Abstract
HIV infection is associated with an increased risk of malignancy, especially B-cell lymphoid malignancies. Many of these lymphomas are further driven by concomitant infection with viruses such as Epstein-Barr virus or Human Herpesvirus 8, the latter being implicated in uncommon types of lymphomas seen in the setting of HIV-1 infection. Treatment outcomes have improved due to infusional chemotherapy, high-dose chemotherapy, and effective antiretroviral therapy. Successful functional cure of HIV-1 infection has been demonstrated with the use of allogeneic hematopoietic stem cell transplantation. This result spurred a change in the field of HIV-1 management so that, ultimately, the goals of therapy would shift from not only curing the underlying lymphoma, but also curing the HIV-1 infection. Treatment options will be discussed with an emphasis on hematopoietic cell-based therapy for the underlying HIV infection.
Learning Objective
To become familiar with the role of Human Herpes Virus-8 and EBV in HIV-associated non-Hodgkin lymphoma
Introduction
It has long been recognized that lymphomas, especially B-cell lymphomas, are more common in immunocompromised individuals, whether from immunosuppression after organ transplantation or from underlying immunodeficiency, either inborn or acquired, such as with HIV-1 infection.1 Since the widespread use of antiretroviral therapy (ART), the incidence of HIV-associated B-cell lymphoma has decreased and the outcome of those affected has substantially improved.
This review focuses primarily on non-Hodgkin lymphoma (NHL), with the exception of primary CNS lymphoma in the HIV-1 setting, which has become relatively rare in the ART era.1 Many paradigm shifts have occurred in terms of treatment and prognosis. We also have more understanding of the biology of these lymphomas and this review discusses some of the roles of other viruses in their pathogenesis. A particular focus will be on outlining the role of hematopoietic stem and progenitor cell transplantation (HCT). This is in part due to the search for future treatments that target both the HIV-1 and the lymphoma, which may allow us to attain the ultimate goal, cure of the lymphoma while also providing at least a “functional cure” of the underlying HIV-1 infection.
Primary effusion lymphoma
Epidemiology
Primary effusion lymphoma (PEL) is a rare subtype, comprising only 3% of HIV-associated lymphomas, and tends to be seen with more advanced underlying HIV-1 disease in great part due to its association with concomitant viral infections such as Human Herpes Virus-8 (HHV-8) and EBV.2 PEL occurs predominantly in the pleural, pericardial, and peritoneal cavities and presents as neoplastic effusions. It should be suspected in patients with HIV-1 infection who present with neoplastic effusions and an absence of solid tumor masses on radiologic imaging.
Pathogenesis
PEL is almost universally linked to prior HHV-8, also known as Kaposi's sarcoma herpes virus (KSHV), and often has evidence of EBV as well.2 Unlike EBV, KSHV does not transform B cells in vitro, so although the virus is essential to the development of PEL, it is probably not sufficient by itself.3 KSHV contributes to the pathogenesis of PEL by virus-encoded gene sequences that mimic several host genes linked to and useful to overriding control of cell proliferation and apoptosis.4
The high rate of concomitant EBV infection suggests that EBV is the important cofactor in PEL development.5 PEL subtypes lack surface expression of B-cell-associated genes, but are derived from the B-cell lineage, as confirmed by the presence of Ig rearrangements. Gene expression profiling confirms the role of KSHV in pathogenesis, but also demonstrates distinct cellular gene expression for KSHV-positive EBV-positive versus EBV-negative PEL.5 In the case of EBV-negative PEL, genes involved in the MAPK pathway are more highly expressed, suggesting that alterations in this pathway may be the drivers of lymphomagenesis rather than EBV.
