Abstract
Assisted reproductive technology is widely used to treat couples affected by infertility. Complications associated with assisted reproduction include venous thromboembolism, ovarian hyperstimulation syndrome, and recurrent implantation failure. It has also been proposed that thrombophilia may be associated with an increased likelihood of these events. Although data are limited, antithrombotic therapy is frequently used to enhance the likelihood of successful assisted reproduction. This chapter reviews the risks of venous and arterial thromboembolism associated with assisted reproduction, as well as available data regarding the impact of thrombophilia on the risks of thromboembolism and failure of implantation. The role of antithrombotic therapy in reducing the likelihood of these events, along with recommendations from various guidelines, are also discussed.
Learning Objectives
To discuss the available data regarding the role of thrombophilia testing in women undergoing assisted reproduction
To describe the state of the evidence with respect to the use of antithrombotic therapy in women undergoing assisted reproduction
Introduction
Assisted reproductive technology, which refers to all treatments or procedures involving in vitro handling of human oocytes and sperm or embryos for the purpose of achieving pregnancy, is widely used to treat the ∼1 in 6 couples affected by infertility.1,2 Techniques used include not only in vitro fertilization, but also intracytoplasmic sperm injection and, much less commonly, gamete intrafallopian transfer, zygote intrafallopian transfer, donor egg or embryo transfer, and surrogacy. These procedures are usually paired with controlled pharmacologic stimulation of ovarian follicles using gonadotropins and gonadotropin-releasing hormones.2
Failure of assisted reproduction
Even with transfer of good-quality embryos, implantation failure is common in patients undergoing assisted reproduction. Overall, only ∼30%-40% of cycles result in clinical pregnancy3,4 and even women <35 years of age have a clinical pregnancy rate per cycle of <50%.2,3,5 Factors that affect the outcome of assisted reproduction include ages of the partners, reason for infertility, type of assisted reproduction technique used, number of oocytes retrieved, quality of the embryos transferred, ease of embryo transfer, and endometrial receptivity.4
Thrombophilia and failure of assisted reproduction
Little is known about the mechanisms responsible for the high frequency of assisted reproduction failure. Theoretically, failure can result from unsuccessful implantation, failed placentation, and/or compromise of early embryonic development (including that of the vasculature). There are no standard criteria for recurrent implantation failure. Proposed definitions include failure of >3 high-quality embryo transfers or failure with transfer of at least 10 embryos in multiple transfers.6
Studies evaluating the relationship between thrombophilia and failure of assisted reproduction have provided inconsistent results. It has been proposed that thrombophilia leads to an increased risk of microthrombosis at the implantation site, impairing initial invasion of maternal vessels by the syncytiotrophoblast.6 A systematic review addressing this issue reported on 6092 patients in 33 studies (23 evaluating antiphospholipid antibodies, 5 studying inherited thrombophilias, and 5 including both).7 Most were case-control studies and the overall methodologic quality was thought to be poor because only a few of the case-control studies used a representative control and consecutive patient sampling was used in <1/2 of the cohort studies, several of which were retrospective. Pooled data from 8 case-control studies showed a 3-fold increased risk of assisted reproduction failure in patients with the factor V Leiden mutation (Table 1); however, an analysis of 3 cohort studies found no significant association. None of the other inherited thrombophilic abnormalities (prothrombin gene mutation, antithrombin deficiency, protein C deficiency, or protein S deficiency) were associated with an increased risk of failure. Although in case-control studies, the presence of one or more antiphospholipid antibodies was associated with a 3-fold higher risk of failure of assisted reproduction, in cohort studies, antiphospholipid antibodies were not associated with a lower risk of positive pregnancy test or live birth (Table 1).
Association between thrombophilia and failure of assisted reproduction

OR indicates odds ratio; and RR, risk ratio.
