Abstract
Donor lymphocyte infusions (DLIs) can induce complete and durable remissions in some patients with hematologic malignancies who have relapsed after allogeneic transplantation, providing definitive evidence of a GVL effect. Despite the great promise initially envisioned for DLI as a method to augment GVL after transplantation, it utility is limited by low response rates in diseases other than chronic myelogenous leukemia and by the development of GVHD, the principal complication of DLI. To maximize GVL potency while minimizing toxicity, cellular effectors active in GVL need to be elucidated. Insight into mechanisms of GVL, such as reversal of in situ T-cell exhaustion, may allow identification of patients who will respond to DLI based on the presence of tumor-infiltrating lymphocytes in the BM. Understanding the clinical factors that influence the effectiveness and abrogate the toxicity of DLI, such as cell dose and timing of DLI after transplantation, will allow further optimization of DLI. This chapter reviews novel strategies that maximize the GVL effect of DLI by enhancing activity while limiting toxicity.
Learning Objectives
To understand the role of DLIs in the treatment and prevention of relapse in patients with hematologic malignancies after allogeneic transplantation
To evaluate new approaches to enhance the efficacy and limit the toxicity of DLI
Introduction
The success of donor lymphocyte infusion (DLI) in inducing long-lasting remissions in patients with chronic myelogenous leukemia (CML) provides direct evidence of a GVL effect. Since the first studies were published, other diseases responsive to DLI have been identified and manipulations to enhance DLI-mediated GVL responses are being explored. Reducing GVHD, the principal complication of DLI, is also a focus of investigation. Despite initial promise, the utility of DLI is limited by lower response rates in relapsed diseases other than CML, disappointing durability in active or more aggressive malignancies, lack of an effective strategy for use as prophylaxis before relapse occurs, and the prevalent toxicity of GVHD. Studies of responses induced by DLI have elucidated various effector cell populations, primarily T cells but also natural killer cells, and identified potential target antigens, both allo-antigens and tumor-specific antigens. Mechanisms of effector cell activity include the novel mechanism of “awakening” endogenous GVL effector cells by reversing T-cell exhaustion in DLI recipients. Several studies have combined DLI with other pharmacologic and immunotherapeutic agents to improve effectiveness. Selection or depletion of specific lymphocytes subsets, activation ex vivo before infusion, and targeting of tumor-specific antigens by genetically modified donor lymphocytes are being pursued. Incorporation of these novel cellular approaches into use of DLI remains an ongoing challenge.
Cellular mediators of the GVL effect
Much of our understanding of GVL derives from studies in CML because of the exquisite sensitivity of this disease to immunologic control. Indirect evidence suggests that T cells play a major role in mediating GVL in humans, because T-cell depletion during hematopoietic stem cell transplantation (HSCT) results in significant loss of GVL. After T-cell–depleted HSCT, relapse rates of 40%–60% are seen in CML patients, compared with 10%–20% after non-T-cell–depleted HSCT. Both CD4+ and CD8+ T-cell subsets demonstrate antileukemic activity in vitro and are implicated as active mediators of GVL in clinical trials of DLI. An increase in HLA class I-restricted CD8+ cells is noted in patients with myeloma responding to DLI. Tumor-specific antigen donor CD8+ T-cell responses are found after DLI in patients with acute myeloid leukemia (AML), myelodysplastic syndrome (MDS), and myelomas that express NY-ESO-1 and other cancer testis antigens such as MAGE and SSX.1 HLA class II-restricted CD4+ cells recognizing minor histocompatibility antigens (mHAg) can exhibit both helper function and cytolytic activity against leukemia cells. CD4+ T cells with selective cytotoxicity against Philadelphia chromosome–positive clones have been identified in vivo.2 In addition to conventional CD4+ and CD8+ T cells, unmanipulated DLI products contain regulatory T cells (Tregs) capable of exerting suppressive effects on effector T-cell proliferation and activity. The role of this regulatory subset in mediating or inhibiting GVL responses is under investigation.
