Abstract
Acute myeloid leukemia (AML) is primarily a disease of the elderly and the numbers of these patients are increasing. Patients ≥60 years of age continue to have poor prognosis. Preliminary results suggest benefit from reduced-intensity allogeneic hematopoietic cell transplantation (HCT) in selected patients 60-80 years of age. However, although patients in this age range comprise >50% of those with AML, they currently constitute only 17% of those offered HCT. In the absence of prospective randomized studies comparing HCT and chemotherapy, the decision to recommend HCT rests on retrospective analyses of the risks of relapse and nonrelapse mortality after each approach. There is strong evidence that pre-HCT comorbidities can predict HCT-related morbidity and mortality. Age alone does not appear predictive and, particularly if the risk of relapse with chemotherapy is high, should not be the sole basis for deciding against HCT. Use of geriatric assessment tools, inflammatory biomarkers, and genetic polymorphism data may further aid in predicting nonrelapse mortality after HCT. Disease status and pretreatment cytogenetics with FLT3-TID, NPM-1, and CEBP-α status are the main factors predicting relapse and these are likely to be supplemented by incorporation of other molecular markers and the level of minimal residual disease after chemotherapy. HLA-matched related and unrelated donor grafts seem preferable to those from other donor sources. Donor age is of no clear significance. Models combining comorbidities with AML risk factors are useful in risk assessment before HCT. In this chapter, we integrated information on AML-specific, HCT-specific, and patient-specific risk factors into a risk-adapted approach to guide decisions about HCT versus no HCT.
Learning Objectives
Age per se is not a barrier to RIC followed by allogeneic HCT in patients 60-80 years of age.
Health status measures, particularly comorbidity indices, should be routinely assessed pre-HCT because they significantly affect risks for NRM.
An adapted-risk approach combining AML disease status, cytogenetics, and molecular markers, together with comorbidities and other patient risk factors, is proposed for decision making about allogeneic HCT for older patients.
Introduction
Acute myeloid leukemia (AML) is the most common form of acute leukemia and is responsible for the largest number of annual deaths from leukemia in the United States.1 In 2013, an estimated 14 590 individuals in the United States developed AML and 10 370 individuals died from the disease.2 AML most commonly affects older individuals, with a median age at diagnosis of 66 years.3 There is an anticipated continued expansion of the elderly population in the United States, together with a continued increase in incidence of AML in that population.4 This is problematic because the prognosis of AML in older patients remains very poor. For example, a recent retrospective evaluation of 2444 older patients who received induction chemotherapy on several protocols reported a 5-year overall survival (OS) rate of only 7%.5 Therefore, finding effective and cost-effective treatments for AML in older patients is a major challenge for clinicians, researchers, payers, and patients. However, efforts in this direction are hampered because only ∼5% of older patients with AML receive treatment in the context of clinical trials,6 resulting in the lack of an evidence base for guiding their treatment choices.7
This poor participation rate may to some extent reflect a degree of nihilism on the part of practicing physicians toward older patients with AML. For example, there may be a reluctance to give these patients “intense” induction therapy out of fear that it will lead to treatment-related mortality. However, treatment-related mortality rates have declined sharply in older patients over the last 20 years, and it is unlikely that these results are entirely due to selection bias such that many older patients now receive azacitidine or decitabine rather than more intense regimens.8 In any event, it is clear that the majority of deaths in older patients with AML occur in the presence of disease, indicating that the major problem in curing AML in these patients is resistance to therapy. As discussed in this chapter, resistance is associated with particular cytogenetic and molecular abnormalities, a history of therapy-related AML, or prior blood disorders. Indeed, the contribution of these factors to resistance is probably greater than that of age.
