Abstract
Excess iron deposition in vital organs is the main cause of morbidity and mortality in patients affected by β-thalassemia and hereditary hemochromatosis. In both disorders, inappropriately low levels of the liver hormone hepcidin are responsible for the increased iron absorption, leading to toxic iron accumulation in many organs. Several studies have shown that targeting iron absorption could be beneficial in reducing or preventing iron overload in these 2 disorders, with promising preclinical data. New approaches target Tmprss6, the main suppressor of hepcidin expression, or use minihepcidins, small peptide hepcidin agonists. Additional strategies in β-thalassemia are showing beneficial effects in ameliorating ineffective erythropoiesis and anemia. Due to the suppressive nature of the erythropoiesis on hepcidin expression, these approaches are also showing beneficial effects on iron metabolism. The goal of this review is to discuss the major factors controlling iron metabolism and erythropoiesis and to discuss potential novel therapeutic approaches to reduce or prevent iron overload in these 2 disorders and ameliorate anemia in β-thalassemia.
Learning Objectives
To provide an overview of the factors that control iron metabolism and erythropoiesis under normal conditions and in disorders associated with iron overload due to hyperabsorption of dietary iron (hereditary hemochromatosis) or increased iron absorption with aberrant erythropoiesis (mainly-thalassemia)
To describe new potential therapeutics that have the potential to modify and improve the clinical management of these disorders
Iron metabolism and erythropoiesis
Iron is one of the most important trace elements in biology, but it has an an ambivalent role. It is involved in important cellular mechanisms such as host defense and erythropoiesis, but in excess it can be extremely harmful.1 Because there are no mechanisms for active excretion of iron from the body, iron metabolism needs to be tightly controlled to prevent toxicity. In humans, the majority of the iron is recycled from senescent RBCs or stored in the liver and just a small part is introduced from the diet. Dietary iron is absorbed at the brush border of duodenal enterocytes. Iron is transported across the apical membrane by divalent metal transporter 1 (DMT1)2 and is exported from enterocytes and other cells by ferroportin (FPN1), the only know iron exporter in mammals. Transferrin (TF), a high-affinity iron-binding protein, is responsible for the transport of circulating iron to sites of utilization or storage. The master regulator of iron homeostasis is hepcidin (HAMP), a 25-aa peptide secreted predominately by the liver in response to iron stores and plasma iron concentration.3 Hepcidin triggers the degradation of FPN1, which is expressed prevalently in duodenal enterocytes, macrophages, and hepatocytes, controlling, respectively, iron absorption, recycling, and storage.4
In normal conditions, HAMP expression is directly related to iron stores and plasma concentration, controlling the homeostatic requirement of this metal for physiological functions. High iron concentration causes increased levels of hepcidin, in turn reducing the release of iron from enterocytes and macrophages into the bloodstream.1 In contrast, in the presence of low iron concentrations, hepcidin levels are reduced, promoting iron absorption and release of iron into the bloodstream.1 HAMP expression can be also increased in response to inflammatory stimuli or decreased by florid erythropoiesis even in the presence of iron overload.5-8
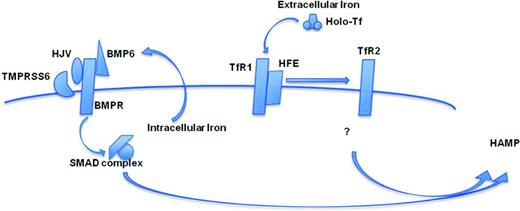
HAMP regulation by iron requires at least 2 major pathways, which are controlled by extracellular and intracellular iron. Holo-TF and cell membrane molecules expressed in the liver, such as HFE, TfR1, and TfR2, mostly regulate the expression of HAMP by sensing the relative concentration of extracellular iron (Figure 1). It has been proposed that, in the presence of increased concentrations of holo-TF, the relative interaction of HFE with TfR2 or TfR1 triggers HAMP expression (Figure 1).1 However, the downstream intracellular mechanisms are not yet certain (Figure 1). In contrast, BMP6 plays a major role in regulating the expression of HAMP by intracellular iron (Figure 1). BMP6, together with BMP receptors (BMPRs), activate the transcription complex SMAD1/5/8-SMAD4 and HAMP expression.9 For the full activation of the SMAD pathway, the BMPR coreceptor hemojuvelin (HJV) is necessary. HJV's activity is balanced by the transmembrane protease serine 6 (TMPRSS6) that cleaves HJV from the cell surface, negatively modulating HAMP expression.9-12 The fundamental antagonistic roles of HJV and TMPRSS6 on hepcidin's activities are reinforced by the observation that patients and mice with mutations affecting 1 of these 2 genes are affected by juvenile hemochromatosis and iron-refractory iron deficiency anemia, respectively.10,13
Graphic representation of the interaction of the factors controlling hepcidin synthesis described in the text.