Data are from Di Nisio et al.7
Although the results of this meta-analysis suggest that women with failure of assisted reproduction are more often positive for the factor V Leiden mutation and antiphospholipid antibodies, these results were not confirmed in a meta-analysis of cohort studies.7 Case-control studies are often limited by incomplete or poor collection of data on potential confounders, as well as differential participation in which more severe cases are recruited. As a result, strengths of association may be overestimated. Although prospective cohort studies may limit these potential biases, they are often underpowered to exclude small but clinically significant associations.7 Reassuringly, a prospective cohort study of 510 women requiring in vitro fertilization published after the above meta-analysis8 also showed no statistically significant differences in the frequencies of implantation, clinical pregnancy, and live birth in women who did and did not carry the factor V Leiden or prothrombin gene mutations. Therefore, at this time, the existence of a definitive relationship between thrombophilia and failure of assisted reproduction remains unproven, although additional large prospective studies are required to definitively disprove an association.
Antithrombotic therapy to enhance the likelihood of success in women undergoing assisted reproduction
Various strategies have been used in an effort to improve pregnancy outcomes in patients with patients with implantation failure, including the use of adjuvant antithrombotic therapy (Table 2). It has been hypothesized that low-dose aspirin might have a positive effect on the success of assisted reproduction by increasing uterine and ovarian blood flow, thereby enhancing implantation and ovarian response to stimulation.9-11 Individual studies have had inconsistent results and the role of aspirin in women undergoing assisted reproduction has remained controversial. Two recent meta-analyses examined this issue.12,13 Randomized controlled studies comparing aspirin with placebo or no treatment in women undergoing in vitro fertilization or intracytoplasmic sperm injection were eligible for both; however, the inclusion and exclusion criteria, as well as the analysis methods, differed between the 2 systematic reviews. The timing, dose, and duration of aspirin use varied between individual studies. In some, aspirin was started at the time of in vitro fertilization or intracytoplasmic sperm injection, whereas, in others, it was started around the time of embryo transfer. Aspirin was continued throughout pregnancy in some studies. In others, it was continued until between weeks 9 and 12 or until laboratory or ultrasonographic confirmation of pregnancy or failure to achieve pregnancy. Neither meta-analysis provided data on bleeding risks in the 2 treatment groups; these data were inconsistently and incompletely documented in the individual studies included in these systematic reviews. Both meta-analyses concluded that there was no good evidence that aspirin improved live birth rate compared with placebo or no treatment.12,13 Although one systematic review showed a small but statistically significant increase in pregnancy rate in women randomized to low-dose aspirin, this difference was not maintained when high-quality studies alone were analyzed.13 Therefore, at this time, the routine use of aspirin in patients undergoing assisted reproduction cannot be recommended. High-quality studies in selected groups of patients may be warranted.
Results of meta-analyses examining the use of antithrombotic therapy in patients undergoing assisted reproduction
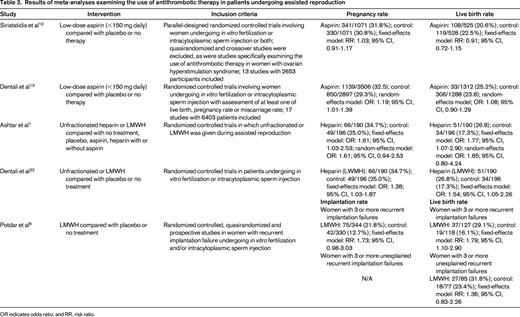
OR indicates odds ratio; and RR, risk ratio.