Natural killer (NK) cells are also identified as potential mediators of GVL, particularly in HLA-mismatched or haploidentical recipient–donor pairings. NK cells appear early during hematopoietic recovery after allogeneic HSCT and mediate cytotoxicity through MHC-unrestricted killing. A correlation between high numbers of circulating NK cells and duration of remission is noted in patients after HSCT. Increased understanding of functionally disparate subsets of NK cells based on KIR (killer-cell immunoglobulin-like receptor) expression patterns and new efforts at ex vivo activation and modification of NK cells highlight a role for NK cells in the effects of DLI.
Reversal of T-cell exhaustion
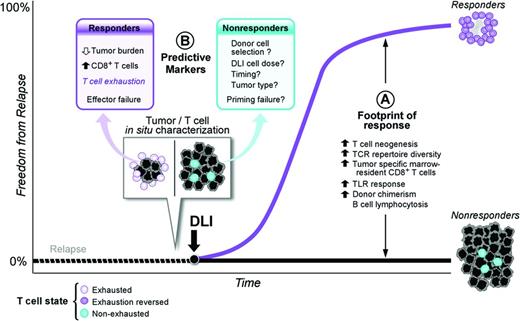
Negative immune feedback on T-cell function through cytotoxic T-lymphocyte antigen 4 (CTLA4)/B7-1 and B7-2 and PD-1/PDL-1 interactions as well as induction of T-cell exhaustion are immune evasion strategies active in solid tumors and hematologic malignancies. T-cell exhaustion is a reversible dysfunctional state triggered by chronic antigen exposure in which T cells lack normal effector and proliferative responses.3 Recent work suggests that adoptively transferred donor lymphocytes may reverse in situ T-cell exhaustion. In patients with relapsed CML after HSCT, increased numbers of CD8+ BM-infiltrating T cells predict disease response after CD4+ T-cell–selected DLI, even in the setting of high leukemic burden.4 Profiling of mRNA expression in the in situ CD8+ T-cell reservoir reveals up-regulation of T-cell exhaustion–specific genes before treatment and a significant down-regulation of those same gene pathways after DLI in responders. Therefore, adoptively transferred CD4+ T cells may mediate GVL activity by reactivating dormant antitumor activity of in situ CD8+ T cells. Other mechanisms under investigation to “awaken” dormant antitumor T-cell populations include vaccination with irradiated tumor cells and the use of anti-PD-1/antiPD-L1 antibodies in lieu of or in combination with DLI (Figure 1).
Model of DLI reversal of CD8+ T-cell exhaustion. (Figure used with permission from Bachireddy et al.4 )
Model of DLI reversal of CD8+ T-cell exhaustion. (Figure used with permission from Bachireddy et al.4 )
Potential targets of DLI and the GVL effect
Potential targets of the GVL effect encompass ubiquitous or hematopoietic-restricted allo-antigens and tumor-specific antigens. Non-disease-specific targets of GVL after HLA-matched allogeneic transplantation include minor histocompatibility antigens and sex-specific H-Y proteins in sex-mismatched donor-recipient pairs. Disease-specific antigens are proteins restricted to the malignant cells. The protein product of the BCR/ABL gene fusion is a potential GVL target in CML, as are other leukemia specific proteins, including the proteinase 3-derived peptide PR-1, CML28, CML66, and survivin.5,6 Other tumor-specific antigens include idiotypic immunogloblins in patients with lymphoma or multiple myeloma. Clinical trials using peptides and antigen-directed CTLs are necessary to prove the role of these potential targets in GVL. A thorough discussion of this topic is beyond the scope of this review.
