Abstract
Because the clinical diagnosis of deep-vein thrombosis and pulmonary embolism is nonspecific, integrated diagnostic approaches for patients with suspected venous thromboembolism have been developed over the years, involving both non-invasive bedside tools (clinical decision rules and D-dimer blood tests) for patients with low pretest probability and diagnostic techniques (compression ultrasound for deep-vein thrombosis and computed tomography pulmonary angiography for pulmonary embolism) for those with a high pretest probability. This combination has led to standardized diagnostic algorithms with proven safety for excluding venous thrombotic disease. At the same time, it has become apparent that, as a result of the natural history of venous thrombosis, there are special patient populations in which the current standard diagnostic algorithms are not sufficient. In this review, we present 3 evidence-based patient cases to underline recent developments in the imaging diagnosis of venous thromboembolism.
Learning Objectives
In a first episode of deep-vein thrombosis, compression ultrasonography is a very accurate imaging method. In recurrent deep-vein thrombosis, this accuracy is less because residual thrombosis is present. A reference compression ultrasonography may help establish an accurate diagnosis. Direct thrombus imaging by magnetic resonance technique, a technique that can actually differentiate between old and new thrombi, is now being validated for this purpose and may become the gold standard.
In elderly patients, the use of an age-adjusted D-dimer cutoff will lead to less need for imaging without jeopardizing safety. For elderly patients with suspected pulmonary embolism and severe renal insufficiency, compression ultrasonography of the legs is a reasonable way to start imaging.
All pregnant patients with clinically suspected pulmonary embolism should undergo a full diagnostic workup; this includes imaging with computed tomography-pulmonary angiography or ventilation–perfusion scan as an alternative.
Venous thromboembolism (VTE), which refers to a diagnosis of deep-vein thrombosis (DVT) and/or pulmonary embolism (PE), is worldwide the third most frequently diagnosed cardiovascular disorder. VTE may be complicated by recurrent thrombosis, anticoagulant associated bleeding, post-thrombotic syndrome, and chronic thromboembolic pulmonary hypertension.1-3 Over the years, the diagnostic approach has evolved from clinical diagnosis to fully standardized diagnostic management algorithms, in which validated pretest clinical decision rules, D-dimer testing, and foremost straightforward diagnostic imaging allow for safe, convenient, and cost-effective management of patients.4 Patients with a low pretest probability and a normal D-dimer can have VTE excluded without the need for imaging, although there is a trend for increased imaging taking place, leading to ever-increasing demands on busy emergency departments.5 Despite all advancements during the past decade, the imaging diagnosis of VTE remains challenging in patients with suspected recurrent VTE disease, pregnant patients, and elderly patients. These 3 distinct clinical presentations that were managed recently at our hospital form the basis for this overview, in which we focus on imaging but consider diagnostic management as a whole.
Case 1
Patient A, a 52-year-old male, contacts his general practitioner because of complaints of progressive pain and swelling of his right leg. The patient has been well until ∼3 days ago, when he noticed pain in his right thigh. Two days later, the pain had worsened and his wife noted that his right leg appeared more reddish than his left leg. The morning of presentation, the leg was clearly swollen and movement was restricted. He reported to be in good health and not to suffer from respiratory symptoms. He had not been immobilized recently, admitted to a hospital, or traveled. He had a history of hypertension and was diagnosed with unprovoked proximal DVT of his right leg 10 years ago, for which he was treated with a vitamin K antagonist for a period of 6 months. Current medications were lisinopril and simvastatin. His general practitioner suspected a recurrent DVT and referred the patient to our emergency ward for additional diagnostic workup. On admission, inspection of his lower extremities revealed painful swelling up to halfway up his right upper leg with 5-cm difference in calf circumference between both legs. In addition, vaguely demarcated erythema was present. Peripheral pulses were normal and the superficial veins were not prominent. The attending physician agrees that recurrent DVT may be an explanation for his symptoms. Would a compression ultrasonography (CUS) of the right leg be the most optimal diagnostic test?
