Abstract
Acute myeloid leukemia (AML) is a genetically heterogeneous clonal hematopoietic stem cell disorder and the majority of patients with AML die from their disease. The treatment paradigms for AML were developed decades ago and, although there have been improvements in the outcomes of selected younger patients and those with specific cytogenetic and molecular genetic characteristics, the overall survival for older patients remains dismal. Over the last few years, next-generation sequencing technologies have identified recurrent mutations in genes encoding proteins involved in the epigenetic regulation of transcription in most patients with AML. This discovery has led to new insights into the role of the epigenome in AML and opens the possibility of epigenetically targeted therapies. This chapter describes how epigenetic dysregulation plays a role in AML and highlights current and future treatment strategies that attempt to exploit epigenetic targets.
Learning Objectives
To be able to identify and describe the most important recurrent mutations in epigenetic regulatory genes in AML
To be able to describe clinical data on the use of hypomethylating agents in AML
To be able to discuss the potential role of epigenetic targeting in AML
Introduction
The title of this article contains 3 of the hottest terms in cancer medicine in 2014: “epigenetic,” “targeting,” and “personalized.” After decades of having limited understanding of pathogenesis, combined with a “one size fits a few” treatment approach using cytotoxic chemotherapy, clinicians, scientists, and patients who struggle with acute myeloid leukemia (AML) are anxious to learn whether this disease will finally be able to join the ranks of other spectacular success stories in the hematologic malignancies. AML is a genetically heterogeneous hematopoietic stem cell disorder characterized by impaired differentiation, clonal proliferation, accumulation of immature myeloid cells, and an aggressive clinical course. It is the most common acute leukemia in adults, with ∼19,000 cases expected in 2014 and a median age at diagnosis of 67 years.1 The prognosis of individual patients is determined by age, cytogenetic and molecular genetic abnormalities, and a host of clinical factors. Over the last few years, identification of gene mutations with known prognostic importance has allowed significant refinements in risk stratification, but therapeutic options are limited and the overall prognosis of the disease remains poor, with median 5-year survival of <10% for patients >60 years old. To date, the most effective anti-AML therapy is allogeneic stem cell transplantation, but even with reduced-intensity conditioning protocols and significant improvements in supportive care, this strategy is applicable to only a minority of patients in first or second remission and an even smaller minority with primary refractory disease.2 The classical “7 + 3” induction regimen of an anthracycline combined with infusional cytarabine has not been surpassed with respect to efficacy despite decades of clinical trials and a wide variety of novel agents. Lower-intensity induction regimens offer alternatives for patients unable to tolerate intensive chemotherapy, but generally yield lower rates of complete remission (CR).3 Finally, there is no effective treatment for relapsed AML.4
An explosion of technologic advances has led to transformative insights into the pathobiology of AML and renewed optimism that the Achilles heel of this devastating disease may now be known and targeted. Whole-genome and exome sequencing studies of somatic genetic alterations have identified recurrent mutations in genes encoding proteins involved in the epigenetic regulation of transcription in >70% of patients with AML.5 Epigenetic gene regulation involves modifications of DNA cytosine residues and posttranslational alterations of histone proteins via the biochemical processes of acetylation, methylation, phosphorylation, and others. Epigenetic modifications are critical for the differential expression of genes, defining cellular identity and the transformation of normal to malignant cells. Importantly, these modifications are heritable, dynamic, reversible, and occur without changes in the underlying DNA sequence. Recurrent mutations in the epigenetic modifier genes DNMT3A (DNA nucleotide methyltransferase 3A), TET2 (ten-eleven translocation 2), IDH1 and IDH2 (isocitrate dehydrogenase 1/2), ASXL1 (the addition of sex combs like 1), and MLL1 (mixed lineage leukemia 1) affect hematopoietic self-renewal and/or differentiation and contribute to myeloid transformation, but are typically not sufficient for leukemogenesis.6,7 It is believed that the epigenome plays an integral and targetable role in AML and its inherent plasticity opens the possibility of therapeutically reprogramming epigenetic modifications by targeting enzymes, transcription factors, and other proteins involved in the epigenetic machinery. The objective of this chapter is to describe how epigenetic dysregulation plays a role in AML and to highlight current and future treatment strategies that attempt to exploit epigenetic targets. The broader topic of personalized and targeted therapy in AML will also be discussed, with the objectives of defining the terms, describing their implications, and suggesting strategies for practical implementation.
