Abstract
A 78-year-old female presents to the emergency department with a traumatic hip fracture. Her past medical history is significant for atrial fibrillation for which she receives rivaroxaban 20 mg daily. Her dose was last taken 12 hours ago. Routine bloodwork conducted in the emergency department shows prothrombin time, international normalized ratio, and activated partial thromboplastin time within the normal range, and estimated glomerular filtration rate of 50 mL/min/1.73 m2 (normal is >90 mL/min/1.73 m2) You are asked by the surgical team to confirm that it is safe to proceed with surgery at this time using neuraxial anesthesia.
Learning Objective
To evaluate existing evidence for the effect of rivaroxaban on routine coagulation tests (prothrombin time, international normalized ratio, and activated partial thromboplastin time)
Introduction
Rivaroxaban is a direct oral anticoagulant that achieves its anticoagulant effect through reversible inhibition of factor Xa.1 After oral administration, peak plasma levels are achieved within 2-4 hours.2,3 Rivaroxaban has a half-life of 6-9 hours in healthy adults and 12-13 hours in healthy elderly subjects.2-5 It is partially excreted by the kidneys (∼36%) and its levels may be affected by inhibitors and inducers of the P-glycoprotein transport protein and CYP3A4 enzyme.5-7 The expected steady-state peak and trough concentrations for patients taking 20 mg of rivaroxaban daily are 270-274 ng/mL and 25-30 ng/mL, respectively.8,9
Unlike vitamin K antagonists, rivaroxaban is administered in fixed doses and does not require laboratory monitoring of anticoagulant effect. However, assessment of the amount of rivaroxaban present in a patient may be desirable in certain clinical situations such as during a major bleeding event or at the time of an urgent invasive procedure.
To evaluate current best evidence for the effect of rivaroxaban on routine coagulation tests [prothrombin time (PT)/international normalized ratio (INR), activated partial thromboplastin time (aPTT)], we conducted a search of MEDLINE from inception to September 10, 2014 using the following keyword search terms and medical subject headings (MeSH) headings: (rivaroxaban OR factor Xa/antagonists and inhibitors) AND (blood coagulation tests/administration and dosage, drug effects, methods OR blood coagulation/drug effects OR prothrombin time OR activated partial thromboplastin time OR laboratory monitoring OR laboratory measurement). Studies were eligible for inclusion if they reported the relationship between rivaroxaban levels in human plasma and PT/INR or aPTT coagulation assays. Pediatric studies, animal studies, abstracts, articles lacking original data, and non-English studies were excluded.
The search yielded 592 articles, of which 14 articles met eligibility criteria (Table 1). Rivaroxaban concentrations in the evaluated samples ranged from 0 to >1000 ng/mL. Samples included normal plasma spiked in vitro with rivaroxaban (n = 9), ex vivo plasma from rivaroxaban-treated patients (n = 3), and plasma from healthy volunteers receiving rivaroxaban (n = 2).
Studies evaluating the effect of rivaroxaban on PT or aPTT
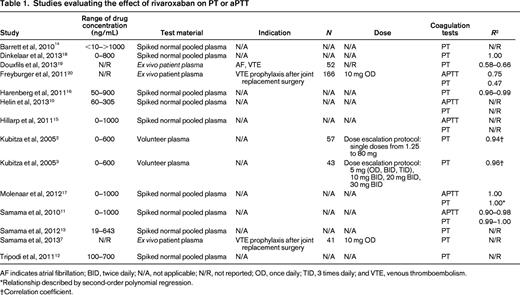
AF indicates atrial fibrillation; BID, twice daily; N/A, not applicable; N/R, not reported; OD, once daily; TID, 3 times daily; and VTE, venous thromboembolism.
*Relationship described by second-order polynomial regression.
†Correlation coefficient.