Diagnosis of recurrent DVT
Pitfalls in current diagnostic management of recurrent DVT
The diagnostic management of suspected first DVT is straightforward. A low clinical pretest probability by the Wells rule for DVT in combination with a normal D-dimer blood test result safely rules out a first DVT, whereas inability to completely compress the common femoral and/or popliteal vein by CUS is the criterion for establishing the diagnosis of a first DVT.6 Conversely, diagnostic management of suspected recurrent DVT is challenging, especially when the same leg is affected again. To start with, the use of clinical decision rules in combination with D-dimer assays has not been evaluated in large cohorts for this specific clinical setting.6-8 Furthermore, it has been shown that ultrasound abnormalities persist in ∼80% of patients 3 months and in 50% of patients 1 year after initial DVT diagnosis, despite adequate anticoagulation.9 Because a sonographer cannot determine whether incompressibility of a specific vein segment is caused by a new DVT or residual thrombosis, the usefulness of CUS in a patient with suspected ipsilateral recurrent DVT is questionable.6,10 Ultrasonographers sometimes describe acute clots as having a fresh appearance versus older clots, which are retracted. However, the sensitivity and specificity of this appearance has never been studied systematically. Other imaging modalities, such as conventional venography, computed tomography (CT) or magnetic resonance (MR) venography, and echo Doppler, are subject to the same limitations. Measurement of residual thrombus diameter by CUS has been suggested to increase the accuracy of diagnosing ipsilateral recurrent DVT.11,12 However, in clinical practice, a reference CUS assessed after treatment cessation to evaluate the presence and exact location of residual thrombosis is not routinely performed and not always available if patients present to different hospitals.13 Furthermore, although 2-mm residual vein diameter has been applied as the criterion for confirming a definite recurrence,6 the absolute mean difference in the measurement between observers is also 2 mm and interobserver agreement of the comparison of 2 CUS examinations is average at best.14 A recent report of clinical practice patterns suggested that recurrent ipsilateral DVT could neither be ruled out nor be confirmed using CUS in 32% [95% confidence interval (CI), 23%-43%] of patients.13
Direct thrombus imaging by magnetic resonance technique as a promising alternative
Direct thrombus imaging by magnetic resonance technique (MRDTI), a technique that is based on measurement of a shortening T1 signal as a result of the formation of methemoglobin in a fresh thrombus and does not require injection of contrast material such as gadolinium, has been shown to be a promising alternative for current diagnostic modalities for suspected ipsilateral recurrent DVT.15-18 The diagnostic accuracy of MRDTI for a first DVT is high (sensitivity, 97%-100%; specificity, 100%), the interobserver agreement between the 2 readers is excellent (κ = 0.98), and the signal is present within hours of thrombus formation and extinguishes completely after 6 months.15-17 The results from the RETURN study, in which MRDTI examination of 39 patients with confirmed recurrent DVT and 42 patients with residual thrombosis were compared with outcomes with compression ultrasound, clearly pointed out that MRDTI differentiates the 2 patient groups with great accuracy and reproducibility (sensitivity, 95%; specificity, 100%), with a negative predictive value of 95% (95% CI, 85-99) in this highly selected cohort with a recurrent DVT incidence of 48% (Figure 1).18 Based on this observation, it was hypothesized that MRDTI has equally high sensitivity but superior specificity than currently available ultrasonography techniques. To confirm this hypothesis, a prospective, multicenter management study, the Theia study, has started recently that will manage 305 patients with suspected ipsilateral recurrent DVT based on the result of MRDTI only, performed within 24 hours of clinical presentation (ClinicalTrials.gov identifier NCT02262052).19 All patients will be followed for the occurrence of symptomatic venous thrombosis during 3-month follow-up. In addition, cost-effectiveness and feasibility of MRDTI in clinical practice will be addressed.
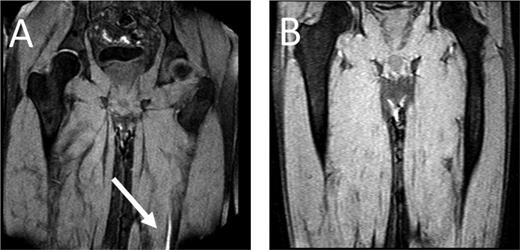
MRDTI of patient from case 1 (A): the white arrow indicates the fresh thrombus in the popliteal vein of the right leg. For contrast, B depicts the MRDTI of a patient with residual thrombosis in the left popliteal and femoral vein: no DTI signal is present.