Therapy
The literature is sparse, with no large trials available. A retrospective study of 28 patients diagnosed between 1993 and 2003 revealed a median overall survival (OS) of only 6.2 months after treatment with a variety of regimens including CHOP (cyclophosphamide + doxorubicin + vincristine + prednisone/prednisolone), high-dose methotrexate, and infusional CDE (cyclophosphamide + doxorubicin + etoposide). Poor prognostic factors include performance status and absence of ART before PEL diagnosis.6 The role of high-dose therapy is undefined; a case report of one of the first patients treated with high-dose therapy and autologous HCT demonstrated that it was well tolerated, but the patient died of recurrent disease soon thereafter.7 Another case report describes the use of a reduced intensity conditioning with melphalan and fludarabine, followed by allogeneic sibling transplantation, with sustained remission at 31 months.8 Preclinical studies have suggested a potential role for brentuximab vedotin, the anti-CD30 monoclonal antibody.9 The presence of EBV-induced cellular proliferation through the NF-κB pathway also suggests the potential for treatment with agents that down-regulate this pathway, such as bortezomib.10 Lenalidomide, an immunomodulatory agent, has also been used successfully.11
Plasmablastic lymphoma
Epidemiology
Plasmablastic lymphoma (PBL) is a distinct variant of diffuse large B-cell lymphoma (DLBCL) initially described in 1997 in HIV-1-infected individuals presenting with tumors in the oropharyngeal area.12 It is a rare subtype of HIV-1-related DLBCL, representing <10% of cases. Since then, plasmablastic NHL has been recognized in both HIV-1-positive and HIV-1-negative individuals.
Pathogenesis
HIV-1-positive PBL patients tend to have a higher incidence of EBV coexpression and higher CD20 and CD56 expression than the HIV-uninfected subgroup.13 Morphologically, cells have features of DLBCL, but usually lack CD20 and CD45 expression. However, HIV-1-positive PBL tends to be more commonly CD20-positive than the HIV-1-negative cases, and this has led to the theory that the HIV-1-positive PBL arises from a B cell at earlier stages of plasmacytic differentiation.
Therapy
Because PBL is a rare subtype, there are no large clinical trials to guide therapy. A review from Memorial Sloan-Kettering Cancer Center of 12 patients (6 HIV-1-positive) in the ART era demonstrated a more heterogeneous clinical presentation than originally described.14 Four of the 12 had bone metastasis and most had advanced stage and high International Prognostic Index disease. Chemotherapy included either CHOP, hyper-CVAD (etoposide + Adriamycin + vincristine + cyclophosphamide + prednisone + cytarabine + methotrexate), or CODOX/M-IVAC (cyclophosphamide + adriamycin + vincristine + methotrexate + ifosfamide + etoposide + cytarabine). The median OS was not reached at time of reporting, with a median follow-up of 15 months, but 7 patients were in remission. Novel agents such as bortezomib have been used in combination with traditional antilymphoma agents and, given the constitutive activation of the NF-κB pathway in non-germinal-center (non-GC) B-cell-derived DLBCL, some degree of response might be expected. A case report of a patient who relapsed after high-dose chemotherapy and autologous HCT describes treatment with bortezomib plus gemcitabine, oxaliplatin, and intrathecal cytarabine, which induced a brief response.15 High-dose chemotherapy and autologous HCT have been used in patients with PBL, but given the rarity of the disease, definitive comments about the role of HCT are not possible. In our initial series of 20 AIDS patients treated with autologous HCT for lymphoma at City of Hope, 3 had relapsed PBL.16 All remain in remission after autologous HCT, with the longest follow-up being 6 years.
DLBCL
Epidemiology
The most common subtype of HIV-1-related lymphomas is DLBCL. In fact, these lymphomas were so common in the pre-ART era that they were considered AIDS-defining illnesses. Since the introduction of ART in the mid-1990s, there has been an overall 50% decrease in the incidence of AIDS-related lymphomas, although the risk still remains far above the general population. The standardized incidence ratio for any lymphoma in the ART era is >70.17 These lymphomas tend to be aggressive in clinical presentation, with advanced-stage disease and extranodal sites of disease being common.
Pathogenesis
We now recognize distinct profiles of DLBCL based on markers of B-cell differentiation in the HIV-1-negative setting. Furthermore, these subtypes carry very different prognoses in HIV-1-negative patients and, although this is likely true for HIV-1-positive patients as well, this has not been as well studied. GC subtypes of NHL express markers associated with GC differentiation, such as CD10 and BCL6. In contrast, the activated B-cell (ABC) subtype of DLBCL has a high BCL2 expression and expression of MUM1. This subtype is linked to overexpression of the NF-κB pathway due to abnormal regulation of upstream proteins. The different subtypes may be linked to the degree of immunodeficiency, with the GC subtype seen in patients with preserved CD4 counts and the ABC subtype generally seen in those with CD4 counts <100/μL. Myc gene overexpression can be seen in ∼20% of HIV-1-associated DLBCL.