Clinical use of unmanipulated DLI
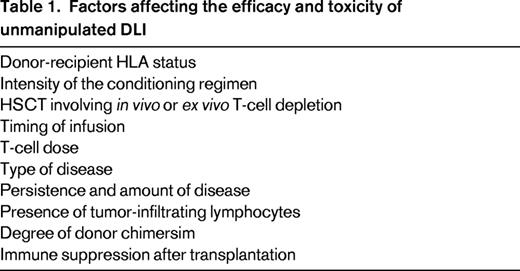
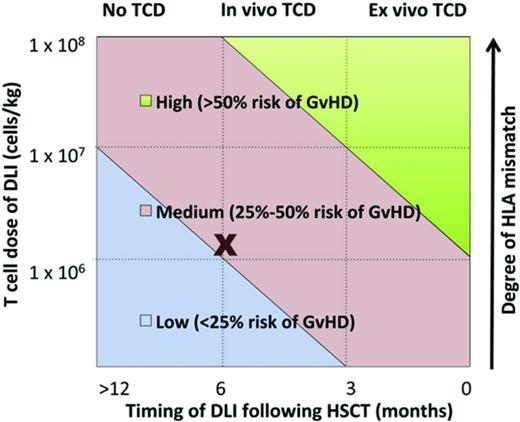
Studies in several disease settings identify a range of therapeutic DLI cell doses effective in inducing remissions with acceptable rates of GVHD. Some researchers hypothesize that prophylactic DLI will reduce relapse in patients who are identified as being high risk based on either persistence of minimal residual disease after transplantation, pretransplantation risk profile, or the presence of mixed chimerism after transplantation. Identifying factors affecting the efficacy and toxicity of DLI is critical (Table 1). Such factors may include intensity of the conditioning regimen, cellular composition of the original graft, donor-recipient HLA status, dose of cells, and timing after HSCT, among others. A complex interplay between these factors, as modeled in Figure 2, influences DLI efficacy and the development of subsequent GVHD.7
Multiple factors determine development of GVHD after DLI. (Figure used with permission from Yun and Waller.7 )
Multiple factors determine development of GVHD after DLI. (Figure used with permission from Yun and Waller.7 )
DLI for CML
Numerous series confirm a >70% durable complete cytogenetic remission rate after DLI for cytogenetic or hematologic relapse of CML after allogeneic HSCT. Studies also consistently demonstrate that patients with CML in more advanced stages of relapse (ie, accelerated phase or blast crisis) have a much lower response rate after DLI. Features predictive of a response to DLI in patients with CML include stage of disease at the time of DLI and the dose of cells infused. Prospective trials of unmanipulated DLI analyzing T-cell number demonstrate a high response rate and low incidence of GVHD in patients receiving 1 × 107 CD3+ cells/kg.8 One trial demonstrated a significantly lower rate of GVHD using an escalating DLI cell dose regimen compared with the single bulk infusion. There was no difference in the remission rates between the 2 dosing approaches. Multivariate analysis of results after CML relapse and receipt of DLIs from HLA-matched related donors versus matched unrelated donors showed that donor source had no difference on rates of durable molecular remission.9
DLI for diseases other than CML
Response rates to DLI are not as high when used in relapse of malignancies other than CML. The overall response rate to DLI in patients with myeloma approaches 45%, with complete responses noted in ∼25% of patients. Durable complete responses are noted in half of patients who obtain an initial complete remission. The response rates and duration of response after DLI in patients with acute leukemia and MDS are disappointing. Only 33%–40% of patients with MDS achieve a remission with DLI. Complete response rates to DLI are only 15%–29% in patients with AML and 5%–18% in acute lymphoblastic leukemia. Most responses are not durable, with, for example, a median survival of 11 weeks after DLI, and come with a high rate of death from treatment-related complications.10 Experience with DLI in patients with chronic lymphocytic lymphoma and low-grade lymphoma is limited; however, these diseases do appear to be sensitive to a GVL effect. In one of the largest retrospective DLI studies to date on 225 patients with a variety of conditioning regimens, intensities, and donor sources, the incidence of GVHD was increased, but overall survival was not improved at CD3+ cell doses of >10 × 107 cells/kg for any disease categories. Receiving DLI from a related versus unrelated or HLA-matched versus HLA-mismatched donor did not significantly contribute to the development of GVHD or overall mortality in multivariate analyses.11
Chemotherapy before DLI
Patients with acute leukemia relapsed after allogeneic transplantation may be treated with chemotherapy before DLI. In many cases, chemotherapy is administered because of rapidly progressive disease or in an attempt to debulk patients before DLI. Although the overall response rate to chemotherapy plus DLI is higher than DLI alone, long-term outcome is not significantly improved. For example, one clinical trial combining chemotherapy and DLI in patients with relapsed myeloid disease, primarily AML, demonstrated an overall complete response rate of 47%.12 Unfortunately, the toxicity associated with this approach was high, with a treatment-related mortality of 23% and a disappointing 2-year overall survival for all patients of 19%.
