Abstract
More than 4 years have passed since the first approval of a target-specific oral anticoagulant (TSOAC) in the United States, and the number of clinicians who have prescribed (or considered prescribing) one or more of these medications is increasing. Although these agents may, in properly selected patients, offer advantages over more traditional therapies, their lack of familiarity can be intimidating. Clinicians who are prescribing the TSOACs face a number of management questions not definitively answered by the registration trials. This chapter reviews some of these situations, including updated information on the periprocedural management of TSOACs and the latest evidence about how to best measure TSOAC effect. The lack of an antidote and other considerations that may be relevant when deciding between newer and more traditional anticoagulant medications are also discussed.
Learning Objectives
To be able to determine whether a patient is a good candidate for target-specific oral anticoagulants (TSOACs)
To be more comfortable with practical issues that arise related to TSOACs, such as assessment of anticoagulant effect or peri-operative use
Introduction
Based on large phase 3 trials,1-10 dabigatran, rivaroxaban, and apixaban have been approved by the U.S. Food and Drug Administration (FDA) for a variety of indications (Table 1). Edoxaban has been studied for stroke prevention and venous thromboembolism treatment and more approvals are expected in the next year.11,12 Although these trials, which collectively involved tens of thousands of patients, have provided very valuable information about the efficacy and safety of oral agents that directly inhibit either thrombin or activated factor X (fXa), many clinicians remain reluctant to prescribe target-specific oral anticoagulants (TSOACs). In part, the slow uptake of these TSOACs is likely attributable to the lack of evidence to answer real-world questions such as: “How and when should the effect of a TSOAC be measured?” “How should a TSOAC be administered before and after invasive procedures?” or “What patients are good candidates for TSOAC use?” This chapter reviews these questions with the aim of providing as many evidence-based recommendations as possible.
Temporary interruptions of TSOAC therapy
An imbalance in stroke rates after study completion in the ARISTOTLE and ROCKET-AF trials raised questions about whether stopping apixaban or rivaroxaban might lead to rebound hypercoagulability; each medication has a prominent warning about this issue in its FDA-approved prescribing information. However, more recently published evidence from ARISTOTLE and ROCKET-AF suggests that the observed differences are likely explained by clinical trial design. Because both trials were double-blinded, patients who finished the study on experimental drug (rivaroxaban or apixaban) and started open-label warfarin were more likely to spend some of the transition period with a subtherapeutic international normalized ratio (INR) than patients who switched at study completion from blinded warfarin to open-label warfarin. This hypothesis is supported by analyses of the post-discontinuation events from both studies, which show that the timing and rate of the thrombotic events among the patients switching from a fXa inhibitor to open-label warfarin are what one would expect in a large group of patients starting warfarin de novo (C. Grander, unpublished data).13 The similar rates of stroke after permanent study-drug discontinuation in all 3 arms (dabigatran 150 mg BID vs dabigatran 110 mg BID vs warfarin) of the open-label RE-LY trial provide additional evidence that trial design, not post-discontinuation rebound hypercoagulability, explains the findings from ROCKET-AF and ARISTOTLE. The lack of stroke difference after RE-LY may also be partly explained by the fact that many patients taking one of the dabigatran doses could elect to continue dabigatran therapy as part of an extension study, an option not available to participants in the clinical trials of fXa inhibitors.
In contrast to these differences in TE rates observed after permanent discontinuation of study drug, the rates of TE during 30 days after temporary study drug interruption in 3 large AF trials were very low and not different between treatment arms.14-16 In all 3 of the large AF trials that have done such analyses, stroke or systemic embolism occurred within 30 days in <1% of TSOAC-treated patients who underwent a procedure and/or had a brief interruption of therapy. As seen in Table 2, there is some variability between (but not within) the different analyses for some key end points such as death and major bleeding. The differences in these rates are likely explained by differences in the way the analyses were performed. For example, the analysis of the ROCKET-AF trial considered only patients who interrupted study medication (whether it was interrupted for a procedure or other reasons). In contrast, the ARISTOTLE analysis included only patients who underwent procedures; many did not interrupt study medication beforehand. Although further studies will be needed to precisely define the optimal timing of the final preprocedure and first postprocedure doses, the data from these large clinical trials suggest that short-term interruption of TSOACs can be done safely, in most cases without a parenteral anticoagulant “bridge.”