Target: DNA methylation
DNMT3A mutations
DNA methylation is an essential component of epigenetic regulation and is generally associated with transcriptional silencing. Hypermethylation and transcriptional repression of a variety of tumor suppressor genes have been identified in acute leukemias and promote leukemogenesis via dysregulated processes of proliferation, differentiation, and survival.8 Data from DNA methylation profiling studies in myelodysplastic syndrome (MDS) suggest that aberrant methylation of tumor suppressor genes may be a dominant mechanism driving the progression of MDS to AML.9 Methylation of cytosine residues in the carbon 5 position of cytosine is catalyzed by DNA methyltransferases (DNMTs) and produces C5-methylcytosine (5-mC). Methylated CpG (cytosine-phosphate-guanine) dinucleotide sequences are recognized by proteins that bind to methylated DNA and promote recruitment of chromatin-remodeling proteins and histone-modifying enzymes, such as histone deacetylases (HDACs) and histone methyltransferases (HMTs). Genomic DNA methylation is established by DNMT3 as hemimethylated templates (de novo methylation) and the fully methylated pattern is maintained by DNMT1 (maintenance methylation). These processes result in distinct, heritable DNA methylation patterns that can discriminate between AML subtypes, predict prognosis, and potentially be used as predictive biomarkers for response to therapy.10 AML can be either globally hypomethylated or hypermethylated, suggesting that both overactivity and underactivity of epigenetic pathways may be important in leukemogenesis.11 Recent data have demonstrated that DNMT1 maintains DNA methylation patterns in leukemia stem cells (LSCs) and is required for their self-renewal.12 DNMT3a has been shown to play a critical role in hematopoietic stem cell self-renewal and differentiation.6 Importantly, loss-of-function somatic mutations in DNMT3A occur in >30% of cytogenetically normal AML and may be associated with poor clinical outcomes, but the impact of these mutations on DNA cytosine methylation and transcription in AML is unclear and loss of DNMT3A alone is insufficient for leukemogenesis.13,14 Most of the mutations involve the heterozygous substitution of arginine 882, which leads to decreased methyltransferase activity in vitro.15 To date, there are no therapies targeted specifically against DNMT3A. Therapeutic demethylation with DNMT inhibitors is discussed in the section “Epigenetically targeted therapeutics.”
TET2 mutations
The Ten-Eleven Translocation (TET) family of dioxygenases contributes to the regulation of DNA methylation by catalyzing the conversion of 5-mC to 5-hydroxymethylcytosine (5-hmC), which in turn blocks the binding of proteins that recognize methylated DNA and serves as a critical step in DNA demethylation.16,17 Loss-of-function mutations in TET2 are identified in ∼10% of patients with cytogenetically normal AML and are associated with inferior overall survival outcomes.18 TET2-deficient mice have increased hematopoietic stem cell renewal and develop proliferative myeloid malignancies similar to chronic myelomonocytic leukemia, but loss of TET2 alone does not result in AML.7
IDH1/2 mutations
DNA methylation is linked to citrate metabolism via the homodimeric enzymes IDH1/2, which catalyze the oxidative decarboxylation of isocitrate to α-ketoglutarate (α-KG). Gain-of-function mutations in IDH1/2 have been identified in 10%–30% of patients with cytogenetically normal AML and result in abnormal enzymatic function and increased production and accumulation of 2-hydroxyglutarate (2-HG), an “oncometablite” that results from reduction of α-KG to 2-HG.19 Both TET2 and the Jumonji-C domain-containing family of histone lysine demethylases are dependent on α-KG and can be impaired by IDH1/2 mutations and the accumulation of 2-HG, thus leading to increases in both DNA and histone methylation.20 Studies of the prognostic impact of IDH1/2 mutations have yielded conflicting results, possibly because of differences in the location of the mutation and/or the presence of concomitant mutations.21 For example, IDH2 mutations at the ARG140 residue are associated with a favorable prognosis22 and cytogenetically normal patients with both NPM1 and IDH1 or IDH2 mutations also have favorable outcomes, with a 3-year overall survival of 89% in a large randomized trial.22 Notably, IDH1/2 and TET2 mutations are mutually exclusive, but AML patients with these distinct mutations share similar methylation profiles.23
