Abstract
The vitamin K antagonists (VKAs) are a widely used class of agent to prevent thromboembolism. In recent years, numerous alternatives to VKAs have been developed, the target-specific oral anticoagulants (TSOACs), which are available in clinical practice. Currently available agents target thrombin and factor Xa. The most significant side effect of these agents, as with VKAs, is the development of bleeding complications. In this review, the risks of major bleeding complications with the TSOACs will be discussed. Data from meta-analyses, randomized controlled trials, and observational studies will be used to highlight bleeding complications associated with TSOACs and warfarin. We highlight the most common causes of major bleeding, GI and intracranial hemorrhage.
Learning Objective
To understand the bleeding risks associated with different target-specific oral anticoagulants
Introduction
Under routine physiologic conditions, the body attempts to maintain an equilibrium between thrombus formation and destruction.1 Historically, the only available oral anticoagulant was warfarin, which inhibits the conversion of vitamin K2,3 epoxide to vitamin K quinone, as well as the conversion of vitamin K quinone to vitamin K quinol, the active form of vitamin K.2,3 This inhibition results in the accumulation of inactive vitamin K precursors and a resultant increase in the international normalized ratio (INR). The risk of vitamin K antagonist (VKA)-associated hemorrhage correlates with the INR.4 It is estimated that 65 000 patients are treated annually in US emergency departments for warfarin-related hemorrhage.5 During warfarin therapy, the risk of major bleeding in patients anticoagulated with warfarin ranges from 0.4% to 7.2% per year.4 In patients with nonvalvular atrial fibrillation (AF), the risk of the most devastating form of major bleeding, intracranial hemorrhage (ICH), is ∼0.8% per year.6
One of the most important factors associated with bleeding is good INR control, often measured as the patient's time in therapeutic range; many clinical trials find that patients are in range only 55%–64% of the time, and this under the controlled circumstances of a clinical trial.7,8 Patients with stable INR control are at lower risk of major bleeding than those without.9 This highlights one of the challenges of warfarin therapy in real-world practice. For ICH in particular, those patients with higher INR at presentations have worse outcomes.10,11 That said, even stable INR is not fully protective and the majority of warfarin-related ICH patients present while in the appropriate therapeutic range,12 so even successful maintenance in the therapeutic range does not prevent this devastating complication. One large observational trial highlighted the importance of ICH as a complication of warfarin; when major bleeding occurred outside the brain, only 3% suffered death or disability compared with 76% when bleeding was inside the brain.13
Target-specific oral anticoagulants
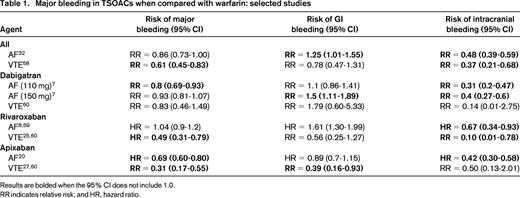
The desire for safer anticoagulants has led to the development of numerous agents, including the factor Xa inhibitors and the direct thrombin inhibitors (DTIs). These have traditionally been termed novel oral anticoagulants, but because they have been available for some time, they are now often referred to as direct or target-specific oral anticoagulants (TSOACs), and we use the term TSOACs in this review. In addition, the major clinical trials have been focused on specific indications and enrolled patients with different ranges of comorbidities. It may be that an individual patient's risk of bleeding is unrelated to the initial indication for anticoagulation; for example, a large observational registry of rivaroxaban use found the major bleeding rates to be approximately 3.4 per 100 patient years, with no clear difference between AF and venous thromboembolism (VTE) patients.14 However, it may also be that the types of patients enrolled in AF trials have different bleeding risks than those enrolled in VTE trials, so in this review, we stratify the discussion by indication. In addition, because the major indications for long-term TSOAC use are AF and VTE, this review focuses first on the bleeding risks specific to each indication and then on risks overall. Table 1 summarizes the rates of major bleeding, GI bleeding, and ICH associated with each agent.