Heparin might improve implantation rates, not only by reducing the risk of implantation site microthrombosis, but also by improving endometrial receptivity and decidualization of endometrial stromal cells, as well as trophoblast adhesion and invasiveness.1,6,14 These non-antithrombotic effects may result from heparin-associated increases in production of prolactin and insulin-like growth factor, inhibition of production of insulin-like growth-factor binding protein, regulation of heparin-binding epidermal growth factor, interaction with cytokines and matrix metalloproteinases, and reduction in the expression of the adhesion molecule E-cadherin.1,6,15
The effect of unfractionated heparin (with aspirin therapy) on in vitro fertilization outcome has been evaluated in several studies, with inconsistent results.16-21 Most of these studies enrolled women with antiphospholipid or other auto-antibodies and were observational in nature.16,18-21 However, one single-center double-blind randomized crossover trial that compared unfractionated heparin 5000 units subcutaneously twice daily and aspirin from the day of embryo transfer with negative pregnancy test or week 14 of pregnancy with placebo in women with recurrent implantation failure and at least one auto-antibody (antiphospholipid antibody or antinuclear antibody) reported no significant difference in pregnancy or implantation rates between treated and placebo cycles (Table 3).17
Randomized and quasirandomized trials evaluating unfractionated heparin and LMWH on in vitro fertilization outcomes
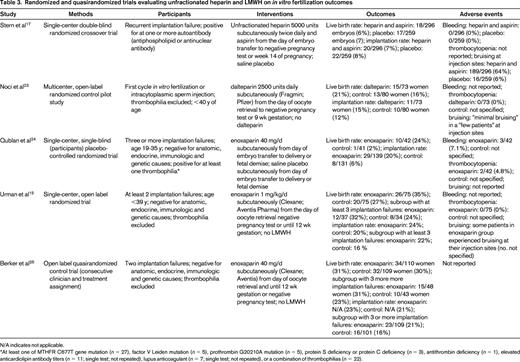
N/A indicates not applicable.
*At least one of MTHFR C677T gene mutation (n = 27), factor V Leiden mutation (n = 5), prothrombin G20210A mutation (n = 5), protein S deficiency or protein C deficiency (n = 3), antithrombin deficiency (n = 1), elevated anticardiolipin antibody titers (n = 11; single test; not repeated), lupus anticoagulant (n = 7; single test; not repeated), or a combination of thrombophilias (n = 22).
The results of 3 recent meta-analyses that investigated whether low-molecular-weight heparin (LMWH) administered around the time of implantation improves clinical outcomes in women undergoing assisted reproduction are shown in Table 2.1,6,22 The characteristics of the individual studies are summarized in Table 3. All 3 meta-analyses used life birth rate per woman as an outcome. Implantation rate (the number of sacs seen per number of embryos transferred) was also determined in one meta-analysis,6 whereas the other 2 calculated pregnancy rate.1,22 Drug-related side effects were also captured in 2 of the meta-analyses.1,6
Two meta-analyses included 3 randomized trials involving 386 women.1,15,22-24 Peri-implantation LMWH administration was associated with improvement in live birth rate compared with placebo or no LMWH; however, the results were sensitive to the statistical methods used.1,22 When a random-effects model, rather than a fixed-effects model, was used; the results were no longer statistically significant.
The third meta-analysis focused exclusively on women with recurrent implantation failure.6 Two randomized trials15,24 and one quasirandomized trial25 met the inclusion criteria. The investigators reported a significant improvement in live birth rate with LMWH therapy in women with a history of 3 or more implantation failures.6 However, if the one study that required the presence of a laboratory thrombophilia24 was excluded; there was only a nonsignificant trend toward improvement. The implantation rate showed a nonsignificant trend toward improvement.6
Although bruising at injection sites, bleeding, thrombocytopenia, and allergic reactions were noted in patients in patients who received LMWH, neither meta-analysis that collected this information conducted a formal comparison of the frequency of these outcomes between the 2 treatment groups1,6 ; however, 1 review commented that the side effects were comparable in both intervention and control groups.6
The authors of all meta-analyses noted that the individual studies were generally small, of low quality, and were highly heterogeneous in terms of inclusion criteria and intervention.1,6,22 The resultant quality of evidence for the main findings was rated as very low using GRADE criteria.1 Therefore, it is not clear whether unfractionated heparin or LMWH may have a beneficial impact in patients undergoing assisted reproduction. Well-designed, adequately powered, randomized trials to assess the efficacy and safety of peri-implantation heparin in improving outcomes after assisted reproduction are required before this intervention should considered for routine clinical use.