MRDTI of patient from case 1 (A): the white arrow indicates the fresh thrombus in the popliteal vein of the right leg. For contrast, B depicts the MRDTI of a patient with residual thrombosis in the left popliteal and femoral vein: no DTI signal is present.
As for our patient, his Wells score for DVT indicated “likely probability” (total of 2 points, 1 point for “calf enlargement >3 cm compared with the other site” and 1 point for “pitting edema”), indicating that a D-dimer test could definitely not be used for ruling out DVT.6,20 Because the patient fulfilled all inclusion and none of the exclusion criteria for the Theia study, he was referred for MRDTI, which showed a clear signal in the right popliteal vein (Figure 1), confirming the diagnosis of recurrent ipsilateral DVT. After 6 months of treatment, a reference CUS was performed and showed persistent incompressibility of the right popliteal vein.
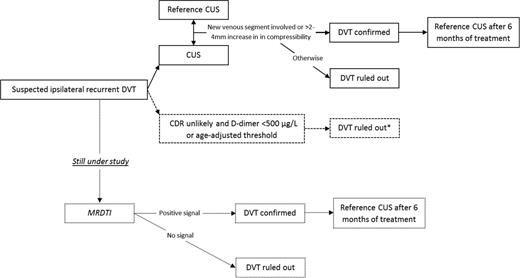
If the patient could not have been included in the study, a CUS would have been made and, if possible, compared with a previously performed reference CUS or the CUS that confirmed the previous DVT to compare current findings with the location and extent of the residual thrombosis or the first DVT. If there are no reference tests (or reports) to compare with and the CUS is abnormal, there is no choice but to assume recurrent DVT and start anticoagulant treatment. The preferred diagnostic algorithm for suspected recurrent ipsilateral DVT is shown in Figure 2.
Preferred diagnostic algorithm for clinically suspected recurrent ipsilateral DVT. Dotted lines and boxes indicate lack of evidence for strong recommendations and/or running studies. CDR indicates clinical decision rule. *Not validated in a large outcome study.
Preferred diagnostic algorithm for clinically suspected recurrent ipsilateral DVT. Dotted lines and boxes indicate lack of evidence for strong recommendations and/or running studies. CDR indicates clinical decision rule. *Not validated in a large outcome study.
Diagnosis of recurrent PE
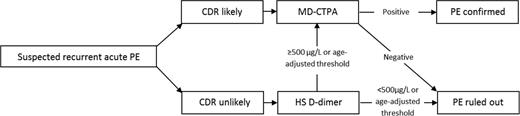
For patients with a suspected recurrent PE, interpretation of the imaging tests of choice, i.e. computed tomography–pulmonary angiography (CTPA), is not as complicated as in suspected recurrent DVT. It has been shown that ruling out recurrent PE based on CTPA is safe, although patients with a normal CTPA had somewhat higher recurrence rates on follow-up than patients with a suspected first PE.21,22 Although in large management studies for suspected first PE episodes the 3-month VTE failure rate is estimated to be ∼1.2%,23 this was 2.8% (95% CI, 1.2%-5.5%) in the REPEAD study by Mos et al21 that focused on suspected recurrent PE. The common belief was that persistent thrombi were prevalent in PE, as is the case after DVT of the leg. Indeed, according to a review in 2006 that summarized the results of the then published mostly small studies of average quality, up to 50% of patients had old pulmonary artery thrombi after 6 months of anticoagulant treatment.24 However, a very recent careful follow-up study in which all patients had repeated CTPA after 6 months of anticoagulant therapy showed that complete PE resolution had occurred in 85%, compared with 71% in the 14 patients with a history of recurrent PE (p = 0.18).25 This result of this study challenges the need for a baseline repeated CTPA imaging after 6 months. The preferred diagnostic algorithm for suspected recurrent acute PE is shown in Figure 3.
Preferred diagnostic algorithm for clinically suspected recurrent acute PE. CDR indicates clinical decision rule; HS, highly sensitive; MD-CTPA, multirow detector CTPA.