Graphic representation of the interaction of the factors controlling hepcidin synthesis described in the text.
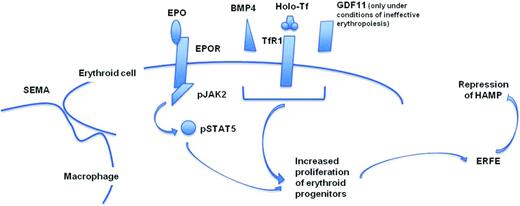
Normal erythropoiesis is a dynamic multistep process, during which erythroid progenitors proliferate and differentiate giving rise to enucleated RBCs. The key molecule is the kidney hormone erythropoietin (EPO), which, after association with its receptor (EPOR), leads to the activation of the cytoplasmic Janus kinase-2 (JAK2; Figure 2).14 Phosphorylated JAK2 activates STAT5, which translocates into the nucleus, activating genes that are involved in differentiation, proliferation, and survival of erythroid progenitors.14 EPO's regulation of erythropoiesis is mediated by hypoxia-inducible factor 2α (HIF2α) in a process that responds to cellular hypoxia.15 Under certain conditions, such as at high altitude or after acute blood loss, there is inadequate tissue oxygenation. In this case, the erythropoietic activity expands significantly, a process commonly known as “stress erythropoiesis,” and erythroid development might extend in extramedullary sites such as the liver and spleen, with massive production of erythroid progenitors and RBCs. Stress erythropoiesis requires several factors to be sustained, such as increased production of EPO and iron supply to newly forming erythroid cells (Figure 2). In mice, increased iron absorption triggered by stress erythropoiesis is mediated by the erythroid factor erythroferron (Erfe), which is secreted by the erythroid progenitors cells and suppresses Hamp in the liver (Figure 2).16 Therefore, as the number of erythroid progenitors increases, the production of Erfe rises and the level of hepcidin decreases (Figure 2). Additional mechanisms play an important role in stress erythropoiesis.15 One of these mechanisms involves the action of bone morphogenic protein 4 (BMP4), which induces (through SMAD5) the proliferation of stress erythroid progenitors (Figure 2).17 Macrophages also have a central role in supporting normal and stress erythropoiesis (Figure 2).18 Erythropoiesis occurs in the bone marrow in niches called erythroblastic islands.18 These can be visualized as a central macrophage surrounded by erythroblasts at different stages of maturation. In mice, depletion of macrophages severely impairs stress erythropoiesis and the recovery from anemia triggered by phlebotomy.18 The erythroid response to Epo is also severely impaired by macrophage depletion, even in the presence of iron supply.18 Upon depletion of macrophages, the number of erythroblasts is significantly reduced, both in steady-state and stress erythropoiesis, suggesting that these central macrophages have a supporting role in erythropoiesis, which takes place through direct cellular interactions.18 Although the cellular molecules involved in this interplay remain unclear, there are several candidates that may be associated with the macrophage–erythroblast crosstalk. Several adhesion molecules are under scrutiny, such as integrin α4β1, which is expressed on developing erythroid progenitor cells and might interact with Vcam-1, present on macrophages.19 Furthermore, focal adhesion kinase (Fak), downstream of the integrin signaling pathway, has been associated with the stress erythropoiesis, probably through the activity of the Epo/Stat5 pathway.20 In summary, this new mechanism has been named “stress erythroid macrophage activity” (SEMA) and emphasizes the importance of the macrophages in modulating the proliferation and differentiation of the erythroid progenitor cells in stress erythropoiesis.18
Graphic representation of some of the factors required to support stress erythropoiesis and modulate hepcidin synthesis in the liver. GDF11 has been associated with the increased proliferation and decreased differentiation observed in ineffective erythropoiesis.