Thrombophilia and ovarian hyperstimulation syndrome
Ovarian hyperstimulation syndrome is an exaggerated response to ovulation induction therapy that occurs in approximately 1/3 of treatment cycles.26 Although symptoms may begin as soon as 24 hours after gonadotropin administration, they usually become most pronounced 7-10 days later.26 Based on clinical features at presentation, this syndrome is classified as mild, moderate, severe, or critical.27-30 Most cases are mild and are associated with mild abdominal pain, abdominal bloating, fluid retention, and ovarian size usually <8 cm. These symptoms are worse in women with moderate ovarian hyperstimulation syndrome, who may also have nausea (with or without vomiting), ultrasound evidence of ascites, and ovarian size 8-12 cm. Severe ovarian hyperstimulation syndrome occurs in 1%–2% of cycles and is also characterized by clinical ascites (occasionally with pleural effusions), oliguria, respiratory distress, hemoconcentration (with a hematocrit of >45%), and markedly enlarged ovaries >12 cm.27-30 Alternative novel techniques for ovulation induction may reduce these risks and complications associated with ovarian hyperstimulation syndrome,31 although though this requires confirmation in larger studies.
The cause of overian hyperstimulation syndrome is incompletely understood; however, it is thought to arise from vasoactive peptides released from hyperstimulated ovaries26,29 that increase vascular permeability, leading to fluid shifts from intravascular to third space compartments.26 In one case-control study, 17 of 20 patients (85%) of patients with severe ovarian hyperstimulation syndrome tested positive for the presence of an inherited or acquired thrombophilia compared with 11 of 41 controls (27%),32 suggesting that thrombophilia was associated with an increased risk of this complication. Thrombophilia may have been overdiagnosed because blood samples were obtained while the patient was symptomatic for severe ovarian stimulation syndrome or during the luteal phase of the treatment cycle. The high frequency of decreased levels of antithrombin and protein S, as well as antiphospholipid positivity, compared with carriage for the factor V Leiden mutation suggests that this may be the case because the results of non-genetic-based assays may be affected in this clinical setting. Two subsequent case-control studies that addressed some of the first study's limitations did not find an increased prevalence of thrombophilia in women with severe ovarian hyperstimulation syndrome.33,34 Given the available data and the fact that severe hyperstimulation syndrome occurs so infrequently that the predictive value of any positive thrombophilia test would be very low, there is no role for thrombophilia testing to influence antithrombotic therapy for the prevention of hyperstimulation in women undergoing ovarian stimulation.35
Venous thromboembolism and assisted reproduction
Assisted reproduction appears to be associated with an increased risk of venous thromboembolism (VTE). Two large retrospective series of patients undergoing in vitro fertilization reported that thrombosis complicated 0.1% [95% confidence interval (CI), 0%–0.3%)36 and 0.3% (95% CI, 0%–0.8%)37 of cycles. A population-based cohort study of all 964 532 inpatient deliveries in Sweden between 1999 and 2008 reported an antepartum incidence of VTE of 0.27% in women receiving in vitro fertilization compared with 0.1% in the background population (odds ratio = 2.7; 95% CI, 2.1%–3.6%).38 The greatest risk of in vitro fertilization-related VTE was seen in the first trimester (0.17% compared with 0.02% in the general population; odds ratio, 9.8; 95% CI, 7.5%–14.3%).38 There was no statistically significant increase in venous thromboembolic risk associated with in vitro fertilization in the second or third trimester or in the postpartum period.38 Women conceiving with frozen embryos were not at increased risk of VTE, presumably due to less frequent or absent ovulation induction.38 A subsequent cross-sectional study that also used Swedish national registry data but controlled for confounders including age, calendar year of delivery, body mass index, parity, smoking, marital status, education, and country of birth and also used outpatient data provided slightly lower risk estimates, but confirmed an increased risk of VTE in pregnancies after in vitro fertilization [hazard ratio (HR) = 1.77; 95% CI, 1.41-2.23), especially during the first trimester (HR = 4.05; 95% CI, 2.54-6.46).39 Although the majority of events were deep vein thrombosis, the risk of pulmonary embolism was similarly increased (overall HR = 1.42; 95% CI, 0.86-2.36; for first trimester events, HR = 6.97; 95% CI, 2.21-21.96).39 These data echo those from a hospital-based case-control study that demonstrated a 4-fold increase in antenatal VTE with assisted reproductive technology for singleton pregnancies and a 6-fold incidence in twin pregnancies, but no statistically significant association with postpartum VTE.40 A Danish cohort study that used data from a national in vitro fertilization register reported a 0.2% risk of VTE in singleton pregnancies and a 0.3% risk in multiple pregnancies.41 Compared with 805 464 pregnancies recorded in the Danish National Patient Registry over the same time frame, pregnancies achieved with in vitro fertilization were associated with a 3-fold increase in VTE (incidence rate ratio = 3.0; 95% CI, 2.1-4.3).41 Interestingly, in this study, venous thromboembolic risk was increased similarly during all 3 trimesters and, in multiple pregnancies only, during the postpartum period as well.39 Therefore, in vitro fertilization appears to be a risk factor for antepartum thromboembolism; however, the overall absolute incidence of symptomatic thrombosis appears to be low.