Allogeneic hematopoietic cell transplantation (HCT) is clearly the most effective therapy for addressing resistance in AML. Reduced-intensity conditioning (RIC) regimens and the use of donors other than HLA-matched siblings have made HCT feasible in many older patients. However, RIC-HCT is underused in older patients with AML for many of the same reasons that lead to reluctance to give intense induction therapy. Nonetheless, as with induction chemotherapy, data suggest that nonrelapse mortality (NRM) after HCT is declining even in older patients.9 There is also a lack of knowledge about the relative benefit of HCT over conventional chemotherapy, leading to a continuous need for better, standardized means to help physicians decide which older patients should receive intensive initial chemotherapy and which should subsequently be treated with RIC-HCT.10,11 Such means are essential if personalized treatment of AML in older patients is to become a reality. A large number of recent retrospective studies have demonstrated the effects of various AML- and patient-specific risk factors on HCT outcomes. In this chapter, we provide a comprehensive and analytical review of these risk factors. This is followed by a discussion of a risk-adapted approach integrating various patient-, HCT-, and AML-specific risk factors to help guide future selection of older candidates for allogeneic HCT and to provide good estimates of their projected survival. By older patients, we mean those ≥60 years of age, which is a commonly accepted criterion in various studies.12
AML- and HCT-specific factors (Table 1)
Outcomes of patients of 60 y or older after allogeneic HCT stratified by AML- and transplantation-specific variables
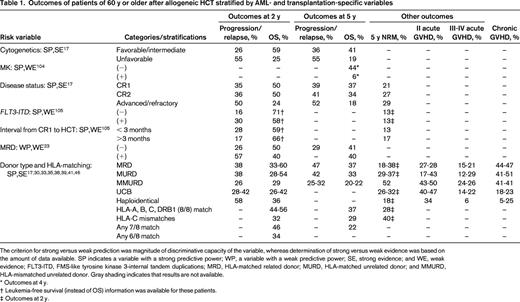
The criterion for strong versus weak prediction was magnitude of discriminative capacity of the variable, whereas determination of strong versus weak evidence was based on the amount of data available. SP indicates a variable with a strong predictive power; WP, a variable with a weak predictive power; SE, strong evidence; and WE, weak evidence; FLT3-ITD, FMS-like tyrosine kinase 3-internal tandem duplications; MRD, HLA-matched related donor; MURD, HLA-matched unrelated donor; and MMURD, HLA-mismatched unrelated donor. Gray shading indicates that results are not available.
* Outcomes at 4 y.
† Leukemia-free survival (instead of OS) information was available for these patients.
‡ Outcomes at 2 y.
Chromosomal aberrations and disease status
Cytogenetic risk groups were initially defined from data on outcomes after chemotherapy given to younger AML patients. However, the same grouping also predicts risks of relapse and mortality in older patients.13-16 In a study examining 1065 older patients (median age 66) treated on the UK AML 11 trial, investigators found 2-year relapse rates of 56% in patients with inv(16) or t(8;21), 78% in those with a normal karyotype, 85% in those with noncomplex abnormalities, and 91% in those with a complex karyotype. Only 7% of patients had inv(16) or t(8;21).13 Farag et al reported similar findings in 635 patients ≥60 years of age, noting that the 7% of patients with inv(16) or t(8;21) had a relapse rate of 70%; therefore, these abnormalities are probably associated with much less “favorable” outcomes in older patients compared with younger patients.14 A common theme appears to be that the same factors that predict poor outcome after chemotherapy also predict relatively poor outcome after RIC-HCT, although outcome generally appears to be better after the latter, subject to considerations of selection bias noted below. For example, in a multicenter retrospective study, outcomes were analyzed for 274 patients with a median age of 60 years who were treated with 2 Gy total body irradiation (TBI) with or without fludarabine.17 Five-year probabilities of NRM, relapse, and OS were 26%, 42%, and 33%, respectively. Unfavorable-risk cytogenetics, as assessed per the Southwest Oncology Group (SWOG) criteria,18 was associated with a 2.7-fold higher risk for relapse compared with favorable-/intermediate-risk cytogenetics. However, the relapse rate of 55% in patients with unfavorable cytogenetics compares favorably with those of 85%–91% without HCT noted above. Similarly, although advanced/refractory AML had a 5.89-fold higher risk of relapse compared with being in first complete remission (CR1), the relapse rate of 50% is almost certainly lower than what would be expected without HCT. Unfavorable cytogenetics and advanced disease risk also predicted higher risks for overall mortality but, again, these rates (∼20% at 4-5 years) were higher than those noted without HCT.14,19,20 Recently, patients whose AML is characterized by monosomal karyotype (MK), defined by the presence of 2 or more autosomal monosomies or a combination of one monosomy with an additional structural abnormality, have been found to have particularly poor prognoses with standard treatments. Among patients >60 years of age, 2-year OS rates were 7% and 22% in those with MK(+)-AML and MK(−)-AML, respectively (P < .0001).21 Fang et al analyzed 432 patients who received HCT; of those, 14% of patients were MK(+)-AML and 21% were >60 years of age. The 4-year OS rate was 25% for MK(+)-AML and 56% for MK(−)-AML (adjusted HR = 2.29, P < .0001).Although this suggested that HCT could reduce the effect of MK, only 17 MK(+)-AML patients were >60 years of age and their 4-year OS was only 6%.