Proportion of patients who experienced a key clinical end point during the 30 days after a procedure and/or temporary interruption of study medication
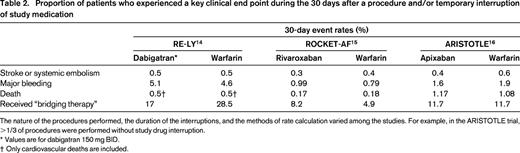
The nature of the procedures performed, the duration of the interruptions, and the methods of rate calculation varied among the studies. For example, in the ARISTOTLE trial, >1/3 of procedures were performed without study drug interruption.
* Values are for dabigatran 150 mg BID.
† Only cardiovascular deaths are included.
Measurement of anticoagulant effect
Although the TSOACs have been developed without routine measurement of their anticoagulant effect, it may be important to determine how much TSOAC effect is present in several clinical situations. For example, when an urgent procedure is contemplated, the risk-benefit tradeoffs of proceeding versus waiting may be better calculated with a quantitative estimate of the degree to which hemostasis might be impaired by the TSOAC. Unfortunately, traditional coagulation times such as the prothrombin time (PT) and partial thromboplastin time (PTT) will provide incomplete (and possibly misleading) information when measured on a patient taking one of the new anticoagulant medications. For example, the PT will sometimes be prolonged if rivaroxaban is present, but a normal PT should not be used to conclude that no rivaroxaban effect is present unless the laboratory performing the test has independently confirmed that the reagent and instrument being used are adequately sensitive.17 Similarly, the PTT is often prolonged in the presence of dabigatran, but can be normal in the presence of clinically important drug concentrations, depending on the specifics of the assay being used.18-20 The thrombin time (TT) is very sensitive to the presence of even very low concentrations of dabigatran. It has been suggested that coagulation laboratories should perform dose-response studies using calibration standards to determine whether their clotting time(s) can reliably detect the presence of any of the TSOACs. However, even if a particular laboratory has determined that its traditional clotting assays are sufficiently sensitive to exclude the presence of a particular TSOAC, the correlation between PT, PTT, or TT prolongation and intensity of TSOAC effect is poor (A. Cuker, unpublished data).
The plasma concentration (and anticoagulant effect) of these TSOACs can be estimated using more specialized testing. For example, the amount of dabigatran present correlates well with a modified TT in which patient plasma is diluted with normal plasma.21 The concentration of rivaroxaban or apixaban can be estimated by measuring the anti-Xa activity using a kit that has been calibrated for the agent of interest.22-26 However, these tests will not be available in all laboratories and may have turnaround times that reduce their clinical utility.
In contrast to the situation with warfarin, the TSOACs do not have a “therapeutic” or “target” range that has been associated with optimal outcomes. Based on pharmacokinetic data, one can define an “expected” range for peak and trough concentrations, but these values may vary significantly from one patient to the next.27 Although it is likely that, for each drug, there is some plasma concentration below which an urgent invasive procedure could be performed with an acceptable bleeding risk, this threshold has not been defined for any of the medications. Furthermore, if a patient were to have a peak or trough value substantially higher or lower than “expected,” there are no evidence-based guidelines about how (or if) the medication dose should be adjusted. For these reasons, routine measurement of anticoagulant effect in an asymptomatic patient is not currently recommended.
Should the lack of antidote be a major concern?
The lack of definitive information about how best to manage patients with TSOAC-associated life-threatening bleeding probably leads many physicians to avoid prescribing TSOACs. Hematologists are especially sensitive to this issue because, in the absence of evidence-based guidelines, it is the hematologist who will often be consulted about how best to manage TSOAC-associated major bleeding. Although this lack of an antidote for the TSOACs is certainly not a trivial concern, I present several reasons why it should not play a major role in the decision to use or not use a TSOAC for most patients.