Target: histone modification
MLL mutations
The MLL gene on chromosome 11q23 encodes an H3K4 methyltransferase (HMT) that is involved in histone remodeling and affects HOX genes and Wnt signaling.24 In-frame partial tandem duplications of MLL occur in 5%–7% of de novo AML cases and are associated with unfavorable outcomes, especially in the setting of deregulated expression of the ecotropic viral integration 1 gene (EVI1).25 The MLL region is a frequent target for chromosomal translocations and rearrangements, which generate many different fusion proteins, several of which are oncogenic. MLL fusion proteins interact directly with several epigenetic modifiers, including the HMT DOT1L, which is responsible for catalyzing the methylation of H3K79. The interaction between MLL fusion proteins and DOT1L has been shown to drive leukemic progression.26
ASXL1 mutations
The polycomb-associated gene ASXL1 is involved in epigenetic regulation via association with the polycomb repressive complex 2 (PRC2), which mediates transcriptional repression of bound genes via trimethylation of histone 3 on lysine residue 27 (H3K27me3). Loss-of-function mutations in ASXL1 are found in 6%–30% of patients with AML and are associated with poor prognosis, advanced age, and a history of antecedent MDS.27 Mutations in ASXL1 result in loss of ASXL1 protein expression, decreased methylation of H3K27, and decreased recruitment of PRC2 to leukemogenic gene targets, resulting in decreased transcriptional repression of these genes.28
Readers, writers, and erasers
Epigenetic pathways also control gene expression by regulating the modification of core histone proteins via methylation, acetylation, phosphorylation, and other biochemical processes. Eukaryotic DNA is packaged around histones to form nucleosomes, the fundamental repeating units of chromatin. Posttranslational modifications alter histone-DNA interactions and cause temporal and spatial changes in histone structure that regulate the accessibility of transcription factors to their target genes. “Writers” and “erasers” govern the addition or removal of these modifications and protein domain “readers” recognize individual or combined modifications.29 Histone acetylation and methylation are reversible processes mediated by histone acetyltransferases, HDACs, HMTs, and histone demethylases. Histone acetylation is generally associated with gene expression, whereas deacetylation leads to transcriptional repression. The proteins of the bromodomain and extraterminal (BET) family contain bromodomain “readers” that bind acetylated lysine residues on the N-terminal tails of histones. BET proteins are involved in the transcriptional regulation of genes critical for leukemogenesis, including consistent down-regulation of BCL2, C-MYC, and IRF8.30 EZH2 (enhancer of zeste homolog 2) is a histone H3K27 methyltransferase and loss-of-function mutations in EZH2 have been identified in MDS and AML.31 Histone lysine methylation is a dynamic process that is regulated by lysine demethylase enzymes (KDMs). In particular, KDM1A/LSD1 is critical for maintaining expression of oncogenic gene programs and blocking differentiation in multiple subtypes of AML.32
Epigenetically targeted therapeutics (Table 1)
Clinical Trials of Epigenetic Therapies in AML from ClinicalTrials.gov, accessed May 2014

*DNMTi=DNA Methyltransferase Inhibitors.
**HDAC=Histone Deacetylase.
***HMT=Histone Methyltransferase.
****BET=Bromo and Extra Terminal.
ß: A=azacitidine, B=bortezomib, BR=brentuximab, C=cytarabine, CL=clofarabine, D=decitabine, DA=daunorubicin, E=etoposide, EN=entinostat, ER=erismodegib, GO=gemtuzumab ozogamicin, HI=hedgehog inhibitor, I=idarubicin, L=lenalidomide, M=mitoxantrone, MD=midostaurin, P=panobinostat, PR=pracinostat, R=rigosertib, RA=rapamycin, RBV=ribavirin, S= selinexor, SI=sirolimus, SO=sorafenib, T=temozolomide V=vosaroxin, VA= valproic acid, VO=volasertib, VOR=vorinostat.