Therapy
Prior studies questioned the usefulness of rituximab in HIV-1 DLBCL due to the higher rate of infectious deaths seen when the AIDS Malignancy Consortium randomized a phase 3 study to CHOP ± rituximab.18 Ultimately, rituximab became standard of care. A meta-analysis of NHL treatment in 1456 patients by Barta and colleagues concluded that rituximab is associated with a higher complete remission rate and improved progression-free survival (PFS).19 In addition, infusional EPOCH (etoposide + prednisone + vincristine + cyclophosphamide + doxorubicin) was associated with better OS in the patients with DLBCL.
Further therapeutic refinements are based on the paradigm of identifying patients who can be spared extended chemotherapy and thus maximally preserve immune response while maintaining disease response. Short-course REPOCH (rituximab + EPOCH) uses a positron emission tomography-directed treatment strategy for reducing the number of chemotherapy cycles such that patients who become positron emission tomography negative after 2 cycles are limited to a total of 3 cycles versus the conventional 6 cycles. This approach has shown a 5-year OS of 68% in DLBCL.20 Future areas of study include incorporating the Bruton's tyrosine kinase inhibitors, which have shown activity in non HIV ABC subtypes of DLBCL.
Burkitt lymphoma
Epidemiology
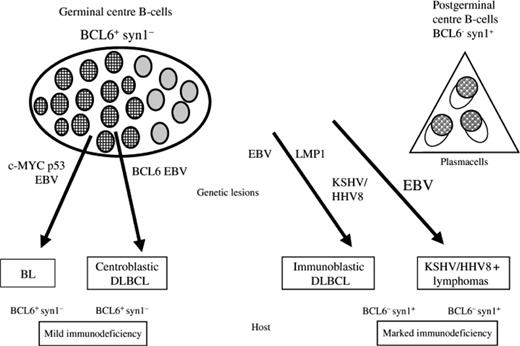
Burkitt lymphoma (BL) or BL-like lymphomas represent between 25% and 40% of HIV-1-associated lymphomas. They tend to develop earlier in the course of HIV-1 infection in patients with higher CD4 counts. Figure 1 summarizes the various subtypes and cell of origin and other associated viruses.21 As with DLBCL, these lymphomas tend to be advanced stage and present with an aggressive clinical course.
Model for the histogenesis of AIDS-related NHL and its linking with the molecular pathways. syn-1, CD138/syndecan-1. Reproduced with permission from Carbone and Gloghini.21
Model for the histogenesis of AIDS-related NHL and its linking with the molecular pathways. syn-1, CD138/syndecan-1. Reproduced with permission from Carbone and Gloghini.21
Pathogenesis
EBV-encoded RNA can be detected in 30% of BL cases, in 50%–70% of BL with plasmacytoid differentiation, and in 30%–50% of BL-like lymphomas. However, in contrast to EBV-encoded RNA-positive DLBCL and PEL, the viral oncogenes latent membrane protein 1 (LMP-1) and EBNA-2 are not expressed.22 This may suggest that EBV does not play the same role in oncogenesis in this subtype of lymphoma and that the oncogenic properties of the HIV-1 virus may predominate. Abnormal overexpression of wild-type RBL2 (Rb2/p130), a tumor suppressor gene, is seen in HIV-1-related BL. Studies have suggested that the function of Rb2/p130 can be negated by interaction with the Tat protein of HIV-1. This may be a potential pathway for the role of HIV-1 proteins acting in synergy with myc activation to fuel the development of lymphoma.