DLI after nonmyeloablative HSCT
The role of DLI after nonmyeloablative or reduced-intensity conditioning (RIC) HSCT is not well defined. DLI has been used after RIC in 2 ways, as treatment for relapse or as a method to convert patients from a mixed chimeric state to full donor chimerism. Two recent studies have detailed the results of DLI after nonmyeloablative HSCT using in vivo T-cell depletion.
Dose-escalating DLI infusions were administered after day 100 to 119 patients with AML or MDS after a T-cell–depleted RIC-HSCT, either as treatment for relapsed disease or as prophylaxis for those with donor CD3+ fractions that were declining or persistently <50%.13 The 5-year overall survival rate was 40% for those receiving DLI for relapse, compared with 80% for those receiving prophylactic DLI. GVHD was noted in 45% of those receiving DLI for relapse, compared with 35% for those receiving DLI as prophylaxis. In a smaller study focusing on prophylactic DLI in patients with acute leukemia after T-cell–depleted RIC-HSCT, the overall survival was 73%, with a median follow-up of >1.5 years. The overall incidence of GVHD rate was 47% and 4 of 15 patients who developed GVHD died of complications.14 Taken together, these studies demonstrate that DLI is capable of reducing the risk of relapse after RIC-HSCT using in vivo T-cell depletion.
Critical determinants of the success and toxicity of DLI after T-cell–replete RIC-HSCT are less clear. There is lack of consensus on dose, timing, and the need for chemotherapeutic intervention before DLI. The high rates of chronic GVHD after T-cell–replete RIC-HSCT hamper the development of a prophylactic DLI strategy in this setting.
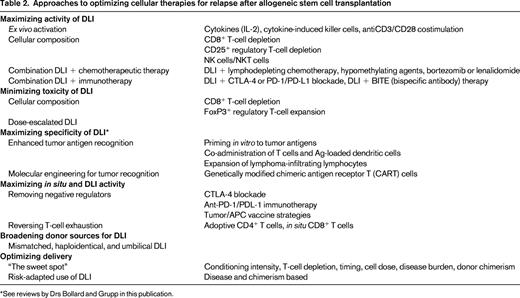
Novel approaches to maximizing GVL activity (Table 2)
Enhancing activity of adoptive cells
To allow administration of effective doses of cells without concomitant increases in GVHD, researchers are fractionating the initial donor lymphocyte product into various cellular subsets. CD8-depleted (or CD4-selected) DLI has been explored, with demonstrated cell-dose–dependent but acceptable rates of GVHD, low treatment-related mortality, and encouraging efficacy and durability, particularly in CML.15 Interestingly, 48% of responding patients in one study never developed GVHD, indicating a potential separation of GVL from GVHD. The use of CD8-depleted products has been studied in a prophylactic manner after T-cell–depleted RIC-HSCT and to enhance duration of remissions and promote immune reconstitution after T-cell–depleted haploidentical transplantation.16,17 Some of the beneficial effects of CD8-depleted DLI may actually reflect reversal of T-cell exhaustion in the in situ CD8+ T cells, thus provoking GVL without excessive GVHD. A direct comparison of CD4+ DLI with unmanipulated DLI administered 6 months after T-cell–depleted HSCT was performed and yielded a significantly lower incidence of GVHD in patients receiving CD4+ DLI.
