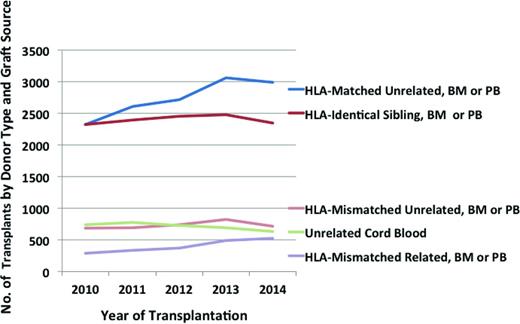
Until now, registry data support that a HLA-matched related or unrelated donor provides superior results compared to HLA-mismatched donors. Preliminary data obtained from the Center for International Blood and Marrow Transplant Research Statistical Center indicate that between 2010 and 2014 the number of US transplants from HLA-matched unrelated donors has exceeded that from HLA-identical siblings, and the number of transplants from HLA-mismatched related donors has increased to approach those from HLA-mismatched unrelated donors and cords (Figure 1). In children, where the required transplant cell dose is lower, unrelated cord blood is used more commonly, followed by HLA-mismatched volunteer donor (marrow is more common than mobilized blood) and similar numbers of haplos. In adults, the use of HLA-mismatched volunteer donors is more common (mobilized blood is more common than marrow), followed by cord blood units and similar number of haplos. The field is gaining greater experience with alternative donor sources, but while awaiting better data, how should we choose between HLA-mismatched related or unrelated donors or cord blood units? In the following paragraphs, I will review the challenges, the state-of-the-art transplant technologies for each of these treatment modalities, pros and cons of each, I will outline planned and ongoing trials directed at advancing the field, and then will point to criteria for selecting the best stem cell source for everyone.
Number of first allogeneic transplants registered with the CIBMTR by US centers. Preliminary data obtained from the Statistical Center of the Center for International Blood and Marrow Transplant Research, not reviewed or approved by the Advisory or Scientific Committees of the CIBMTR. Cases with unknown matching are not presented.
Number of first allogeneic transplants registered with the CIBMTR by US centers. Preliminary data obtained from the Statistical Center of the Center for International Blood and Marrow Transplant Research, not reviewed or approved by the Advisory or Scientific Committees of the CIBMTR. Cases with unknown matching are not presented.
Overcoming the HLA barrier for engraftment: the greatest challenge is for cord blood
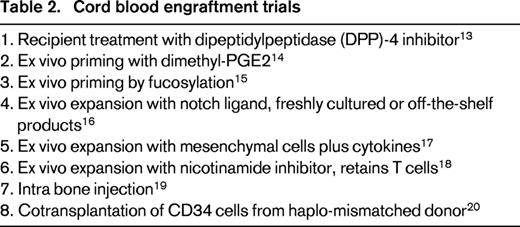
Both HLA-disparity and low cell dose contribute to the increased risk of graft failure after hematopoietic cell transplantation.9 Based on both human and experimental animal model data, graft failure of HLA mismatched transplants is caused by host rejection via innate and adaptive immunity by cells residual after conditioning. Within a few hours after transplantation, murine natural killer cells of the host destroy a fixed fraction of transplanted donor hematopoietic cells lacking host-type MHC class I antigens. Antibodies to both class I and class II MHC mediate early rejection of incompatible stem cell grafts in animal models. Assays to identify pathogenic antibodies have been established in humans.10 T cells recognize both class I and II MHC mismatches and minor histocompatibility antigens presented as peptides by the shared MHC. Overcoming these immune mechanisms would facilitate engraftment of any HLA-disparate hematopoietic cell source in humans. Nucleoside analogues, alkylators, irradiation, and antibodies are used in various combinations with improved efficacy. Nevertheless, cord blood engraftment is delayed and graft failure is more frequent than after adult donor allografts, contributing to increased patient morbidity and mortality. The very low dose of hematopoietic cells, about one-tenth of marrow grafts, represents a unique challenge for engraftment of HLA-disparate cord blood. Transplantation of 2 cord blood units, partially HLA-matched to the host has been used to augment cell dose and facilitate engraftment, but it is ineffective at improving outcomes, if a single cord has an adequate cell dose.11,12 At least 8 distinct approaches are being tested in clinical trials to facilitate engraftment of HLA-disparate cord blood transplantation by silencing the host immune response, ex vivo cord blood priming or expansion, intra-marrow injection or cotransplantation of third-party progenitors (Table 2).13-20