Therapy
BL remains a challenge in both the HIV-1-negative and HIV-1-positive patient, with treatment remaining controversial. Concern exists about the use of the dose-intense regimens of CODOX or hyper-CEVAD that are used in the HIV-1-negative setting. The AIDS Malignancy Consortium did a phase 2 trial of modified dose-intensive R-CODOX-M/IVAC in HIV-1 BL, reported in abstract form only, and showed that 14% of patients were taken off study due to adverse events even though there was no treatment-related mortality. The efficacy of this complex, intensive regimen remains unproven.23 R-EPOCH has also been used in this patient population either in the traditional 6 cycles or the short-course paradigm. The National Cancer Institute series of 29 patients with BL treated with R-EPOCH included 10 who were HIV-1-positive and the compete response rate and OS rate were an impressive 100% at 57 months of follow-up.24
High-dose therapy and HCT
The advent of ART made this approach feasible in HIV-1-related lymphoma as well. Before ART, the need for alternate approaches was paramount because the median survival for relapsed HIV-1-related NHL with standard chemotherapy salvage was <1 year.25
The City of Hope experience specifically excluded chemotherapy refractory patients, similar to the institution practice in HIV-1-negative patients.16 The initial series of 20 patients with either HL or NHL were required to have HIV-1 viral loads <10 000 gc/mL by RT-PCR on ART. The majority of patients received high-dose chemotherapy-based conditioning of CBV (cyclophosphamide + carmustine + etoposide). Only 9 of the 20 were able to continue ART; the others had either nausea or mucositis that prevented good tolerance of oral medication. At the time of initial reporting, the PFS was 85% and OS 81%. The cohort has expanded to 28 patients and, with a median of 41 months follow-up, the PFS remains high at 78%. In addition, transplantation-related mortality was low and no patient succumbed to opportunistic infection. Current practice is to adjust ART before conditioning to minimize antiretroviral and chemotherapy drug interactions and to maximize efforts to continue antiretrovirals through the period of the transplantation. The approach to the use of antiviral agents is shown in Table 1.
Approach to the use of antivirals*

NNRTI indicates Nonnucleoside reverse transcriptase inhibitor; and PI, protease inhibitor.
Antiretrovirals are to be administered only when it is anticipated that they can be taken consistently.
A recent abstract describes a multiple myeloma patient who was an elite controller, defined as an individual with no detectable virus in the blood without ART; before autologous HCT and with high-dose melphalan therapy, there was viral rebound at day 6 with a peak of 28 000 c/mL and a return to <50 c/mL by day 41 corresponding to subsequent engraftment of peripheral blood lymphocytes. Regain of viral control was associated with a potent CD8 response.26 This experience underscores the essential T-cell immunity of the elite controller and suggests the need to continue ART throughout the transplantation period to control virus for those without such immunity.
The Italian Cooperative Group on AIDS and Tumors (GICAT) reported long-term results on 50 patients with HIV-1 and relapsed/refractory lymphoma, either NHL or HL.27,28 Forty-six patients were already on ART, although 2 patients started at the time of study enrollment and 2 at the time of stem cell mobilization. The viral loads at study entry therefore ranged from 204 to 750 000 copies/mL. Thirteen patients withdrew before stem cell collection, the majority due to progressive disease. Ultimately, 27 of the initial 50 patients underwent HCT. The 3-year PFS for the patients who proceeded to HCT was similar to the City of Hope group at 76.3%. Multivariate analysis for prognostic factors for survival showed that marrow involvement, performance status <2, and CD4 count <100 cells/μL were significant.
The Bone and Marrow Transplantation Clinical Trials Network (BMT-CTN) has recently completed enrollment in a multicenter trial addressing the feasibility and efficacy of autologous HCT in HIV-1-related NHL or HL patients with persistent or relapsed disease (www.ClinicalTrials.gov identifier #NCT01141712). The primary end point of the trial is OS at 1 year; analysis is pending.
HIV reservoir
The observation that HIV-1 can be isolated from blood of patients during ART therapy, when the HIV-1 RNA levels are below the level of clinical detection, suggested that a sanctuary site exists from which infection could always be recovered.29 The tissue repository of HIV-1-infected cells from which this active replicating HIV-1 is derived is considered the HIV-1 reservoir.30 The theoretical space that is considered the HIV-1 reservoir consists of CD4+ T-lymphocytes and dendritic cells (DCs). DCs, predominantly infected by R5 strains of virus or by dual-tropic infection with R5 and CXCR4 strains, undergo stimulation by cytokines, leading to the expression of cellular coreceptors needed for further viral replication and spread. Therefore, factors that stimulate cytokine release, including mucosal damage from high-dose chemotherapy, coinfection with microbes, and microbial translocation from the GI tract, can influence the activation of DC and CD4+ T cells, ultimately influencing the size of the HIV-1 reservoir and pathogenesis of AIDS in the host.31
The favorable results of high-dose therapy and autologous in HIV-1-associated lymphoma raised the question of whether the apheresis or the high-dose chemotherapy provided any beneficial effects on HIV-1 infection. For example, could the apheresis have depleted the reservoir or could myeloablative chemotherapy ablate the infected lymphocyte pool? To address this, Cillo et al32 reported a retrospective analysis of frozen PBMC specimens from AIDS lymphoma HCT recipients who remained on ART during much of the HCT. Patients had no detectable HIV-1 RNA in the plasma at variable time points after HCT. Specimens from blood, studied before HCT and at one other post-HCT time, used assays for HIV RNA and DNA having single-copy sensitivity, a surrogate measure of the HIV-1 reservoir. No HIV-1 RNA was detected in plasma by routine assays, but with the more sensitive assay, 9 of 10 patients were viremic after HCT while on ART, with a range of <0.15 to 26 HIV-1 gene copies/mL, and 9/10 were found to have detectable HIV-1 DNA. This finding may have been due to 2 factors: (1) the autologous graft likely had infectious virus in contaminating T-cells and (2) the endogenous reservoir was still present despite the cytoreductive conditioning regimen.