Risk factors for and mechanisms behind VTE associated with assisted reproduction
In a review of thrombosis associated with assisted reproductive technology, Chan et al identified 61 reports of venous thrombosis (of which 49 cases involved thrombosis of the veins of the neck and arm) and 35 reports describing arterial events.42 Ovarian hyperstimulation syndrome was reported in 90% of arterial cases and in 78% of venous events. In 98% of cases, thrombosis occurred after ovulation induction. Venous events were delayed compared with those involving the arterial circulation (42.4 days and 10.7 days after embryo transfer, respectively).42
The mechanism behind the increased frequency of VTE associated with assisted reproduction remains unknown.27 The high estrogen levels associated with ovarian stimulation may induce a procoagulant effect by increasing levels of coagulation factors such as von Willebrand factor, factor VIII, factor V, and fibrinogen while decreasing levels of the anticoagulants protein S and antithrombin.43 However, the clinical relevance of these changes is unclear because most variables remain within the normal range.43 It has been suggested that the increased frequency of upper extremity thrombosis seen in these patients may result from drainage of increased peritoneal fluid with inflammatory properties through the thoracic duct into the subclavian vein, with resultant down-regulation of local thrombomodulin and up-regulation of tissue factor, thereby promoting thrombosis.38,44 It has also been proposed that fluid-filled branchial cysts developing close to the jugular or subclavian veins in patients with ovarian hyperstimulation syndrome might impair circulation.38,45 Data regarding the risk of VTE in women with thrombophilia or prior VTE who undergo assisted reproduction are lacking.
Ovarian hyperstimulation syndrome has been associated with an increased risk of first trimester thrombosis and incidences of 1.7% in admitted patients38 and 4.1% (95% CI, 1.1%–13.7%) in severe cases37 have been reported. Intravascular fluid depletion, increased blood viscosity,26 and immobilization may explain the additional VTE risk seen in these patients.
Thrombosis prophylaxis in assisted reproduction
Studies that address the impact of thrombosis prophylaxis in assisted reproduction have important limitations and the number of patients who have received anticoagulants is too small to draw any conclusions about safety and efficacy.15,17,23-25,46 Whether bleeding risks are increased in this population with antithrombotic prophylaxis is uncertain.47
There have been no randomized trials demonstrating that prophylactic anticoagulation prevents VTE in patients with severe ovarian hyperstimulation syndrome. However, based on the risk estimates above and the generally low risks of bleeding associated with prophylactic LMWH in pregnancy, several guidelines recommend short-term prophylaxis in these patients (Table 4).26,43,47-49
Summary of Guideline Recommendations Related to Thrombophilia Testing and Thrombosis Prophylaxis in Patients undergoing Assisted Reproduction
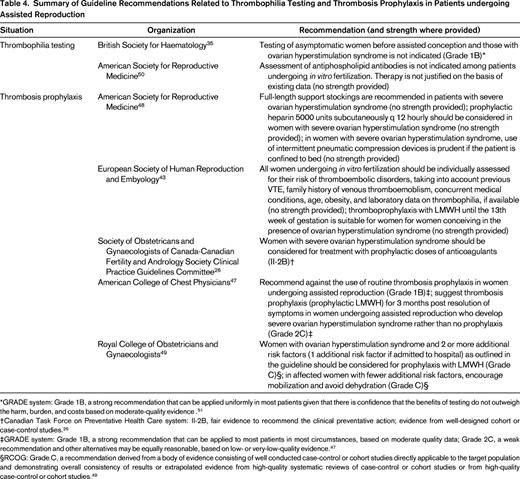
*GRADE system: Grade 1B, a strong recommendation that can be applied uniformly in most patients given that there is confidence that the benefits of testing do not outweigh the harm, burden, and costs based on moderate-quality evidence .51
†Canadian Task Force on Preventative Health Care system: II-2B, fair evidence to recommend the clinical preventative action; evidence from well-designed cohort or case-control studies.26
‡GRADE system: Grade 1B, a strong recommendation that can be applied to most patients in most circumstances, based on moderate quality data; Grade 2C, a weak recommendation and other alternatives may be equally reasonable, based on low- or very-low-quality evidence.47