Bleeding risks in AF
Overall
In a recent meta-analysis of several large clinical trials of AF, major bleeding occurred in 5.2% of patients on TSOACs compared with 6% of those on warfarin [OR = 0.86; 95% confidence interval (CI), 0.73-1.0].7 However, not all bleeding was equal; those patients on TSOACs had a reduced risk of ICH (OR = 0.48; 95% CI, 0.39-0.59; P < .0001) but an increased risk of GI bleeding (OR = 1.25; 95% CI, 1.01-1.55; P = .04).7
Direct thrombin (factor IIa) inhibitors
The DTIs are a class of drugs available for both oral and intravenous administration. The major oral DTI is dabigatran. Overall, the rate of major bleeding with dabigatran 150 mg bid in the RE-LY trial was 3.1% per year, which was similar to that associated with warfarin.7,15 However, not all major bleeding is equal. In the RE-LY trial, the risk of GI bleeding with dabigatran was 1.1%–1.5% per year, whereas that of intracranial bleeding (typically associated with much more devastating outcomes) was only 0.23%–0.3% per year (compared with 0.74% per year in the warfarin group).7 Similar findings come from a recent communication from the US Food and Drug Administration (FDA) that reviewed data from >134 000 Medicare patients. They found that the incidence rate of ICH on dabigatran is 3.3 per 1000 person years compared with 9.6 for warfarin [hazard ratio (HR) = 0.34; 95% CI, 0.26-0.46]. There was a signal suggesting an increased incidence of major GI bleeding of 34.2 versus 26 per 1000 person years on dabigatran versus warfarin, respectively (HR = 1.28; 95% CI, 1.14-1.44)16 Therefore, even if the major bleeding rates are similar, the type of bleeding appears to be shifted to less devastating GI bleeding with dabigatran. Consistent with this, it appears that when major bleeding develops, those randomized to dabigatran have shorter intensive care unit stays and a trend toward improved mortality compared with patients on warfarin.17
Factor Xa inhibitors
These drugs inhibit factor Xa, the first step in the common pathway of the coagulation cascade, in a dose-dependent fashion.18 Overall data from a recent Cochrane review of major AF trials suggests a risk of major bleeding of 46 per 1000 patient years, of which 11 per 1000 are intracranial.19 The risk is slightly lower with factor Xa inhibitors than warfarin (OR = 0.89; 95% CI, 0.81-0.98). Importantly, the risk of the most severe type of bleeding, ICH, was substantially lower with TSOACs (OR = 0.56; 95% CI, 0.45-0.70) and this effect was robust across sensitivity analyses. Therefore, although this class of agents is associated with important major bleeding risks, these risks appear to be consistently lower than those associated with warfarin. It is not yet clear whether there are clinically relevant differences in bleeding risks between different factor Xa inhibitors.
Rivaroxaban
In the ROCKET AF trial, GI hemorrhage was more common in those in the rivaroxaban group (3.2% per year) than in the warfarin group (2.2% per year).8 It is important to note that, for the more devastating bleeding complication, ICH rates were lower in rivaroxaban than warfarin (0.5% per year vs 0.7% per year; HR = 0.67; 95% CI, 0.47-0.93).8
Apixaban
The ARISTOTLE trial highlighted how major bleeding risk can appear different based on different definitions.20 Using the International Society on Thrombosis & Haemostasis (ISTH) definitions, major bleeding occurred in 2.13% per year versus 3.09% per year in the warfarin group (HR = 0.69; 95% CI, 0.6-0.8; P < .001); using the GUSTO definition, severe bleeding occurred in 0.52% per year versus 1.13% per year (HR = 0.46; 95% CI, 0.35-0.6; P < .001); and using the TIMI definition, major bleeding occurred in 0.96% per versus 1.69% per year (HR = 0.57; 95% CI, 0.46-0.70; P < .001). Most importantly for the clinician, it is clear that the HR for bleeding (compared with warfarin) is similar irrespective of definition used and that ICH is less common in those receiving apixaban (0.33% per year vs 0.8% per year; HR = 0.42; 95% CI, 0.3-0.58).20
Edoxaban
At the time of this writing, edoxaban is not yet available in the United States. The ENGAGE AF-TIMI 48 trial21 noted that the rate of major bleeding was 3.43% per year in the warfarin group, 2.75% per year in the high-dose edoxaban group (HR = 0.8; 95% CI, 0.71-0.91; P < .001), and 1.61% per year in the low-dose edoxaban group (HR = 0.47; 95% CI, 0.41-0.55; P < .001). This highlights the fact that, as with the other factor Xa inhibitors, the risk of bleeding is lower than with warfarin and this risk is dose dependent.
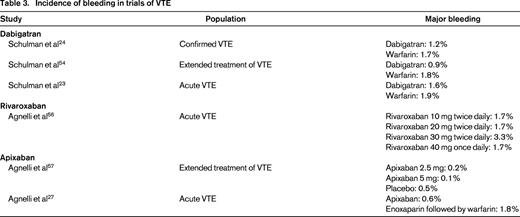
Bleeding risks in VTE
Overall, the risk of major bleeding associated with VTE therapy appears lower with TSOACs than with warfarin [relative risk (RR) = 0.62; 95% CI, 0.45-0.85).22 Specific factors that modify this risk are highlighted below, but it appears that particular caution should be applied to those who are older, those with renal insufficiency, and those on concomitant antiplatelet agents or NSAIDs.