Tregs, which are characterized by CD4+CD25+ expression, are thought to ameliorate the development and activity of GVHD but, given their tolerogenic effects, are potentially inhibitory to GVL. A phase 1 trial of DLI products depleted of CD4+CD25+ Tregs demonstrated safety, feasibility, no significant elevation of GVHD incidence, and antileukemic efficacy in AML (S.N. et al, unpublished data).18 Infusions of CD8+CD44high memory T cells are being investigated as potential mediators of GVL activity without GVHD, given the hypothesis that naive T cells are the primary effectors in GVHD.
NK cells can also exert potent GVL effects. Alloreactive haploidentical NK cells have been transferred in elderly high-risk AML patients unable to undergo allogeneic HSCT, with clinical trials ongoing to confirm safety and efficacy.19,20 Early after haploidentical HSCT, prophylactic infusion of donor-derived NK cells can significantly reduce leukemia progression and shows no increase in transplantation-related mortality or GVHD rates.21
Combination with novel targeted therapies
The addition of DLI to novel agents active in multiple myeloma, such as bortezomib and lenalidomide, has been performed in patients with residual disease after allogeneic HSCT. These combinations are well tolerated, do not increase risk of GVHD over DLI alone, and result in deep remissions.22,23 The combination of 5-azacytidine followed by DLI in older patients with relapse of AML or CML after allogeneic HSCT is feasible and induces a 66% response rate, even in patients with poor-risk cytogenetics.24 Other combinations of disease-modifying agents with DLI can be explored, such as sorafenib plus DLI for relapsed AML or the immunomodulatory agent lenalidomide plus DLI outside of the myeloma setting.
Ex vivo activation
Activation of DLI effector cells before infusion may maximize GVL effect. Culture of lymphocytes with IFN-γ, IL-2, and anti-CD3 results in “cytokine-induced killer cells” (CIKs), cytotoxic effector T cells expressing CD3 and CD56 that recognize targets through NKG2D. Allogeneic CIK cells demonstrate response rates of 30% in several trials of relapsed AML, sometimes with longer duration of response than was observed after the original HSCT.25 IL-2 has been combined with DLI infusions, but with unclear efficacy in early studies and resulting chronic GVHD rates of almost 50%. Costimulation with CD3/CD28-coated beads to expand and activate donor lymphocyte products ex vivo before infusion is safe and feasible in early-stage trials without excessive GVHD, although efficacy is not high.26
Tumor/APC vaccines
Originally, tumor vaccines such as the GVAX platform (adenoviral vector-mediated GM-CSF gene transfer as a vaccine adjuvant into irradiated autologous AML cells) were used to generate lymphocytes primed ex vivo to tumor antigens. These were reinfused with an autologous stem cell graft. Recently, an autologous GVAX vaccine has been manufactured to stimulate T cells in situ after allogeneic HSCT for MDS/AML.27 Leukemia blasts were collected before HSCT, transduced, irradiated, and reinfused between days +30 and +45 after HSCT. Two-year overall survival rates were 56% in patients who received at least one vaccination, compared with 18% in an historical control cohort. Alternatively, DLI can be combined with recipient dendritic cells, either unmanipulated or pulsed with tumor antigens, to enhance T-cell activity.
Immune blockade
CTLA-4 blockade to inhibit this negative regulatory pathway over T-cell activation has efficacy in relapsed and refractory malignancies, including non-Hodgkin lymphoma. Blockade with ipilimumab, an anti-CTLA-4 antibody, during relapse after allogeneic HSCT results in increased CD4+ T-cell counts and activation without increasing Tregs. In a phase 2 trial, use of ipilimumab was feasible in 29 patients without induction of significant GVHD.28
Similarly, blockade of the PD-1/PD-L1 inhibitory interaction may release inhibition exerted by AML cells on minor histocompatibility antigens-specific CD8+ T cells. Blockade of this pathway may enhance in vivo activity of T cells to an autologous dendritic cell/myeloma fusion vaccine manufactured ex vivo.29 In murine models, PD-L1 blockade combined with late adoptive transfer of antigen-specific CD8+ T cells restored GVL efficacy and persistence without triggering GVHD.30 Therefore, immunotherapies combined with DLI may enhance activity of both adoptively transferred and in situ “exhausted” T cells (Figure 1).