Preferred diagnostic algorithm for clinically suspected recurrent acute PE. CDR indicates clinical decision rule; HS, highly sensitive; MD-CTPA, multirow detector CTPA.
Case 2
An 82-year-old female, with a history of mild chronic obstructive pulmonary disease, is visited by her general practitioner because she complained of acute onset of left-sided thoracic pain and dyspnea. She has been fully ambulant in the previous months but has been in bed for the past week because of what seems to have been an intercurrent viral pneumonia with fever up to 38.5°C. She did not complain of pain in the calf or arrhythmia. On physical examination, her blood pressure is 135/90 mmHg with a regular pulse of 76/minute. Some pleural friction at the left side is heard but no cardiac bruits. The pocket electrocardiogram reveals a sinus rhythm of 76 beats/min. The point-of-care D-dimer test is 890 ng/mL (cutoff level of 500 ng/mL; STA-LIA, Stago, France). A recent laboratory examination had shown her estimated creatinine clearance to be 26 mL/min. What is the next diagnostic step: should the attending physician consider acute PE as a possible explanation for her symptoms and refer her for imaging tests, or is this diagnosis safely excluded by the D-dimer test?
Diagnosing acute PE in elderly patients
Clinical decision rule and D-dimer testing
The clinical presentation of acute PE varies widely among patients. Elderly patients have a high prevalence of acute PE, being ∼1% per year.26 Although younger patients, generally without cardiopulmonary comorbidity, often present with typical complaints, including rapid-onset dyspnea or pleuritic chest pain, wheezing, or a nonproductive cough, symptoms in elderly patients are more nonspecific. In retrospective case series, syncope was reported to be present more often, whereas pleuritic chest pain was less frequent.27-29 Proven algorithms such as those used in the Christopher Study consist of a validated clinical decision rule, a D-dimer blood test, and imaging by either CTPA or ventilation–perfusion (V–Q) lung scan.30-33 As in younger patients, the diagnosis should always start with assessing clinical probability by one of the validated decision rules. Whether these decision rules perform differently in elderly patients has not been studied systematically. A challenging difficulty is the physiologic increase of D-dimer with age. As a result, in patients older than 80 years, normal D-dimer rules out the diagnosis of PE in only 5% compared with >50% in patients younger than 50 years.34
Attempts have been made to increase the number of patients in whom PE can be ruled out without imaging tests. This is especially appealing to elderly patients in whom transfer to an imaging facility can be spared. Thus, an age-dependent D-dimer cutoff level was proposed: age × 10 μg/L in patients >50 years of age.35,36 A recent large, prospective management outcome trial demonstrated the safety of applying this age-dependent D-dimer cutoff.37 The 3-month VTE incidence in patients with “PE unlikely” probability and a D-dimer level ≥500 μg/L but below the age-adjusted threshold was only 0.3% (95% CI, 0.1-1.7). The absolute increase of the patients >50 years old that could be managed without CTPA was 11.6%. Particularly patients of ≥75 years were shown to be more frequently managed without CTPA in a safe manner.
Imaging
For both younger and elderly patients, multirow detector CTPA has very high sensitivity and specificity for diagnosing PE. Several outcome studies have demonstrated the safety of withholding anticoagulant therapy in patients with a negative CTPA alone. In a meta-analysis, the pooled 3-month VTE incidence after a normal CTPA alone in patients in whom CTPA was indicated based on a validated clinical decision rule and/or D-dimer test was 1.2% (95% CI, 0.8-1.8) and 1.1% (95% CI, 0.6-2.0) after normal CTPA in combination with normal lower bilateral limb CUS, indicating that the safety of a normal CTPA could not be improved further by excluding asymptomatic DVT after a normal CTPA.23 Although an important drawback of CTPA is the risk of contrast-induced nephropathy,38 a recent study randomly allocated patients to short prehydration with sodium bicarbonate or placebo.39 The mean creatinine clearance was ∼50 mL/min, and the study included patients with clearances as low as 15 mL/min. No significant difference in the rate of worsening of creatinine clearance or contrast-induced acute kidney injury was seen, suggesting that preventive hydration could be withheld safely in patients with chronic renal insufficiency, who are referred for CTPA to rule out acute PE. This will facilitate management of these patients and prevents delay in diagnosis, as well as unnecessary start of anticoagulant treatment while receiving volume expansion, which in itself may lead to acute heart failure.