Engraftment of HLA-disparate grafts from adult related or unrelated donors has improved by increasing the dose of donor hematopoietic and immune cells. The content of CD34 cells is on average 3-fold greater and the T-cell content 10-fold greater in granulocyte-colony stimulating factor (G-CSF)-mobilized blood compared with marrow grafts. Therefore, G-CSF-mobilized blood progenitor cells have been added to or have replaced marrow for transplantation of HLA-disparate related grafts that employ CD34-selection protocols or in vivo T-cell depletion, and most unrelated donor grafts, and have decreased the risk of graft failure.21-24 However, the higher T cell content of G-CSF-mobilized grafts has increased the risk of acute and chronic GVHD.24 Marrow alone is still used predominantly with the post-transplant cyclophosphamide protocol.31
GVHD prevention: the greatest challenge is for haplos
Mature donor T cells cause GVHD in immunodeficient conditioned hosts. Attempts at removing T cells from the graft have met with graft failure, relapse of malignancy, and opportunistic infections. Current trials are testing redesigned grafts that contain small numbers of mature T cells sufficient to reconstitute immunity: in some centers, T cells are modified with a suicide gene that can trigger their immediate death in the case of GVHD, in others conventional T cells are supplemented by regulatory T cells that dampen GVHD without interfering with immune reconstitution and graft anti-tumor effects.25,26 In vivo T-cell depletion by antibodies has significantly decreased the risk of acute and chronic GVHD, and when combined with multi-drug post-grafting immune suppression has permitted transplantation from HLA-disparate relatives achieving results similar to HLA-identical sibling transplantation.22 Initial anti-T cell globulin data from Beijing in haplos has later been reproduced in Korea and Italy.27,28
Post-transplant cyclophosphamide for GVHD prevention
Great enthusiasm for haplos has developed in the US and Europe using post-transplant cyclophosphamide to eliminate alloreactive, GVHD-causing T cells, while sparing regulatory T cells.29 Such an approach, spearheaded by Fuchs and Jones at Johns Hopkins, is effective by itself in inducing a stable, GVHD- and immunesuppression-free, tolerant state in approximately one-half of HLA-matched transplant recipients.30 In most trials, post-transplant cyclophosphamide is used with maintenance immune suppression with mycophenolate mofetil for 1 month and tacrolimus or sirolimus for 6-12 months. A phase 3 multicenter trial is testing post-transplant cyclophosphamide followed by a calcineurin-inhibitor plus mycophenolate mofetil versus standard calcineurin-inhibitor plus methotrexate versus ex vivo T-cell depletion in HLA-matched transplants (BMT CTN 1301). Results of this trial will establish whether post-grafting cyclophosphamide should become standard of care for GVHD prevention in HLA-matched transplants. The combined regimen is especially robust across HLA-disparity, it is associated with very low risk of acute and chronic GVHD and 10% transplant-related mortality.31 Immune reconstitution is preserved against cytomegalovirus and Epstein-Barr virus. When used after minimal intensity conditioning regimens, post-transplant cyclophosphamide has been associated with high risk of leukemia relapse because it almost totally eliminates alloreactivity and T-cell–mediated graft-versus-leukemia effects.31 When used after ablative regimens, it is also effective in preventing GVHD, with an apparent lower risk of leukemia relapse.32 The putative risk of cyclophosphamide selecting for clonal hematopoiesis has not been realized within 15 years of human trials, but more data and longer follow-up will quantify such a risk.
Natural killer cell recognition of HLA-disparate hematopoietic cells: anti-leukemia activity without GVHD.