Novel approaches toward maximizing antigen specificity of DLI, namely expansion of naturally occurring lymphocytes directed toward tumor-specific antigens such as BCR-ABL, PR1, and WT1 or genetic engineering of tumor-specific cells against CD19, CS-1, NY-ESO 1, CD123, WT1, and p53 are currently at the forefront of graft-versus-malignancy research, as reviewed by Drs Bollard and Grupp in separate chapters in this publication.
Alternative donor DLI sources
Use of unmanipulated DLI from alternative donors is also being explored. Comparison between trials of prophylactic and therapeutic DLI from HLA-mismatched and haploidentical donors is complicated by the wide range and schema of cell doses administered, the presence of prior T-cell depletion, variations in the degree of mismatching, mobilization with G-CSF, and the use of immune suppression for GVHD prevention after DLI. Rates of acute GVHD grades III-IV range from 14% to 32%. No consistent correlations between degree of HLA mismatching and either acute or chronic GVHD or efficacy are noted. Comparative efficacy versus DLI from matched sources is difficult to evaluate because large, head-to-head prospective trials have not been performed, but appeared similar when mismatched DLIs are administered in escalating doses starting at 1 × 105 to 1 × 106 CD3+ cells/kg.31,32 Umbilical cord blood units are usually prohibitively small and donors cannot be contacted for donation after relapse. However, expansion and activation of cord T cells to recognize allogeneic targets is feasible, indicating that cord T cells may be a future source of DLI.33
Risk-adapted use of DLI
Cytogenetic and molecular determinants are critical factors affecting decisions to pursue allogeneic HSCT in AML. Disease-intrinsic factors and stage at time of HSCT comprise a disease risk index that can be used to stratify patients for risk of relapse after HSCT and thus predict the benefit of pursuing HSCT.34 Analogously, predictors of DLI success based on chimerism after HSCT and pre-DLI lymphocyte counts have been postulated.35 The use of prophylactic DLI from matched-related donors when stratified for risk of relapse based on the presence of mixed chimerism was studied in 50 patients with predominantly high-risk chronic lymphocytic leukemia. This approach achieved a 4-year progression-free survival of 65% and overall survival of 75% (60% and 61%, respectively, in very-high-risk patients with 17p deletions). Rates of severe GVHD and treatment-related mortality were 29% and 6.4%, respectively.36 A risk-adapted algorithm in pediatric leukemia involving preemptive DLI for mixed chimerism until full donor chimerism was achieved demonstrated superior rates of relapse and survival versus patients whose mixed chimerism was not addressed with DLI.37
Future directions
Although responses to DLI, particularly in CML, demonstrate the potential potency of a GVL effect, a clear and effective role for DLI after allogeneic HSCT for other malignancies is still to be defined. Efforts to improve the efficacy of DLI include obtaining a better understanding of effector cells and targets of the GVL response to enable more selective therapies. Aspects of administration such as cell dose, timing after HSCT, and degree of tolerable HLA mismatch need to be optimized. Manipulation of the T cells adoptively transferred during DLI to enhance activity and antigen specificity is ongoing, as are attempts to reverse T-cell exhaustion of donor effector cells lying dormant in the recipient. Combinations of novel chemotherapeutic agents and immunotherapies with DLI offer exciting avenues of investigation. Future efforts should focus, not just on the DLI product, but also on identifying a risk-adapted approach to DLI to allow earlier intervention and maximize clinical outcomes in the setting of relapse after allogeneic HSCT, which at the present time entails such a poor prognosis.
Disclosures
Conflict-of-interest disclosures: The authors declare no competing financial interests. Off-label drug use: Chemotherapy agents used for transplantation.
Correspondence
Edwin P. Alyea, MD, Dana-Farber Cancer Institute, 450 Brookline Ave., Mailstop: DA-02048, Boston, MA 02215; Phone: (617)632-3903; Fax: (617)632-5168; e-mail: edwin_alyea@dfci.harvard.edu.