HCT for HIV-1
It has been demonstrated successfully that a large proportion of patients with relapsed HIV-1-associated lymphoma can be cured by high-dose therapy and autologous HCT. However, cure of HIV-1 remains the Holy Grail and HCT may provide us the route to this goal. Infusion of resistant allogeneic hematopoietic cells or manipulation of autologous hematopoietic cells to render them resistant to the HIV-1, followed by reinfusion after ablative chemotherapy that depletes the endogenous reservoir and allows engraftment, are suggested methods. To date, the most successful method was the use of allogeneic hematopoietic cells from a donor genetically resistant to HIV-1 into an AIDS patient with acute myelogenous leukemia. The unrelated donor was homozygous for a 32-bp deletion in the chemokine receptor 5 gene (CCR5Δ32/Δ32). Individuals who are homozygous at this locus do not express CCR5 on their cell surface and therefore cannot be infected with CCR5 (R5) tropic HIV-1.33 The donor cells were infused after myeloablative conditioning, and the recipient attained complete hematopoietic reconstitution with the donor hematopoietic cells. ART was suspended early after HCT, and the patient remains with undetectable HIV-1 RNA in blood and undetectable HIV-1 DNA in tissues using single-copy sensitive PCR methods for more than 7 years after treatment.34,35 Despite the success of this single case, widespread use is limited by the rarity of both the homozygous CCR5Δ32/Δ32 mutation and HLA-compatibility, not to mention the morbidity of allogeneic HCT. Alternately, genetic modification of autologous hematopoietic cells circumvents the issues of identifying donors and, given the far lower morbidity of autologous HCT, allows a more viable approach to hematopoietic cell therapy for HIV-1.
A large phase 2 trial of gene-modified autologous hematopoietic cells infused without conditioning therapy showed only minimal detection of gene-marked cells in peripheral blood and a lack of significant effect on HIV-1 levels.36 The study did use analytical treatment interruption (ATI) of ART to assess more accurately the effects of the genetically modified cells on the HIV infection. There were no long-term deleterious effects of the ATI, so this trial set the platform for this approach in future trials.
At City of Hope, we wished to test an ablative conditioning regimen because we felt that this would be the best milieu to allow maximal engraftment of the genetically modified hematopoietic cells. At the same time, the challenge was also to find a suitable patient population for whom myeloablation could be justified. The lymphoma population was perfectly suited, especially given the poor long-term survival of patients with relapsed HIV lymphoma. The pilot trial treated 4 relapsed HIV-1-positive NHL patients with hematopoietic cells transduced with a lentivirus encoding 3 anti-HIV-1 RNAs, namely TAR, siRNA to tat/rev, and a ribozyme targeting CCR5.37 The cells were infused in combination with unmodified cells after high-dose CBV therapy. There were no serious adverse events associated with the genetically modified cells, but, similar to the aforementioned Mitsuyasu study,36 levels of gene marking, although present in all patients in the peripheral blood, were low at 0.35% (Figure 2).37,38 This was surmised to be due in part to competition with the much higher levels of unmodified hematopoietic cells that were infused concomitantly with the genetically modified product.