HLA-disparity confers hematopoietic cell grafts additional innate immune effector mechanisms for potent anti-leukemia activity without GVHD.33 Killer Ig-like receptor (KIR) genes on human chromosome 19 codify for inhibitory and activating KIRs that regulate NK-cell activation. KIR2DL1 recognizes HLA-C alleles with lysine in position 80 (ie, HLA-Cw4), whereas KIR2DL2 and KIR2DL3 recognize HLA-C with an asparagine in position 80 (ie, HLA-Cw3). KIR3DL1 recognizes HLA-B alleles sharing isoleucine in position 80 and arginine in position 83, both of which together characterize the common Bw4 specificity. During development, HLA class I molecules select for functional NK-cells with self-tolerant KIRs. After haplo transplantation, alloreactive NK cells that do not express inhibitory KIRs against the recipient HLA class I mismatches kill the recipient hematopoietic cells, including leukemic cells.33 Relapse of acute myeloid leukemia, and in some reports relapse of acute lymphoblastic leukemia, is lower and survival is improved if the donor is HLA class I incompatible, NK cells are alloreactive, and donor T cells are depleted ex vivo or in vivo.33,34 NK cells only lyse hematopoietic cells, and therefore they cannot cause GVHD. NK cell alloreactivity may also occur in HLA class I mismatched transplants from unrelated donors or cord units. However, studies show no advantage in transplantation from KIR ligand-mismatched unrelated donors if bone marrow or G-CSF-mobilized blood is T-cell replete. HLA-A, -B, and -C sequencing predicts for KIR-ligand mismatch with a good approximation, but KIR sequencing is helpful to confirm that the donor has the relevant KIR gene, as not all KIR genes are present in every individual. Some allelic variants in the HLA-Bw4 inhibitory NK receptor gene KIR3DL1 do not allow receptor expression, therefore, KIR gene sequencing may be necessary for optimal donor selection. The receptors for most activating KIRs remain unknown. Donors differ in their content of activating KIR genes: the “A” haplotype is almost devoid of activating KIRs, whereas the “B” haplotype is rich of activating KIRs. Donors with activating KIR2S1, and one or both “B” KIR haplotypes are associated with lower risk of recipient AML relapse.35,36 Clinical trials are ongoing to validate prospective protocols for selection of HLA-matched or mismatched donors based on the content of inhibitory and activating KIRs (NCT01288222 and NCT02450708).
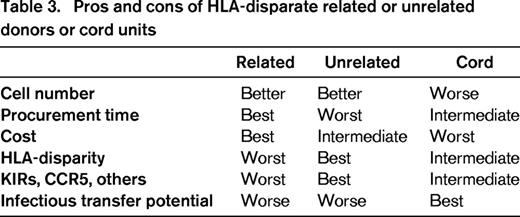
Pros and cons of HLA-disparate related or unrelated donors or cord units
For adults, the dose of progenitor cells confers a benefit to related or unrelated donors compared with cord blood units (Table 3). The search duration and procurement time is the shortest for matched and mismatched related donors, intermediate for cord, and the longest for unrelated donors. The cost is also favorable to related donors, intermediate for unrelated donors and the worst for the cords, especially if two units are needed for transplant. Based on the evidence that HLA matching is beneficial for survival after transplantation, the large pool of unrelated volunteer donors confers an advantage as it allows selecting the closest match, compared to banked cord blood units, whose pool is smaller so that the average match is not as good, and to haplos that are chosen among a few mismatched relatives. The same ranking preference for the donor pool size applies for selection of the graft secondary characteristics, such as CMV serostatus and ABO matching, desirable KIR genotype, or a mutant CCR5 genotype for HIV positive patients. Cords are in general the safest from the infection transfer viewpoint, as the fetus remains unaffected by adult diseases, although cord blood recipients may be more susceptible to viral infections. Overall, unrelated donors offer immunogenetic advantages, related donors offer prompt availability and cost advantages, and both donor types offer cell-dose advantages over cord blood units.
Unrelated mismatched adult grafts versus unrelated mismatched cord grafts: results in adults
Data on all US cord and adult donor marrow and blood transplants are reported to the Stem Cell Transplant Outcome Database and presented on the Health Resources and Services Administration website (http://bloodcell.transplant.hrsa.gov). Retrospective registry and single center analyses have compared outcomes of mismatched unrelated adult marrow or peripheral blood to cord blood grafts in transplantation of adult patients. Most reported data show comparable results between unrelated adult marrow or blood and cord blood sources.37 For example, in acute lymphoblastic leukemia in first or second complete remission, Marks and collaborators found no differences in the 3 year probabilities of survival between recipients of cord blood (44%), matched adult donor (44%), and mismatched adult donor (43%) transplants.38 Cord blood transplants engrafted slower and were associated with less grade 2-4 acute but similar chronic graft-versus-host disease, relapse, and transplant-related mortality. The survival of cord blood graft recipients was similar to that of recipients of matched or mismatched unrelated adult donor grafts and so cord blood should be considered a valid alternative source of stem cells for adults with acute lymphoblastic leukemia in the absence of a matched unrelated adult donor. Although multiple centers have confirmed the Johns Hopkins data demonstrating effective GVHD prevention with high-dose cyclophosphamide after haplo, there is little experience using the same regimen after HLA-mismatched unrelated donor transplantation. A phase 2 trial is being planned by the RCI BMT in HLA-disparate unrelated donor transplantation.