In elderly patients who often have renal insufficiency and are thus prone to develop contrast nephropathy, performing CUS of the legs to screen for symptomatic DVT as an alternative to CTPA is attractive, because it does not involve radiation and injecting contrast.40,41 In a patient with suspected PE, the presence of a DVT is highly predictive of PE (positive likelihood of 42), thus obviating the need for CTPA imaging in a hospital-based setting.42 Although the yield for CUS is low in younger patients with suspected PE, only 7% of patients younger than 40 years had a proximal DVT as shown by CUS, proximal DVT was found in 25% of patients with suspected PE older than 80 years.34 This means that the number to test with CUS is only 4 to rule out one PE without additional imaging. Importantly, after normal CUS, CTPA is still necessary to rule out PE. Thus, CUS as the initial imaging test will obviate the need for CTPA only in a limited number of patients.
Applying the adjust PE rule in our patient indicated a D-dimer cutoff level of 820 ng/mL. The general physician concluded that she had a high probability of PE. Because this patient had severe renal insufficiency, he first ordered a CUS, which was normal in both legs. CTPA imaging was performed without prehydration; it showed multiple segmental PE in the left lung. She was started on low–molecular–weight heparin, adjusted for her creatinine clearance, followed by vitamin K antagonists, and she had an uneventful follow-up.
Case 3
A 26-year-old, 31-week pregnant female patient is evaluated at the emergency unit because of suspected PE. She woke up at night 4 hours earlier because of sudden shortness of breath, which has since then worsened. Two years ago, she has had an idiopathic PE for which she had taken vitamin K antagonists for a period of 6 months. She had been treated with 2850 IU of prophylactic nadroparin subcutaneously once daily since she became pregnant and has been very compliant with the injections. She does not complain of dizziness, arrhythmia, or pain in the calf. At the physical examination, the patient is very anxious and alarmed. She has a blood pressure of 120/75 mmHg with a pulse rate of 96/minute, her central venous pressure is not increased, and no abnormal lung or heart sounds are heard. The legs do not display signs of DVT. What is the next step in the diagnostic process?
VTE diagnosis in the pregnant patient
The risk of VTE is increased during pregnancy, and VTE remains one of the main causes of maternal mortality in developed countries.43 Although the absolute risk of VTE in pregnancy is modest, there is generally a high concern among physicians and the patient on presentation with symptoms of VTE. Leg or thoracic symptoms are often experienced in pregnancy and are sometimes clinically indistinguishable from those found in patients with DVT and PE. Hence, the clinical diagnosis is inaccurate, and accurate diagnostic testing is essential to exclude or diagnose VTE. As a result, many physicians use a low imaging test threshold. A recent meta-analysis demonstrated that this led to a low 4.1% prevalence of VTE among suspected pregnant patients compared with 12.4% in nonpregnant patients.44 Moreover, many of the common diagnostic tests, including CUS, V–Q lung scan, and CTPA, that have been investigated extensively in nonpregnant patients have not been validated appropriately in pregnancy. Extrapolating results of diagnostic studies of DVT and PE in nonpregnant patients to those who are pregnant may not be correct because of physiologic changes during pregnancy and the possibility of differences in pathophysiology and presentation of VTE in pregnancy. In addition, imaging is complicated by a concern for reduced accuracy of CUS, whereas both CTPA and V–Q lung scanning involve radiation with associated concern for unwanted side effects to the mother and/or child.4