Direct thrombin (factor IIa) inhibitors
Factor Xa inhibitors
Rivaroxaban
For patients on rivaroxaban for VTE, major bleeding occurred in 0.8%–1.1% compared with 1.2%–2.2% in those on warfarin.23-25 The largest trial, EINSTEIN-PE, found this risk to be significantly lower with rivaroxaban (HR = 0.49; 95% CI, 0.31-0.79); the others found similar point estimates but with CIs that crossed 1.0.23-25 Again, the major driver of fatal bleeding was ICH, which appears to be lower with rivaroxaban than with warfarin (RR = 0.48; 95% CI, 0.31-0.74).26
Apixaban
The AMPLIFY study found that major bleeding occurred in 0.6% of apixaban patients versus 1.8% of those on warfarin for acute VTE (RR = 0.31; 95% CI, 0.17-0.55)27 Apixaban therefore appears to show a lower bleeding risk than warfarin for acute VTE management.
Indirect comparisons
Although numerous individual studies compare the bleeding rates between drugs and for different indications (Tables 2 and 3), it is somewhat difficult to compare the bleeding rates between different agents due to differences in individual trial reporting and populations with different bleeding risks. For example, the ROCKET-AF population showed higher CHADS2 scores than other trials, suggesting a higher-risk population, so that the event rates for rivaroxaban may reflect differences in population rather than agent.8
Incidence of bleeding in trials of AF
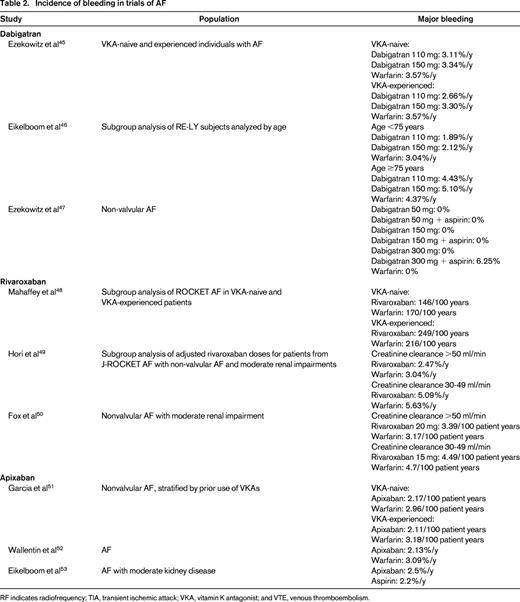
RF indicates radiofrequency; TIA, transient ischemic attack; VKA, vitamin K antagonist; and VTE, venous thromboembolism.
Some groups have attempted to compare different agents indirectly using a common comparator (warfarin). One found ICH risk to be lower with dabigatran than with rivaroxaban, but this was only significant for the lower 110 mg dose (OR = 0.15; 95% CI, 0.03-0.67).28 This suggested that a patient at high risk of ICH may be safer with a lower dose of dabigatran (although this offers decreased protection against thromboembolism). They also noted the risk of major bleeding to be lower with apixaban than with high-dose dabigatran (OR = 0.19; 95% CI, 0.13-0.28) and rivaroxaban (OR = 0.19; 95% CI, 0.14-0.28). Another group found similar results, with apixaban being associated with a lower risk of major bleeding than dabigatran (RR = 0.42; 95% CI, 0.21-0.87), but not significantly different from rivaroxaban (RR = 0.57; 95% CI, 0.29-1.15).29 Finally, others have suggested that apixaban demonstrates a lower overall bleeding risk than other agents.30 Apixaban may ultimately prove to be a lower-risk option.
Individual factors that can modify bleeding risk
Although knowledge of overall bleeding risk can help to guide the choice of therapy, patient-specific factors likely influence this risk and may be useful in guiding the choice of agent. Although not designed specifically for any particular agent, the HAS-BLED bleeding score, which assigns points based on the presence of hypertension, abnormal renal and liver function, prior stroke, prior bleeding history, labile INRs, elderly (age >65 years), and concomitant use of drugs or alcohol has been shown to be a useful risk score to determine bleeding risk in patients anticoagulated for AF.31 However, a few key patient features repeatedly arise that the clinician should keep in mind.