Graphic representation of some of the factors required to support stress erythropoiesis and modulate hepcidin synthesis in the liver. GDF11 has been associated with the increased proliferation and decreased differentiation observed in ineffective erythropoiesis.
Disorders of iron overload: primary (hemochromatosis) and secondary (β-thalassemia)
Dysregulation in iron metabolism associated with low levels of hepcidin can lead to some of the most common disorders characterized by iron overload, including hereditary hemochromatosis (HH) and β-thalassemias. Iron overload can lead to severe organ damage. HH are heterogeneous genetic diseases that result in inappropriately low levels of hepcidin synthesis and, eventually, iron overload. The most common form of HH is associated with mutations in the gene HFE, which has a low penetrance and is normally treated with phlebotomy.21 However, if HFE-related hemochromatosis is left untreated, it may cause iron overload, resulting in liver fibrosis, cirrhosis, congestive cardiomyopathy, and diabetes.21
β-Thalassemias are inherited genetic disorders caused by different mutations in the β-globin gene or its promoter.22,23 These mutations lead to a partial or complete lack of β-globin synthesis with consequent ineffective erythropoiesis (IE) and anemia.22,23
IE is a process in which erythroid precursors undergo apoptosis before completing the maturation process into RBCs.22,23 Responsible for this premature death in β-thalassemia is the imbalanced synthesis of α- and β-chains that leads to the formation of inclusion bodies (hemichromes). They ultimately lead to the formation of reactive oxygen species that induce oxidative stress and death in the RBCs and progenitors.22,23 In β-thalassemia, anemia is associated with increased serum EPO and increased numbers of erythroid progenitors as a reaction from the body that tries to compensate for the reduced capacity of the blood to transport oxygen.22,23 β-Thalassemia can be defined as chronic stress erythropoiesis in which erythropoiesis is ineffective.22,23 In mice, the increased erythropoiesis is associated with high levels of Erfe, causing suppression of Hamp.16 IE exacerbates progressively over time, leading to a more severe anemia and extramedullary hematopoiesis and worsening of the iron overload.22,23
Patients with the most severe form of the disease, also called β-thalassemia major or Cooley's anemia, require lifelong transfusions to treat severe anemia and suppress IE.22,23 However, chronic blood transfusions can cause massive iron overload in the absence of iron chelation. Moreover, suppression of IE mediated by blood transfusion is often incomplete and patients might develop extramedullary hematopoiesis, splenomegaly, and increased intestinal absorption.22,23 Patients affected by a milder form of the disease, β-thalassemia intermedia (also called non-transfusion-dependent thalassemia), usually do not require blood transfusion but develop iron overload due to increased iron absorption from the gastrointestinal tract.22,23
Rationale to target abnormal iron absorption and ineffective erythropoiesis
Excess of iron deposition in organs of patients affected by HH and β-thalassemia is one of the main causes of morbidity and mortality.21-23 Recent studies have been focusing on the molecules involved in iron metabolism and erythropoiesis to develop new strategies for the management of iron overload in both HH and β-thalassemia and, eventually, to improve erythropoiesis in the latter disease.
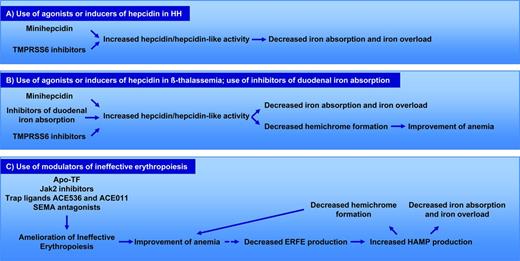
Because iron overload is associated with low levels of hepcidin, several studies have suggested that use of hepcidin agonists, modulators of HAMP expression, or duodenal iron absorption could represent alternative strategies to reduce or prevent iron overload in HH (Figure 3A) and in β-thalassemia (Figure 3B).22,23 In the latter disease, targeting iron metabolism could be beneficial not only in reducing iron overload, but also in improving IE (Figure 3B). In fact, reduced iron absorption limits TF saturation, decreasing erythroid iron intake, heme synthesis, and the formation of hemichromes. This eventually could improve IE and anemia (Figure 3B). Conversely, agents that improve IE could be beneficial not only to ameliorate the anemia, but also to reduce the component of chronic stress erythropoiesis that suppresses HAMP (Figure 3C). In other words, as IE and the anemia improve, it is expected that fewer erythroid progenitors will be generated, leading to the reduction in the production of ERFE, increased synthesis of hepcidin, and amelioration of inappropriate iron absorption (Figure 3C). Some of experimental observations supporting these models are documented in several studies, as described in the next section.