Another study assessed outcomes of 113 patients given a regimen of fludarabine plus reduced-dose busulfan (n = 93) or TBI of 4-8 Gy (n = 20).22 Disease-free survival (DFS) at 2 years was longer in patients with <5% (49%) compared with those with 5%–20% (24%) or >20% blasts (14%). As in the first study, patients in CR1 at time of RIC-HCT had better OS (52% at 2 years) than patients in CR2 (40%) or beyond (<20%). In addition, recent evidence suggests that recipients of nonmyeloablative regimens who, despite having <5% blasts and/or being in CR, have minimal residual disease (MRD) as detected by multiparametric flow cytometry and have 4-fold higher risks of relapse compared with those with MRD-negative AML.23
New molecular markers
Although, as with cytogenetics, the prognostic impact of nucleophosmin gene (NPM-1) mutations and internal tandem duplications of the FMS-like tyrosine kinase gene (FLT3-ITD), was first established in patients <60 years of age, the relative favorable effect of NPM mutations and the unfavorable effect of FLT3-ITD compared with those of other markers also seem present in older patients.20,24 However, much as is the case with inv(16) or t(8;21), older patients with the NPM-1+/FLT3-ITD− genotype seem to have, in general, worse prognosis that younger patients with the same genotype. Therefore, although NPM-1+/FLT3-ITD− patients (all age <60 years) experienced no improvement in DFS in genetic randomization studies using a donor/no donor approach (HR = 0.92), reflecting their low relapse rate without chemotherapy, the same might not be true in NPM-1+/FLT3-ITD− patients aged ≥65 years, in whom the 1-year relapse rate was 71% and the 2-year OS rate was 19% in a combined SWOG/MRC data analysis.25 It is likely that the negative effect on relapse and survival of FLT3-ITD per se, although also present after HCT,22 can be substantially reduced after HCT; however, whether the same will apply in studies relatively free of selection bias and focusing on older patients remains to be seen. The same applies to recent data suggesting that HCT is useful in patients with double CEBP-α (the gene encoding CCAAT/enhancer-binding protein-α) mutations.26 Additional mutations that carry adverse prognosis have been characterized recently, but are awaiting further confirmation in collaborative studies (for review, see Cornelissen et al27 ).
Conditioning regimens
Conditioning regimens can be divided into 3 levels of intensity: high-dose, RIC, and nonmyeloablative.28 Center for International Blood and Marrow Transplant Research (CIBMTR) data indicate that 65% of older patients receive either RIC or nonmyeloablative regimens (Marcelo Pasquini, Center for International Blood and Marrow Transplant Research, personal communication). A group of older patients, especially those 60-65 years of age who are otherwise relatively healthy, might still benefit from high-dose HCT.29 A clinical trial (www.ClinicalTrials.gov identifier #NCT00322101) randomized patients up to age 65 to either high-dose or RIC conditioning followed by HLA-identical sibling grafts. However, accrual was stopped after Data Safety and Monitoring Plan analysis suggested a benefit from high-dose regimens. New models for risk assessment before allogeneic HCT29 should prove very useful in informing future trials comparing nonmyeloablative and RIC for patients ≥65 years of age.
Donor type
Grafts from HLA-identical siblings have been traditionally favored for myeloablative high-dose HCT due to lessened risks for acute GVHD and NRM compared with other graft sources. In the setting of RIC-HCT, however, grafts from HLA-matched unrelated donors and HLA-identical siblings resulted, on average, in comparable outcomes in retrospective analyses. For example, a study analyzing data from 221 and 184 recipients of HLA-matched related and unrelated donor grafts, respectively, after nonmyeloablative conditioning found no significant differences in NRM (HR = 0.98), relapse (HR = 1.04), or OS (HR = 0.99) between the 2 groups.30 However, another study in 433 patients receiving fludarabine and IV busulfan RIC found that the use of unrelated grafts lessened risks for relapse (HR = 0.67, P = .002), but with comparable OS.31
Stem cells sources other than HLA-matched sibling or matched unrelated donors are required for ∼40% of Caucasians and 80% of ethnic minorities32 or for those requiring urgent HCTs because of a high risk for progression or relapse. Another alternative donor source is the HLA-mismatched donor. Historically, this graft type has been associated with higher risks for GVHD and increased risks for NRM and overall mortality compared with HLA-matched grafts. Despite the use of nonmyeloablative regimens followed by 1 antigen ± 1 allele HLA class I mismatch or 2 HLA class I allele mismatches, this approach continues to be limited by a relatively high incidence of grades II-IV acute GVHD and NRM.33 The use of novel combinations for GVHD prophylaxis might help in maximizing the benefit from these mismatched HCTs. A CIBMTR study evaluated outcomes in 1933 unrelated donor recipients, of whom 49% had AML, 42% were ≥50 years of age, and 35% received RIC-HCT. High-resolution typing for HLA-A, HLA-B, HLA-C, HLA-DRB1, HLA-DQA1, and HLA-DQB1 was done for all patient–donor pairs.34 In adjusted comparisons, 8/8 matching for HLA-A, HLA-B, HLA-C, and HLA-DRB1 alleles was associated with better OS at 1 year compared with any 7/8 HLA-matched pairs (56% vs 47%). HLA-C antigen mismatches (n = 189) predicted increased risk for overall mortality [relative risk (RR) = 1.41, P = .0005], NRM (RR = 1.61, P = .0002), and grade III-IV acute GVHD (RR = 1.98; p<.0001). No other statistically significant associations with OS could be detected between other single antigen/allele mismatches versus 8/8 HLA-matched pairs, although there were few patients in some of these subgroups. Another study from the National Marrow Donor Program confirmed worse OS for a single mismatch compared with 8/8 match, but highlighted higher risks for mortality with HLA-A and HLA-DRB1 mismatches compared with HLA-A and HLA-C.35 Further studies are warranted to clarify the role of using mismatched HCTs among older recipients of RIC regimens.