§RCOG: Grade C, a recommendation derived from a body of evidence consisting of well conducted case-control or cohort studies directly applicable to the target population and demonstrating overall consistency of results or extrapolated evidence from high-quality systematic reviews of case-control or cohort studies or from high-quality case-control or cohort studies.49
Given the low baseline risk of VTE associated with assisted reproduction, women with low-risk thrombophilias or prior VTE associated with major transient risk factors will receive only very small benefit from prophylaxis.47 Those with higher-risk thrombophilias or unprovoked or hormone-associated VTE are more likely to benefit.47
Dosage and duration of thromboprophylaxis during and after assisted reproductive therapy has not been well studied. It has been suggested that prophylactic heparin and LMWH should not be given 12-24 hours before oocyte retrieval and for at least 6-12 hours afterward to reduce the risk of bleeding.14
Approach to thrombophilia testing and thrombosis prophylaxis in patients undergoing assisted reproduction
Women undergoing assisted reproduction are often prepared to undertake any measure to improve their chances of a live birth. Although health care providers should provide couples with every opportunity to achieve a successful pregnancy, it is essential that recommended treatments do actually provide benefit. There is no comprehensive set of guidelines addressing thromboembolic issues in patients undergoing assisted reproduction. However, various societies have issued individual recommendations relevant to this patient population.35,43,47-50 These are summarized in Table 4.
My approach to thrombophilia testing and the use of antithrombotic therapy in patients undergoing assisted reproduction is consistent with the available guideline recommendations. I do not perform thrombophilia testing in or provide antithrombotic therapy to women with recurrent implantation failure. I initiate prophylactic LMWH in women with severe ovarian hyperstimulation syndrome and continue prophylaxis for approximately 3 months after resolution of their symptoms. Women with less severe hyperstimulation syndrome and additional risk factors for VTE (eg, admission to hospital) are candidates for prophylaxis until their additional risks resolve. I suggest LMWH prophylaxis during ovarian stimulation in women with prior unprovoked or hormone-related VTE and in those with higher-risk thrombophilias (eg, homozygosity for the factor V Leiden mutation or prothrombin mutation) or lower-risk thrombophilias and a strong family history of venous thrombosis. However, given the lack of clinical outcome data in this setting, I accept that women may decline prophylaxis if they are averse to subcutaneous injections and/or comfortable with modest risk of developing deep vein thrombosis or pulmonary embolism. I do not generally recommend prophylaxis to women with a prior VTE event associated with a major transient risk factor that has resolved or a low-risk thrombophilia (eg, heterozygosity for the factor V Leiden or prothrombin gene mutation) unless it is clear that they indicate a strong preference for prophylaxis because they are afraid of recurrent VTE and understand that the benefit obtained from intervening with prophylaxis may be very small.
Disclosures
Conflict-of-interest disclosure: The author has received honoraria from Leo Pharma and Pfizer Canada. Off-label drug use: LMWH and aspirin use to increase the success of assisted reproduction would be considered off-label usage.
Correspondence
Dr. Shannon M. Bates, Department of Medicine, McMaster University, 1280 Main Street West, Room HSC 3W11, Hamilton, Ontario L8S 4K1, Canada; Phone: (905)521-2100, ext. 73928; Fax: (905)521-4997; e-mail: batesm@mcmaster.ca.