Mechanisms of action of MH and TMPRSS inhibitors in HH and β-thalassemia. (A) Description of potential mechanisms of action and benefits by the use of TMPRSS6 inhibitors or minihepcidins in HH. (B) Description of potential mechanisms of action and benefits by the use of TMPRSS6 inhibitors or minihepcidins in β-thalassemia. (C) Description of potential mechanisms of action and benefits by the use of modulators of ineffective erythropoiesis in β-thalassemia. The broken line indicates a correlation that has not been demonstrated yet.
Mechanisms of action of MH and TMPRSS inhibitors in HH and β-thalassemia. (A) Description of potential mechanisms of action and benefits by the use of TMPRSS6 inhibitors or minihepcidins in HH. (B) Description of potential mechanisms of action and benefits by the use of TMPRSS6 inhibitors or minihepcidins in β-thalassemia. (C) Description of potential mechanisms of action and benefits by the use of modulators of ineffective erythropoiesis in β-thalassemia. The broken line indicates a correlation that has not been demonstrated yet.
Targeting iron absorption: experimental observations and preclinical data
Previous studies in mice have indicated that excessive hepcidin synthesis is detrimental to iron absorption and erythropoiesis.24 Conversely, more recent studies have indicated that moderate increases in Hamp expression or administration of hepcidin agonists could avoid excessive iron absorption in models of HH and β-thalassemia, highlighting the fact that a beneficial effect would depend upon the level of hepcidin activity attained.25-27
Due to the important role of TMPRSS6 in suppressing HAMP, whether increased expression of Hamp observed in Tmprss6−/− mice could be beneficial in animals affected by HH has been investigated. Indeed, the absence of Tmprss6 in a mouse model of Hfe-mediated HH (Tmprss6−/−Hfe−/−) exhibited amelioration of iron overload.28 Additional compounds that mimic hepcidin's activity can also be used for this purpose. These compounds, called minihepcidins, are short peptides mimetic of hepcidin that are synthetized based on the sequence of endogenous hepcidin that bind ferroportin, thereby promoting its internalization and degradation.26 Minihepcidins have been studied in mouse models of severe HH caused by complete lack of hepcidin expression (Hamp−/−), as well as in mice affected by Hfe-mediated HH.26,27 These peptides have demonstrated a beneficial effect on reducing iron absorption and inducing redistribution of iron from different organs into splenic macrophages with mobilization of iron from the liver and heart.26,27
Mice showing a β-thalassemia intermedia phenotype, characterized by IE, anemia, extramedullary hematopoiesis, splenomegaly, bone abnormalities, and iron overload, were kept on a low-iron diet or crossed with animals with genetically altered synthesis of hepcidin.25,29 In each case, decreased iron intake mediated by dietary means or increased levels of hepcidin improved hepatic and splenic iron concentration, together with amelioration of anemia, extramedullary hematopoiesis, and splenomegaly.25 Mice affected by β-thalassemia intermedia (Hbbth3+) were crossed with Tmprss6−/− animals, generating Tmprss6−/−Hbbth3+ animals.30 In these mice, loss of the most important HAMP suppressor led to improved anemia, IE, and splenomegaly compared with the Hbbth3+ mice.30 This was also associated with an increase in hepcidin levels and marked reduction of iron stores to levels similar or lower than wild-type animals. Mean corpuscular volume and mean corpuscular hemoglobin were also reduced in thalassemic mice lacking Tmprss6, probably as a result of reduced heme synthesis in erythroblasts, an index of reduction in iron availability.30
Based on these initial observations, it has been investigated whether pharmacological inhibition of Tmprss6 could have a beneficial effect on iron-overloaded animals affected by HH and β-thalassemia intermedia by 2 different approaches. Targeting Tmprss6 expression pharmacologically using LNP-formulated siRNA or RNaseH-mediated antisense oligonucleotide activity (Tmprss6-ASO) increased Hamp expression in mouse models of HH and β-thalassemia intermedia, leading to the reduction of serum iron and liver iron overload in both models.31,32 In addition, the treatment improved anemia and reduced splenomegaly (by limiting iron availability for erythropoiesis) in the β-thalassemia mice.