Inhibition of DNMT
There are 2 DNMT inhibitors currently approved by the U.S. Food and Drug Administration (FDA), azacitidine (5-azacitidine or Vidaza) and its deoxy-derivative dectabine (5-aza-2′deoxycytidine). Although it is known that these agents, first developed in the 1960s, are cytosine analogs that inhibit DNMTs at low doses and incorporate into DNA (decitabine) or both DNA and RNA (azacitine) at higher doses, their mechanisms of action in patients are still not completely understood. Both drugs are approved for the treatment of MDS, but are also routinely used for AML based on results from several clinical trials.
In a multicenter phase 2 study, decitabine 135 mg/m2 total dose was administered IV over 72 hours every 6 weeks for up to 4 courses to 227 newly diagnosed patients with AML.33 Some patients also received all-trans retinoic acid during course 2 and/or a maintenance regimen of decitabine 20 mg/m2 for 3 consecutive days every 4–6 weeks. The overall response rate was 26% regardless of karyotype, with a median overall survival of 5.5 months and a 1-year survival of 28%.33 In another multicenter phase 2 study, decitabine 20 mg/m2/d administered IV for 5 consecutive days every 4 weeks resulted in a CR rate of 24% and an overall median survival of 7.7 months in 55 patients >60 years of age with AML (>20% BM blasts).34 Responses were seen in patients with poor prognosis cytogenetics and antecedent hematological disorders. The same dose and schedule of decitabine were compared with supportive care or low-dose cytarabine in a randomized phase 3 trial, which showed a significantly improved CR rate of 17.8% versus 7.8% for the control population (supportive care or low-dose cytarabine), but no statistically significant benefit in overall survival.35 A subsequent, unplanned analysis did show a survival benefit that reached statistical significance and the aggregate of the data convinced the European Medicines Agency, but not the FDA, to approve the use of decitabine for AML.36 Finally, decitabine 20 mg/m2 for 5 days was well-tolerated as an “epigenetic priming” agent before conventional induction with daunorubicin and cytarabine,37 and a randomized phase 2 trial of standard induction with and without decitabine priming is under way (www.ClinicalTrials.gov identifier #NCT01627041).
Subsequent single-arm, single-institution studies have increased the duration of treatment with decitabine to 10 days in similar patient populations and improved the CR rates to >40%, again including patients with poor-prognosis baseline features.38-40 In one study, 10 days of decitabine was combined with the CXCR4 inhibitor plerixafor in an effort to mobilize LSCs and enhance their sensitivity to decitabine.41 A randomized trial comparing 10 days of decitabine with and without bortezomib has completed accrual (www.ClinicalTrials.gov identifier #NCT01420926) and another comparing 5-day versus 10-day induction in older patients is ongoing (www.ClinicalTrials.gov identifier #NCT01786343). In all of the 10-day decitabine trials to date, responding patients and some with stable disease were treated with ongoing, approximately monthly maintenance cycles of decitabine, but the specific impact of these additional treatment cycles on remission duration or overall survival could not be assessed. A randomized trial of postremission therapy with decitabine versus observation, low-dose cytarabine or prolonged intensive chemotherapy demonstrated the feasibility of ongoing cycles of decitabine maintenance, but was terminated early and had too few patients to draw conclusions regarding efficacy.42 The feasibility of ongoing cycles of decitabine maintenance after allogeneic stem cell transplantation is also under investigation (www.ClinicalTrials.gov identifier #NCT00986804).