Unrelated mismatched marrow versus unrelated mismatched cord: equipoise in children
Estimates of disease-free survival after transplantation from HLA-mismatched donor marrow or cord blood are similar based on registry data.39 Unrelated donor peripheral blood transplantation is not often considered in children, because there is increased risk of chronic GVHD and the marrow cell dose is usually adequate.24 There is also a paucity of comparative data between cord blood and haplo transplantation in children. Development of approaches to overcome the HLA barrier for engraftment of cord blood transplantation with low cell dose, and for prevention of GVHD after marrow or cord blood transplantation should be high priorities. Viral but not bacterial and fungal infections are more common with cord blood. If GVHD could be prevented and glucocorticoids avoided, one anticipates that the long-term infectious risk would be also decreased.
Unrelated adult donor versus cord: how to choose
For patients ineligible for a clinical trial, 8/8 HLA-matched unrelated cord blood units are rarely found yet preferred based on improved survival over HLA-matched unrelated donor marrow.39 When a single HLA-A, -B, and -DRB1 locus mismatched cord blood unit with an adequate cell dose (>2.5 × 107 per recipient body weight in kg) and a 7/8 or 8/8 HLA-A, -B, -C, and -DRB1 matched unrelated donor marrow are both available to the patient, the choice relies on timely cell source acquisition and secondary characteristics of the grafts. Closer HLA-A, -B, -C, and -DRB1 matching and higher cell dose provide cord blood transplant recipients a significant survival advantage, whereas cotransplantation of 2 cord blood units does not improve outcomes.9,11,12,37,38 For adult unrelated donors, favorable features for survival are younger age, matching for HLA-HLA-A, -B, -C, -DRB1, -DRB3/4/5, -DQB1, permissive DPB1, matching for CMV serotype and ABO, whereas favorable features for mitigating the GVHD risk are male gender and no history of female parity. An algorithm is needed to compute all the significant factors for choosing the most favorable donor.
Related mismatched versus unrelated mismatched cord: equipoise in adults
Two parallel multicenter phase II trials have tested haplos (BMT CTN 0603) and double-cord blood transplantation (BMT CTN 0604) in adults without an HLA match.31 Both trials included minimal intensity conditioning regimens. Post grafting immune suppression was high dose cyclophosphamide followed by tacrolimus/mycophenolate mofetil after haplo, and tacrolimus or cyclosporine plus mycophenolate mofetil after cord. Transplant related mortality was higher with cord and post-transplant relapse of malignancy was higher with haplo. Disease-free survival at 1 and 3 years after transplantation was similar in the 2 groups.40 A phase 3 trial is testing cyclophosphamide plus calcineurin inhibitor/mycophenolate mofetil after haplo versus calcineurin inhibitor/mycophenolate mofetil after cord in patients treated with a reduced intensity protocol (BMT CTN 1101). Results of this trial will establish whether post-grafting cyclophosphamide will make haplos preferable to cord blood transplantation in adults after a reduced intensity conditioning. Candidate patients should be enrolled into this trial, if available to the transplant center; otherwise patients should be enrolled into available trials addressing cord blood engraftment (Table 2), or prevention of GVHD and relapse for haplos. Until BMT CTN 1101 is complete, there is no guidance about the preferred of these 2 stem cell source for patients that have to be treated outside a clinical trial. Such patients should be treated with the approach most familiar to the transplant team.
Correspondence
Claudio Anasetti, Moffitt Cancer Center, 12902 Magnolia Drive, Tampa, FL 33612; Phone: 813-745-2557; Fax: 813-745-8468; e-mail: claudio.anasetti@moffitt.org.
References
Competing Interests
Conflict-of-interest disclosure: The author declares no competing financial interests.
Author notes
Off-label drug use: None disclosed.