All of these data confirm that manipulation of HAMP expression can be an effective treatment for human diseases associate with iron dysregulation. This new approach could be used together or as an alternative to traditional therapies (phlebotomy or chelation). In particular, suppression of Tmprss6 in mouse models of HH mice reduced iron overload with only minimal effects on the RBC and hemoglobin levels, indicating that a lower dose of these agents could achieve a reduction in iron absorption and iron overload after phlebotomy without an effect on erythropoiesis. In β-thalassemia, these approaches also led to a significant improvement of anemia, IE, extramedullary hematopoiesis, and a significant reduction of spleen size associated with improved spleen architecture. Similar results were observed using minihepcidins, showing that these molecules represent a valid therapeutic approach with a disease modifier's end point.33 In both cases, thalassemic mice also showed significant reduction in serum iron, TF saturation, reactive oxygen species, and hemichrome formation, confirming that these approaches might be able to interrupt the cycle leading to the worsening of IE in this disorder. Furthermore, these approaches may be superior to phlebotomy for HH and iron chelators for β-thalassemia because they are only effective in inducing a temporary reduction of iron levels but do not target the underlying pathophysiology that is responsible for the increased iron absorption, the inappropriately low level of HAMP expression.
Alternatively, intestinal iron absorption could be reduced, targeting molecules responsible for controlling iron absorption in the duodenum. In mice, intestinal Hif2α, under hypoxia conditions, controls expression of Dmt1 in enterocytes.34 These proteins, together with Fpn1, are essential for the excess of iron observed in organs of mice affected by β-thalassemia.29,34 Indeed, thalassemic mice had significant improvement in tissue iron levels and anemia after disruption of intestinal Hif2α, suggesting that this, together with duodenal Dmt1 and Fpn1, may be novel therapeutic targets in this disorder.34
Targeting ineffective erythropoiesis: experimental observations and preclinical data
Erythroid iron uptake is mediated by the uptake of holo-TF by TfR1. Under normal circumstances, TfR1 has a greater affinity for holo-TF than monoferric-TF. However, this affinity diminishes as the iron supply decreases.1 Because monoferric-TF is the predominant form of TF in circulation when TF saturation is lowered, each molecule of monoferric-TF will deliver less iron to erythroid precursors than holo-TF.1 Therefore, this will lead a large number of erythroid precursors to receive a smaller quantity of iron than in normal circumstances. If this is detrimental in normal balanced globin synthesis, it might reduce the production of hemichromes in β-thalassemia (Figure 3B). In fact, administration of apo-TF to animals affected by β-thalassemia intermedia showed amelioration of IE, anemia, and restored appropriate Hamp expression, which in turn decreased parenchymal iron overload.35
The short life span and inefficient oxygen-carrying ability of the abnormal erythrocytes in β-thalassemia causes chronic anemia that stimulates erythropoietic activity and results in chronic stress erythropoiesis. This suggests the existence of an autocrine amplification loop of the erythroid progenitors responsible for extramedullary hematopoiesis, splenomegaly, and, over time, exacerbation of the anemia and iron overload.
In mice, the hypoxia and consequent increased synthesis of Epo in β-thalassemia is associated with a physiological increase of Jak2 activity.36 This suggests that Jak2 inhibition may be an excellent therapeutic strategy, especially if administration of these inhibitors might be used in an acute setting, such as to reverse splenomegaly. Indeed, Jak2 inhibition has demonstrated successful reduction of IE and splenomegaly in β-thalassemic mice.36 Amelioration of IE could, in principle, also reduce iron absorption. Therefore, JAK2 inhibition might have positive clinical consequences in β-thalassemia patients in whom excessive IE, causing iron overload and splenomegaly, are severe problems.1
It has also been shown that chronic stress erythropoiesis, and the consequent overproliferation of erythroid progenitors in β-thalassemic mice, is modulated by SEMA. Upon depletion of macrophages in these animals, the number of erythroblasts was significantly reduced, with major improvement of IE, anemia, iron metabolism, and splenomegaly.18 This was also associated with normalization of the ratio between proliferation and differentiation of erythroid progenitor cells, suggesting that IE is not only characterized by cell death, but is also a progressive expansion of the erythron mediated by a decreased ability of the erythroid progenitors to differentiate. However, depletion of macrophages is detrimental to the innate immune system and is potentially associated with a greater risk of opportunistic infections. For this reason, macrophage depletion may not represent a valid clinical approach. However, specifically interrupting the interaction between macrophages and erythroblasts, therefore sparing the immunological function of macrophages, may be a very feasible approach to limiting chronic stress erythropoiesis. Ongoing studies to more fully characterize the molecules involved macrophage–erythroblast interactions and to identify new mediators involved in this mechanism may lead to new therapies aimed at limiting SEMA and, in turn, reducing or preventing chronic stress erythropoiesis in β-thalassemia.