Level of gene marking expressed as number of copies of vector (WPRE) per 100 blood cells analyzed over time. Unique patient identifiers are listed in the upper right corner of the graph. Limits of quantification (stippled) and limits of detection (diagonal lines) values were determined for each amplification reaction and typically were in the range of 0.05% (500 cells/million) to 0.01% (100 cells/million), respectively. Reproduced with permission from DiGiusto et al.38
Level of gene marking expressed as number of copies of vector (WPRE) per 100 blood cells analyzed over time. Unique patient identifiers are listed in the upper right corner of the graph. Limits of quantification (stippled) and limits of detection (diagonal lines) values were determined for each amplification reaction and typically were in the range of 0.05% (500 cells/million) to 0.01% (100 cells/million), respectively. Reproduced with permission from DiGiusto et al.38
The evolving paradigm is that success is contingent upon high levels of only genetically modified hematopoietic cells being infused or selection of the protected cells after engraftment. Ultimately, the test of this method will be in the true target population: those with HIV and no malignancy. We have adopted a stepwise approach, with initial trials being done using nonmyeloablative conditioning in patients in remission from HIV-1-related NHL. The rationale for this is that these patients have already been exposed to chemotherapy risks, so the addition of nonmyeloablative conditioning beyond their NHL chemotherapy would not significantly increase risk. In addition, infusion of only genetically modified hematopoietic cells could be justified in the nonmyeloablative setting because early or late graft failure in this setting would not have the same devastating consequences as after ablative chemotherapy. A pilot trial using busulfan at nonmyeloablative doses in first-remission HIV-1-positive NHL patients is open at City of Hope (www.ClinicalTrials.gov identifier #NCT01961063).
Busulfan is not considered an antilymphoma therapy, so its use in the current trial is solely to facilitate engraftment. Clinical trials in human genetic diseases, especially in pediatric populations, using solely gene-modified stem cells have provided guidance on the use of busulfan-based regimens. Candotti et al39 correlated busulfan dosing with area under the curve (AUC) measurements demonstrating doses ranging from 65-90 mg/m2 (equivalent to ∼4 mg/kg) achieved an AUC in the range of 4000-4800 mU/min and was associated with engraftment. Toxicity was mild, with transient neutropenia, mild thrombocytopenia, and transient elevated liver enzymes. The ultimate step for the HIV-1 trials is to use this busulfan-based conditioning in HIV-1-infected patients without malignancy. Such a trial is planned at City of Hope and will use AUC-targeted busulfan dosing, followed by infusion of a CCR5-negative product edited by a zinc finger nuclease (ZFN).
The ZFN construct has already been tested successfully with autologous T lymphocytes.40 T lymphocytes are attractive targets for gene therapy because they are easily obtained from the donor's peripheral blood and can be expanded to large numbers in vitro. Indeed, limitations of cell number have been one of the challenges of using CD34+-selected stem cells. Targeting mature T lymphocytes for gene therapy has the added advantage that the effect of the therapeutic gene can be rapidly monitored for effects on cell survival, viral load, and other parameters. ZFNs combine the DNA recognition specificity of zinc finger proteins with the strong enzymatic activity of the cleavage domain of the restriction enzyme FokI.
The T-cell trial with this approach included 12 patients who had chronic aviremic HIV-1 infection.40 No conditioning was used before infusion of the CCR5-negative ZFN-edited T-cell product. Patients in cohort 1 (n = 6) underwent a 12-week ATI of ART beginning 4 weeks after the T-cell infusion. HIV-1 viral load was undetectable at the start of ATI and became detectable in 4 of the 6 patients at 2-4 weeks after cessation of c-ART. There was a decline of CD4 counts during ATI, but this decline in CCR5-modified cells (1.81 cells/day) was significantly less than that of unmodified cells (7.25 cells/day). Only one serious adverse event was associated with the cell infusion and was attributed to a transfusion reaction. This trial has thus successfully set the platform for ZFN editing of cells as a viable and well-tolerated approach that may lead to in vivo resistance of these CCR5-edited cells to HIV-1. The future trial of ZFN-1-modified hematopoietic cells in conjunction with nonmyeloablative busulfan conditioning in selected HIV-1-positive patients may be the ultimate proof of principle of this concept.
Disclosures
Conflict-of-interest disclosures: A.K. declares no competing financial interests. J.A.Z. is a data safety monitoring member for CALIMMUNE and CytoDyn. Off-label drug use: None disclosed.
Correspondence
Amrita Krishnan, MD, Department of Hematology and Hematopoietic Cell Transplantation, Beckman Research Institute, City of Hope Medical Center, 1500 E Duarte Road, Duarte, CA 91010. Phone: 626-359-8111; Fax: 626-301-8973; e-mail: AKrishnan@coh.org.