Workup of suspected DVT in pregnancy
In DVT diagnosis during pregnancy, the performance of CUS is challenged by a higher proportion of isolated iliac DVT. In a recent review of the literature, it was found that 62% of all DVTs in symptomatic pregnant women were in the iliofemoral veins, 17% were in the iliac vein alone, and 6% were in the calf veins.45 In contrast, in the general population, >80% of DVTs involved calf veins, and iliofemoral DVTs or isolated iliac veins are uncommon (<5%).46 Three management studies do support the use of either serial proximal ultrasound or one whole-leg ultrasound, both combined with a Doppler examination of the iliac veins, to rule out DVT in pregnant women.47-49 In the largest study with a 7.7% prevalence of DVT (17/221 patients), 65% (11/17) of patients had isolated iliofemoral DVT, and 12% (2/17) had isolated iliac DVT.49 If no Doppler examination is available, shielded CT venography or MR venography are alternative imaging methods. Finally, the role of pretest probability assessment and D-dimer testing in the diagnosis of suspected DVT in pregnancy has yet to be defined. A prospective management study in which patients' management is based on the results of the so-called “LEFt” clinical decision rule in combination with D-dimer and CUS is currently under way and recruiting patients (ClinicalTrials.gov identifier NCT01708239). LEFt is the acronym for symptoms in the left leg (L), calf circumference difference ≥2 cm between asymptomatic and symptomatic legs (E), and first trimester presentation (Ft). All 3 variables were shown to be highly predictive of DVT in pregnant patients.50
Workup of suspected PE in pregnancy
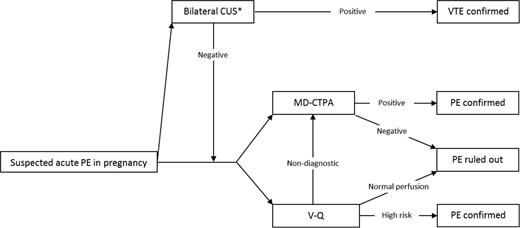
As for suspected DVT in pregnancy, no established clinical decision rule exists for PE, leaving imaging as the logical first choice. Three options for imaging exist: (1) to start with bilateral CUS; (2) to start with V–Q lung scanning; or (3) to start with CTPA. This first approach has the advantage that additional imaging of the chest may be omitted: an abnormal CUS is sufficient to start anticoagulant treatment, whereas a normal bilateral CUS should be followed by additional imaging. Of note, although never studied specifically, the yield of CUS in patients presenting with PE symptoms during pregnancy without signs of DVT is likely low (Figure 4).51
Diagnostic algorithm for clinically suspected acute PE in pregnant patients. MD-CTPA indicates multirow detector CTPA. *Strategy with very low yield, unless symptomatic DVT.
Diagnostic algorithm for clinically suspected acute PE in pregnant patients. MD-CTPA indicates multirow detector CTPA. *Strategy with very low yield, unless symptomatic DVT.
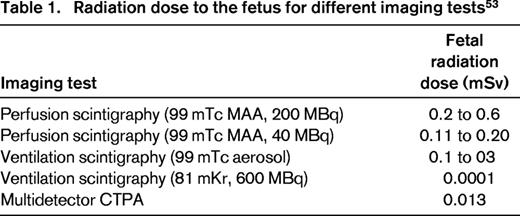
According to North American guidelines on diagnosis of PE in pregnancy, V–Q lung scintigraphy is to be considered as the first-line diagnostic test, especially in the presence of a normal chest x-ray, and rules out the diagnosis of PE when the result of the perfusion scan is normal.52 However, some argue that, because fetal radiation is higher with V–Q lung scan than with CTPA, the latter method is to be preferred (Table 1).53 When a high-probability V–Q lung scan is obtained, the diagnosis of PE can be considered confirmed. All other abnormal V–Q scan results are nondiagnostic and the diagnosis of PE needs to be confirmed or excluded by additional testing with a CTPA.