Azacitidine 75 mg/m2 administered subcutaneously for 7 days in 28-day cycles resulted in a statistically significant improvement in overall survival compared with conventional care regimens (best supportive care, low-dose cytarabine, or intensive chemotherapy) in patients with high-risk MDS, including those with 20%–30% BM blasts, who would now be classified as AML according to the guidelines of the World Health Organization.43 The improvement in survival was achieved despite a CR rate of only 18%, which was not statistically different from that of the control group (16%). Secondary end points such as transfusion requirements, use of IV antibiotics, and days of hospitalization also favored the azacitidine group.43 In France, 149 newly diagnosed older patients with AML were treated with the same dose and schedule of azacitidine on a compassionate use protocol, with an overall response rate of 33%, CR or CR with incomplete platelet recovery rates of 23%, and an overall survival of 9.4 months.44 A smaller study of 35 untreated AML patients treated with azacitidine on an Italian compassionate use protocol showed similar results, with 31% CRs and an overall survival of 9 months.45 As with decitabine, most responding patients in the AML trials received ongoing treatment cycles with azacitidine until progression or toxicity. Maintenance treatment with azacitidine has also been shown to be feasible and possibly effective in reducing or delaying relapses after intensive AML induction chemotherapy46 and after allogeneic stem cell transplantation.47
Inhibition of HDAC
HDAC inhibitors alone have shown limited clinical activity in MDS and AML, but combinations with DNMT inhibitors have been widely anticipated to act synergistically via enhanced release of transcriptional repression. Multiple HDAC inhibitors, including valproic acid, mocetinostat, panobinostat, vorinostat, and others, have been evaluated in combination with azacitidine or decitabine and many clinical trials are still ongoing, but the results so far have been disappointing. For example, the addition of entinostat to azacitidine in a randomized phase 2 trial of 149 patients with MDS and AML failed to improve responses.48 Importantly, in this trial, the 2 agents were administered simultaneously, whereas previous in vitro work suggested that optimal synergy was achieved with sequential administration first of the DNMT inhibitor, followed by the HDAC inhibitor. Etinostat is a potent cell cycle inhibitor and may have inhibited incorporation of azacitidine, resulting in suboptimal promoter demethylation.48
Inhibition of IDH1/2
Early-phase clinical trials of small-molecule inhibitors targeting mutant IDH1/2 enzymes are ongoing for AML patients with IDH1/2 mutations. The inhibitors are believed to decrease production of 2-HG, induce demethylation of H3K9me3, and increase expression of genes associated with differentiation.49
Inhibition of protein methyltransferases
Enzymatic activity of the DOT1L HMT has been shown to be a driver of leukemogenesis in MLL-rearranged leukemia. Inhibition of DOT1L results in decreased H3K79 methylation and MLL-fusion target gene expression and a potent, highly selective DOT1L inhibitor has entered clinical trials.50 Potent and selective inhibitors of the HMT EZH2 are also under development.
Inhibition of lysine acetylation readers
Bromodomain-containing proteins recognize lysine residues on histone tails and these interactions can be targeted by small-molecule inhibition. BET inhibitors, several of which are in clinical trials, have been shown to inhibit leukemia and stem and progenitor cell proliferation and can block MLL-mediated transformation.51
Inhibition of lysine demethylases
KDM1A/LSD1 inhibitors are effective against AML cell lines and primary human cells in vitro, especially in combination with HDAC inhibitors and all-trans retinoic acid. Although the in vitro effects were highest in MLL leukemias, other subtypes of AML and other myeloid malignancies were also sensitive, and clinical trials in AML and MDS are under development.32
Challenges in developing epigenetically targeted therapy in AML
What to target?
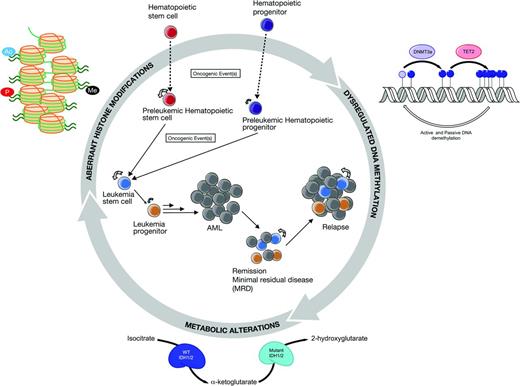
AML is driven by an array of abnormalities and the disease itself probably has too many “targets” to be “targetable” by a single agent, including dysregulated epigenetic pathways, gene mutations, surface markers, signaling pathways, LSCs, the BM microenvironment, and the list goes on. The situation has become even more complex recently because studies of the clonal architecture of AML have demonstrated the clonal heterogeneity that evolves with disease progression and relapse.52 Therefore, the therapeutic targets in AML may be “moving targets” that require different agents at different times. For example, the LSC, blast, and progenitor subpopulations at the time of a patient's initial diagnosis are likely to be genetically and epigenetically distinct from each other and also from the clones that survive and evolve after chemotherapy (Figure 1). Furthermore, whole-genome and capture-based deep sequencing studies of the clonal architecture of primary AML samples have also demonstrated significant functional heterogeneity in genetically defined subclones and LSCs.53 The challenge is to identify and eradicate all clones, rather than to apply selective pressure onto a predominant population using an approach that is too narrowly targeted.