For many patients with TSOAC-associated bleeding, an antidote would not be used even it were available
In patients with normal renal function, the anticoagulant effect of a TSOAC will dissipate very rapidly after the drug is discontinued. This is illustrated by an analysis of the open label RE-LY trial, during which 399 patients taking dabigatran 150 mg BID experienced a major hemorrhage. Although the RE-LY clinical trial protocol listed prothrombin complex concentrate (PCC) and recombinant factor VIIa (rFVIIa) as “options to be considered at the discretion of the treating physician” for life-threatening bleeding, only 2 (0.5%) and 7 (1.8%) of the dabigatran 150 mg patients with major bleeding received either PCC or rFVIIa, respectively.28 This is indirect evidence that, in the majority of nearly 400 cases of dabigatran-related major bleeding, the treating physician saw no need to escalate beyond supportive care such as RBC transfusion, volume support, etc.
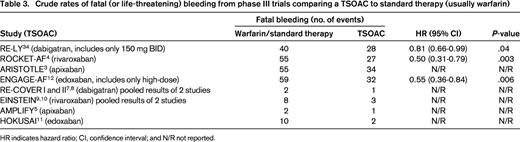
TSOAC-treated patients had fewer fatal bleeds than did warfarin-treated patients
In all of the phase 3 tudies comparing a TSOAC with conventional therapy, the crude rate of fatal bleeding was lower with the TSOAC (Table 3). Although this difference is partly explained by the ∼50% relative risk reduction in intracranial bleeding (a type of bleeding that is often fatal in anticoagulated patients), intracranial bleeds are overall much less common than major gastrointestinal bleeds. For dabigatran 150 mg BID and rivaroxaban 20 mg QD, the end point of gastrointestinal bleeding occurred more frequently among those on a TSOAC vs those on warfarin. Despite these differences and despite the lack of an antidote for TSOACs, fatal bleeding was numerically less frequent in the TSOAC arm of each trial. Furthermore, a prespecified analysis of data from ARISTOTLE shows that the combination of bleeding followed by death within 30 days occurred significantly more commonly in the warfarin arm compared with apixaban29 and, of the >1000 patients with a first major bleed in the RE-LY trial, 30-day mortality was numerically lower in the dabigatran group (9.1%) than in the warfarin group (13.0%) (pooled odds ratio, 0.68; 95% confidence interval, 0.46-1.01; P = .057).28
Evidence that the rapid “reversal” of VKA effect will affect patient-important outcomes is weak
Although IV vitamin K can, in combination with PCC, restore the INR of most warfarin-treated patients to a normal value within minutes,30 >10% of patients hospitalized for warfarin-associated major bleeding die within 90 days.31,32 Furthermore, although PCC can reduce the INR much faster than fresh-frozen plasma, the landmark trial that led the FDA to approve a 4-factor PCC (KCentra) was unable to demonstrate a statistically significant difference in mortality or time to hemostasis between the PCC and fresh-frozen plasma treatment arms.30 It is possible that this lack of mortality difference is explained by a lack of statistical power and that the high 90-day mortality rate among patients who experience major warfarin-associated bleeding is certainly explained, in part, by the numerous comorbidities known to be associated with major anticoagulant-related hemorrhage. Nevertheless, these facts highlight both the imperfect nature of warfarin reversal and the importance of avoiding anticoagulant-related bleeding in the first place.
Based on the above, the lack of a proven reversal strategy for TSOACs should not be a major reason to choose a vitamin K antagonist (VKA) over a TSOAC. That notwithstanding, there are other reasons why a VKA may be preferred: cost, presence of a prosthetic heart valve, concerns about adherence, and extremes of body weight. Advanced kidney disease should lead the clinician to consider the risks and benefits of any anticoagulant (including VKAs) with special caution.33
Disclosures
Conflict-of-interest disclosure: The author has consulted for Daiichi-Sankyo, Janssen, Pfizer, Bristol Meyers Squibb, Boehringer Ingelheim, and CSL Behring. Off-label drug use: None disclosed.
Correspondence
David Garcia, MD, Division of Hematology, University of Washington School of Medicine, Box 357710, 1705 NE Pacific Street, Seattle, WA 98195-7710; Phone: (206)543-3360; Fax: (206) 543-3560; e-mail: davidg99@uw.edu.