Additional studies support the notion that modulation of IE, in particular limiting the proliferation and improving the ability of erythroid progenitors to differentiate, can be beneficial in β-thalassemia. In mice, this has been underscored by studies using 2 different drugs, RAP-011 and RAP-536, ligand traps based on the extracellular domains of the activin receptor IIA (ActRIIA) and ActRIIB, respectively. Complexes containing ActRIIA, ActRIIB, or Tgfβ type II receptor regulate gene expression primarily by activating the Smad2/3 subfamily of intracellular effectors.37,38 Overactivation of the Smad2/3 complex has been associated with an abnormal ratio between immature and mature erythroblasts, leading to expansion of the erythron and abortive erythroid precursor maturation.37,38 Both RAP-011 and RAP-536 inhibit Smad2/3 signaling in erythroid cells. In particular, RAP-011 acts by targeting Gdf11, which is up-regulated in thalassemic erythroid cells and is associated with increased Smad2/3 activation.37,38 Ultimately, these 2 drugs corrected the abnormal ratio between immature and mature erythroblasts in animal models of β-thalassemia intermedia, with amelioration of IE, anemia, iron overload, splenomegaly, and bone pathology.37,38 In conclusion, targeting mechanisms of IE or iron absorption may trigger a beneficial and synergistic loop that will culminate in amelioration of erythropoiesis and iron overload.
Future directions
New agents that decrease iron absorption have the potential to improve the management of iron overload in both HH and β-thalassemia intermedia by limiting excessive iron absorption. It is also conceivable that the combination of these agents to conventional iron chelation might be beneficial in accelerating the unloading of organs when iron deposition is already severe. This might be the case in some patients affected by HH, but, most likely, by β-thalassemia intermedia, as indicated by some preliminary observations.39 Additional agents that modulate IE could also be beneficial in β-thalassemia intermedia. These drugs might improve RBC synthesis and, eventually, iron overload.
For individuals affected by β-thalassemia major, iron overload is primarily the result of repeated transfusion. However, the levels of hepcidin concentrations in these patients are still low, suggesting that iron absorption is abnormally elevated.40 Therefore, these agents might block the unnecessary iron absorbed from the duodenum and, in combination with iron chelation, improve the management of iron overload. In addition, in the presence of blood transfusion, they might further suppress the IE and prevent or reverse the splenomegaly. This could decrease transfusion volume and frequency and result in significant less transfusional iron burden. Therefore, also in β-thalassemia major, these drugs have the potential to transform the management of this disease.
In conclusion, the focus of many investigators is to identify new mechanisms and strategies to prevent or reverse iron overload and target IE. Hopefully, these investigations will lead to new and improved therapeutics, advancing the management of these disorders and improving the quality of life of the affected patients.
Disclosures
Conflict-of-interest disclosures: S.S. has received research funding from Bayer Pharmaceuticals, Isis Pharmaceuticals, and Merganser Pharmaceuticals; has consulted for Bayer Pharmaceuticals and Isis Pharmaceuticals; and has equity ownership in Merganser Pharmaceuticals. C.C. has received research funding from Isis Pharmaceuticals and Merganser Biotech LLC. Off-label drug use: None disclosed.
Correspondence
Stefano Rivella, Department of Pediatrics, Weil Medical College, Cornell University, Belfer Research Bldg, 413 East 69th St, Rm 1202, Box 284, New York, NY 10021. Phone: 646-962-6248; Fax: 646-962-0574; e-mail: str2010@med.cornell.edu.