Complex interplay between aberrant epigenetic processes and leukomogenesis. Constructed in collaboration with Monica Guzman and Eric Sturgill.
Complex interplay between aberrant epigenetic processes and leukomogenesis. Constructed in collaboration with Monica Guzman and Eric Sturgill.
Which patients to target?
This is a highly complex, but absolutely critical question. Patients with AML can be divided into a variety of subgroups, with advantages and disadvantages for each grouping. Historically, patients have been divided by age between those younger and older than 60 years. This arbitrary division was mostly meant to divide patients into those who could and could not tolerate intensive chemotherapy, but with modern supportive care strategies and the increasing “fitness” of older patients, it has become clear that numerical age alone is inadequate to guide treatment decisions. Nevertheless, age remains an important prognostic factor and age-based decision making has been standard in AML clinical trials for decades. Clinicians (and regulatory authorities) are accustomed to interpreting efficacy data for novel therapies by comparing historical data from age-based subgroups. Therefore, it is likely that age will continue to be a part of AML clinical trials until a well-tolerated, “magic-bullet” agent or regimen emerges to erase its relevance.
AML patients can also be “targeted” by prognosis. Early phase trials of novel agents are frequently conducted in relapsed/refractory patients, but there has been a distressing pattern of drug development in AML in which novel agents are tested in this population, fail to show an efficacy signal (notwithstanding that efficacy should not really be evaluated in phase 1 studies), and then are withdrawn from further development. Relapsed/refractory AML patients are biologically and clinically different from those with newly diagnosed disease and, for the sake of both the drug and the patient, it is important to get new drugs into untreated patients. Newly diagnosed patients who have poor outcomes with standard therapies can be defined using increasingly complex risk stratification systems that incorporate cytogenetics, molecular genetics, and, more recently, epigenetic data. For example, one study identified 7 genes with high methylation levels in differentially methylated regions and low expression that correlated with better clinical outcomes independently of age.54 Other investigators have defined an epigenetic signature of healthy hematopoietic stem cell commitment that is an independent predictor of poor overall survival in AML.55 Newly diagnosed patients who are expected to have a poor prognosis with standard treatments can and should clearly be “targeted” for clinical trials.
It would be ideal to target patient subgroups based on the predicted biology of their disease, combined with the predicted biology of a particular epigenetic modifier, but this is not straightforward. For example, although azanucleosides are known to inhibit DNMT, it is unclear to what extent this plays a role in their efficacy and, to date, efforts to use methylation patterns to predict responders or guide treatment have been unsuccessful.56 Conversely, DNMT3A mutations seem to correlate with favorable responses to decitabine in AML.57 To lump or to split? If we select narrow subgroups for clinical trials based on assumptions about disease biology, accrual will be challenging and we risk missing responses in patients whose disease might have been “correct” for the trial for unpredictable reasons. However, if we give a truly targeted drug to an unselected group of patients, we may fail to see the benefit of the drug unless the study population is specifically enriched for the subgroup predicted to respond.
When to target?