CTPA has the advantage over a V–Q of low fetal radiation exposure, although some studies warned about the increased risk of breast cancer in pregnant women exposed to CTPA.51,54 CTPA is associated with a low number of nondiagnostic results, although it has been claimed to involve a higher inadequacy rate during pregnancy because of the underlying physiologic changes in pregnancy, such as alterations in cardiac output and changes in plasma volume.55 The ability to make an alternative diagnosis that can explain the patient's complaints by imaging other chest structures (lung parenchyma, mediastinum, etc.) is an advantage of the CT, although its significance for patients' management has been debated recently.56 The safety of using helical CT as a sole imaging method to exclude PE during pregnancy has been investigated in a recent cohort follow-up study in 134 patients. None of the patients with normal CTPA returned with recurrent VTE (upper 95% CI, 2.5%), whereas there was a low 6% inadequacy rate of CTPA imaging.57 It should be noted that the prevalence of PE in this study was only 3.7%, stressing the need for studies in which better triaging of these patients by means of clinical decision rules and D-dimer testing is evaluated. In general, the radiation exposure risks are lower than the consequences of a misdiagnosis in PE [risk of (fatal) untreated PE and risk of (fatal) bleeding in case of unnecessary anticoagulant therapy], and it is stressed that all pregnant women with suspected PE should undergo a complete workup if relevant, including a CTPA. Of note, pregnancy was one of the most common reasons for not following diagnostic algorithms, which has been shown to be associated with less favorable outcome. Specifically, patients in whom diagnostic algorithms were not strictly followed had higher rates of a symptomatic VTE diagnosis during 3-month follow-up than patients who were managed according to the validated diagnostic algorithms.58
In our patient, a bilateral CUS did not show any incompressible deep venous segments. The subsequently performed CTPA showed no new pulmonary emboli, so the diagnosis of acute PE could be excluded, and she remained on prophylactic dose nadroparin and was not treated with therapeutic intensity anticoagulation. Eight weeks later, she gave birth to a healthy boy. The postpartum period was without complications.
Emerging imaging tests for diagnosing VTE
With concerns for contrast nephropathy and radiation exposure associated with CTPA, contrast-enhanced or not-enhanced magnetic resonance imaging (MRI) may be an alternative imaging method.59-62 Initial studies reported sensitivity of 77% and a specificity of 96% of magnetic resonance pulmonary angiography (MR-PA) for acute PE.59 However, a recent, carefully performed study confirmed the average sensitivity (70%) and good specificity (100%) but showed a high inter-individual variability, with κ = 0.59.60 An additional and important practical drawback of MR-PA was a 30% proportion of technically inadequate tests. Given the current techniques and evidence from clinical studies and despite the attractive potential of MR-PA, it is not yet a suitable alternative for CTPA in the daily management of patients presenting with clinically suspected PE.62 However, this modality could still be useful in patients with contraindications to CTPA or in pregnant women. An ongoing prospective management study is evaluating the diagnostic performance of a combination of contrast-enhanced MRI and bilateral CUS of the leg veins (ClinicalTrials.gov identifier NCT02059551).
Normal V–Q lung scanning has the drawback of many indeterminate results. V–Q single photon emission tomography (SPECT) scanning is a new technique, very similar to conventional planar V–Q, but it allows for tomographic imaging, which may lead to better contrast resolution. Initial results show a reasonable accuracy for PE diagnosis when compared with CTPA imaging, but validation of predefined interpretation criteria is required, as well as management outcome studies, in which a normal V–Q SPECT scan as the sole test is used to rule out PE.63
Because both venous and arterial thrombosis and inflammation are intimately linked, the inflammatory component of VTE may allow the use of fluorodeoxyglucose positron emission tomography/CT in the detection of thrombotic process. A pilot diagnostic study in 100 consecutive patients showed disappointing accuracy (sensitivity, 3%; specificity, 99%), and this method proved not yet suitable for clinical practice.64
Conclusions
After decades of dedicated research, diagnostic management of acute VTE has become standardized but remains challenging for special patient groups. Much benefit can be expected if adherence to validated algorithms in day-to-day clinical practice can be improved. Strictly adhering to these strategies will limit the number of necessary imaging tests and are associated with lower recurrence rates and a reduction in healthcare costs, as well as complications. Furthermore, enhanced imaging methods are being developed and validated for specific circumstances, including recurrent VTE.
Correspondence
Dr. M. V. Huisman, Leiden University Medical Center (C-7-Q), Albinusdreef 2, 2300 RC, Leiden, The Netherlands; Phone: + 31 71 5263761; e-mail: m.v.huisman@lumc.nl.
References
Author notes
This article was selected by the Blood and Hematology 2015 American Society of Hematology Education Program editors for concurrent submission to Blood and Hematology 2015. It is reprinted with permission from Blood 2015, Volume 126.
Competing Interests
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Off-label drug use: None disclosed.