Advanced age
Older patients appear to be at consistently higher risk of bleeding complications while being anticoagulated.14 Therefore, any anticoagulant should be prescribed with caution. That said, such patients are also at higher risk for thromboembolism and can often derive substantially more benefit than harm from anticoagulation. Overall, it appears that TSOACs are associated with lower bleeding risks than warfarin for younger patients (RR = 0.79; 95% CI, 0.67-0.94), but this effect may be less pronounced in those >75 years of age (OR = 0.93; 95% CI, 0.74-1.17)32 A meta-analysis specifically examining older patients found that major bleeding occurred in 6.4% of TSOAC patients versus 6.3% of warfarin patients, which was not statistically significant, and there was no clear effect of type of agent (rivaroxaban: OR = 1.18; 95% CI, 0.64-2.19; apixaban: OR = 0.80; 95% CI, 0.43-1.51; dabigatran: OR = 1.07; 95% CI, 0.90-1.28).33 In contrast, a subgroup analysis found that, although the OR for bleeding was similar, apixaban showed lower odds of bleeding than warfarin even in older patients (OR = 0.65; 95% CI, 0.52-0.80), suggesting that it may be of particular value in this population.30
Renal insufficiency
Patients with renal insufficiency appear to be at increased major bleeding risk with anticoagulants.14 Among patients with mild renal insufficiency, the risk of major or clinically relevant bleeding appears lower with TSOACs than with warfarin (OR = 0.81; 95% CI, 0.72-0.90), with no significant difference between TSOACs.34 Those with moderate renal insufficiency are at even higher risk, with rates of major bleeding of 6.8% versus 4.8% in those with mild insufficiency. However, the apparent benefit of TSOACs was less obvious (OR for major bleeding = 0.82; 95% CI, 0.59-1.14). It is likely that this finding is due to smaller numbers of patients with moderate renal insufficiency. However, the point estimate is similar and the trend toward lower bleeding rates with TSOACs is relatively robust.
Dual-agent therapy
Some patients are at such increased thromboembolic risk that consideration is given to providing both an oral anticoagulant and antiplatelet therapy. This issue appears to be most common after an acute coronary syndrome (ACS). However, such an approach should be considered with caution because concomitant therapy increases major bleeding risk. Such risk can outweigh benefit, as was found in the APPRAISE-II trial of apixaban with antiplatelet therapy for ACS. This trial was halted early because any benefit was outweighed by major bleeding, which increased from 0.5% of patients receiving antiplatelet therapy alone to 1.3% of those receiving apixaban as well (HR = 2.59; 95% CI, 1.5-4.5).35 In addition, analyses of dabigatran trial data suggested that use of aspirin or nonsteroidal anti-inflammatory agents increased major bleeding risk.17 Although similar bleeding concerns were noted in the ATLAS ACS-2-TIMI-51 trial of rivaroxaban for ACS, major bleeding occurred in 0.6% of patients on antiplatelet therapy alone versus in 2.2% of those with rivaroxaban added (HR = 3.63; 95% CI, 1.7-7.6); the trial still found a net decrease in risk of cardiovascular death,36,37 so it is likely that careful patient selection can allow patients to derive more benefit than harm from combined TSOAC and antiplatelet use.
Drug levels
For the most part, unlike warfarin, no clinically available tools exist to detect drug levels of the TSOACs. However, it is likely that plasma concentration influences bleeding risk. A substudy of the RE-LY study of dabigatran for AF found that the risk of major bleeding increases with increasing trough serum levels of drug.38 There has been concern that this information was not made available in a timely manner to the public.39,40 Although it is likely that high serum concentrations of TSOACs, like high INR levels in warfarin patients, carry elevated bleeding risks, it has been consistently found that appropriate TSOAC use (without monitoring) carries equivalent or lower bleeding risk than warfarin use with monitoring. Furthermore, at this time, serum drug levels are not routinely available to clinicians nor are therapeutic drug target ranges established by prospective outcome studies.
Minimizing ICH risk
ICH is the most devastating complication of TSOAC therapy, and management of individual risk factors may help to mitigate this risk. Some modifiable factors include high levels of alcohol intake, drug use, and cigarette smoking.41-43 Perhaps counterintuitively, low cholesterol levels may actually increase ICH risk, but the benefit of cholesterol management in preventing cardiovascular disease often outweighs this risk.44 Finally, the most important modifiable factor is hypertension44 ; one randomized trial actually demonstrated that antihypertensive therapy can significantly lower the risk of ICH (HR = 0.44; 95% CI, 0.28-0.69). Therefore, all patients on anticoagulant therapy should be strongly considered for aggressive blood pressure management.
Summary
In recent years, several novel oral anticoagulants have been developed. A direct comparison of bleeding rates is difficult due to different bleeding definitions and the fact that each major trial used warfarin rather than another agent as the comparator. Nonetheless, it appears that the TSOACs have slightly lower rates of overall major bleeding and ICH in particular, but may carry slightly higher rates of GI bleeding. Clinicians seeking to minimize the risk of major bleeding should consider avoiding concomitant antiplatelet therapy, controlling ICH risk factors such as hypertension, and carefully weighing the risks and benefits in patients with renal insufficiency.
Disclosures
Conflict-of-interest disclosures: M.L. declares no competing financial interests. J.N.G. has received research funding from and has consulted for CSL Behring. Off-label drug use: None disclosed.
Correspondence
Joshua N. Goldstein, MD, PhD, Department of Emergency Medicine, Massachusetts General Hospital, Zero Emerson Place, Suite 3B, Boston, MA 02114; Phone: (617)724-3290; Fax: (617)724-0917; e-mail: jgoldstein@partners.org.