AML therapy is generally divided into induction, consolidation, and postremission strategies. Despite the observation that azanucleosides may modestly improve overall survival in AML in the absence of complete remission (CR), this end point should not be abandoned as a therapeutic goal because it is strongly correlated with both survival and quality of life. Because epigenetically targeted drugs are likely to work better in combination with other agents than alone, it is frustrating not to have widely accepted “backbone” induction regimens onto which new drugs can be added. For example, the Medical Research Council in the United Kingdom has defined a specific regimen of low-dose cytarabine as its standard induction for older patients with AML and an ongoing clinical trial adds novel agents to this backbone in a “Pick-A-Winner” design.58 Because DNMT inhibitors may work well with other epigenetic therapies, the United States could consider deciding that azacitidine 75 mg/m2 × 7 days and decitabine 20 mg/m2 × 10 days are, for now, its standard induction therapies for AML patients, irrespective of age, who cannot tolerate intensive chemotherapy. Similarly, because no intensive chemotherapy regimen is meaningfully better than 7 days of infusional cytarabine and 3 days of idarubicin, quibbling over distinctions without a difference should stop and this regimen could be adopted as the backbone regimen for patients undergoing intensive induction. New agents could then be added to these regimens or evaluated as single agents, and patients not treated on clinical trials would receive 1 of the 3 standard regimens at the discretion of their treating physicians. This would provide an ongoing, real-world “control” group by trying to eliminate unnecessary heterogeneity in induction approaches.
Regardless of which drugs are used for induction, it is likely that postinduction residual leukemic populations are biologically different from the untreated disease. Therefore, it is counterintuitive to administer repeated cycles of the same treatment, as is often current practice. There may be an important role for epigenetically targeted therapies in controlling evolving leukemic clones in the postremission setting, both as postchemotherapy and posttransplant maintenance. Because these compounds are, so far, largely noncytotoxic drugs, they may have a better chance of succeeding in the absence of fulminant disease. Significantly increased participation of AML patients in clinical trials will be required to demonstrate the efficacy of new drugs in these settings.
How and when to assess efficacy?
Epigenetically targeted therapies may require prolonged administration, perhaps weeks or months, to show efficacy. For example, CRs to DNMT inhibitors are often not seen for several months. Furthermore, standard morphology and immunophenotype-based assessments will probably prove to be inadequate for these agents. Efforts to identify biomarkers that predict response and improve disease monitoring are under way using DNA methylation signatures, gene expression profiles, mass spectrometry, and other techniques, but there are still no epigenetic biomarkers in routine clinical practice.
Combinations and endless permutations
Although the clinical activity of single-agent azacitidine and decitabine in AML is undisputed, there is plenty of room for improvement and many more questions than answers remain regarding their optimal dose, schedule, combination partners, and duration of administration, as seen by the numerous trials listed in Table 1. Furthermore, in the absence of an obvious, spectacular success, the utility of multiple small, single-arm, single-institution, phase 1 and 2 studies in answering these questions is unclear. Finally, there are many examples of the investigational agents that are predicted to work well together, for example, HDAC inhibitors and bromodomain inhibitors, but logistics often become convoluted or impossible when multiple pharmaceutical companies are involved in the same trial.
Conclusions
The identification of recurrent somatic mutations in epigenetic modifiers in patients with AML has unleashed a flood of discoveries about the critical and complex role of epigenetics in the biology of the disease. Epigenetically targeted therapeutics are already changing clinical practice and there is much optimism that matching an individual patient's disease pathophysiology with a specific, targeted therapy can and will become a reality. However, there are many significant challenges to this ideal, most importantly the highly heterogeneous nature of both AML biology and AML patients. Creative clinical trials with robust correlative scientific studies and massive efforts to increase physician and patient participation in AML clinical trials are required to ensure that the results lead to “personalized medicine,” not “anecdotal medicine.”
Disclosures
Conflict-of-interest disclosure: The author has consulted for Celgene, Glaxo SmithKline, Astra Zeneca, Sunesis, Novartis, Teva Oncology, Agios, and Astex. Off-label drug use: Mention of several different early-phase investigational compounds.
Correspondence
Gail J. Roboz, MD, The Leukemia Program, Weill Cornell Medical College, New York Presbyterian Hospital, 520 East 70th St, New York, NY 10021; Phone: (646)962-2700; Fax: (646)962-0115; e-mail: gar2001@med.cornell.edu.