PT/INR
The effect of rivaroxaban on the PT was evaluated in 14 studies. Only 6 studies reported a correlation coefficient (R2) to describe the relationship between rivaroxaban levels and the PT, which varied from 0.47 to 1.00 (Table 1). Correlation coefficients appeared lower in ex vivo patient samples (0.47-0.66) compared with spiked normal human plasma (0.99-1.00). In plasma from patients receiving rivaroxaban and plasma spiked with rivaroxaban there was a linear, concentration-dependent prolongation of the PT. However, in patients receiving rivaroxaban, the effect on PT was modest and variable, with lower concentrations approximating trough levels (41-60 ng/mL) and higher concentrations approximating peak levels (219-305 ng/mL), which increased the PT by 6%–19% and 50%–135%, respectively.10-13 There was significant variability with respect to assay sensitivity depending on the thromboplastin reagent used.12-16 Use of an international sensitivity index (ISI) specific for rivaroxaban reduced interassay variability, whereas conversion to an INR used for monitoring vitamin K antagonist (VKA) therapy increased variability and demonstrated reduced rivaroxaban responsiveness.10-12,14,16 The instrumentation and method of data analysis for determining the international sensitivity index of thromboplastin reagents for rivaroxaban also appear to affect variability between PT reagents.16
aPTT
The effect of rivaroxaban on the aPTT was evaluated in 5 studies (Table 1). Although rivaroxaban prolonged the aPTT in a dose-dependent manner, the relationship between rivaroxaban concentration and aPTT was nonlinear.10,15,17 Furthermore, there were conflicting results between studies with regard to the concentration ranges over which nonlinearity was most pronounced. Three studies reported correlation coefficients using rivaroxaban-spiked normal human plasma (n = 2) and plasma from rivaroxaban-treated patients (n = 1), which ranged from 0.75 to 1.00. Similar to the PT, correlation coefficients appeared lower with plasma from rivaroxaban-treated patients (0.75) compared with rivaroxaban-spiked plasma (0.90-1.00). There was significant variability in results by reagents used and between individual laboratories.10,15,17 In one study, the aPTT assay was insensitive at low drug levels (25 ng/mL).15
Taken together, these results demonstrate that rivaroxaban can prolong the PT, but assay results vary markedly with different thromboplastin reagents. Assay methodology, including data analysis, and instrumentation may also affect results. Because some PT assays demonstrate low sensitivity, a normal PT does not rule out the presence of clinically significant rivaroxaban concentrations; however, a prolonged PT provides a qualitative indication of drug presence. Conversion of PT results to an INR used for VKA monitoring reduced sensitivity and increased variability, making it unsuitable for assessment of rivaroxaban anticoagulant effect. Finally, the nonlinear relationship of aPTT with rivaroxaban concentration, poor sensitivity, and significant variability between reagents makes it unsuitable for assessing the presence of rivaroxaban.
Our results suggest that dose-response studies using calibration standards should be conducted by individual laboratories to determine the sensitivity of PT assays to rivaroxaban, with results communicated to clinicians. In addition, results should be interpreted by practicing physicians with attention to timing of last administration, advanced age, renal insufficiency, and concomitant medications, which are known to alter the expected concentration of drug present in circulation. In the setting described in this case, we would recommend against the use of the neuraxial anesthesia 12 hours after a dose of rivaroxaban because significant amounts of drug are likely to be present given the predicted half-life of rivaroxaban, the patient's age and renal function, and the potential insensitivity of the PT/INR assay to detect clinically significant rivaroxaban concentrations (grade 2C).
Disclosures
Conflict-of-interest disclosures: The authors declare no competing financial interests. Off-label drug use: None disclosed.
Correspondence
Dr. Barbara A. Konkle, Director, Clinical and Translational Research, Medical Director, Hemostasis Reference Laboratory, Puget Sound Blood Center, 921 Terry Ave., Seattle, WA 98104; Phone: (206)689-6191; Fax: (206)292-8030; e-mail: barbarak@psbc.org.