Grafts from HLA-mismatched umbilical cord blood (UCB) were associated with similar and lessened risks for acute GVHD compared with matched and mismatched BM grafts, respectively, with no differences in GVL effects.36 The negative impact of HLA mismatch on hematopoietic recovery and OS after UCB-HCT can be mitigated by increasing the cell dose of the infused UCB.37 Current efforts to accomplish this involves infusion of 2 partially mismatched units or by ex vivo expansion of 1 of 2 units. Results of UCB HCT after RIC suggested reduced incidences of chronic GVHD and relatively similar OS outcomes compared with other HLA-matched grafts.38,39 However, this information requires validation in randomized studies. Moreover, median ages in these studies ranged between 51 and 60 years.38,39 Clearly, studies more dedicated to older patients are required to better evaluate the role of UCB-HCT in older patients.
The majority of patients will have a parent, sibling, or child that is HLA-haploidentical matched. Haploidentical transplantations have been facilitated by using T-cell-depleted grafts to ameliorate risks of GVHD with acceptable rates of engraftment and disease control.40 Another approach is the use of 2 Gy TBI and fludarabine, 150 mg/m2, in addition to cyclophosphamide administered before to facilitate engraftment and afterward to delete alloreactive donor T-cell clones presumably involved in GVHD. Results have been encouraging with regard to NRM but at the expense of weakened GVT effects.41 Other approaches to HLA-haploidentical transplantation continue to be explored.42
Donor age
The effect of donor age on the quality and quantity of transplanted hematopoietic cells and, therefore, the resulting outcome is an important question. Advanced donor age was shown to increase risks for GVHD43 and shorter survival44 when high-dose regimens were used. However, results are different when RIC regimens are used in older patients. For example, among 125 recipients of nonmyeloablative conditioning, increasing donor age was only associated with lower day +28 donor T-cell chimerism (P = .02), but not relapse or OS.45 A large CIBMTR study compared outcomes using an older HLA-matched donor or a younger allele-level 8/8 HLA-matched unrelated donor.46 Patients had leukemia/lymphoma and were ≥50 years of age. Grafts from younger unrelated donors conferred higher risks for grades II-IV acute GVHD (HR = 1.63, P < .001) and chronic GVHD (HR = 1.48, P < .0001). Survival benefit for older sibling donors was limited to those with performance status (PS) of 90%–100%. Results suggest that donor age should not be factored into risk assessment.
Other factors
Grafts from a female donor to a male recipient carry higher risks for GVHD and mortality. The impacts of donor race, parity, and CMV serology status are controversial. B haplotypes of activating KIR “killing immunoglobulin-like receptors” are associated with better OS.47
Patient-specific factors (Table 2)
Outcomes of patients of 60 y or older after allogeneic HCT as stratified by patient-specific variables
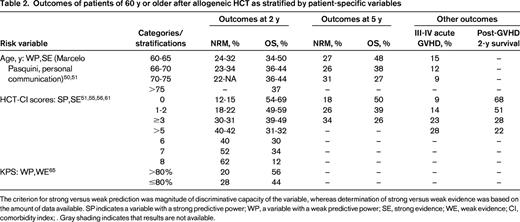
The criterion for strong versus weak prediction was magnitude of discriminative capacity of the variable, whereas determination of strong versus weak evidence was based on the amount of data available. SP indicates a variable with a strong predictive power; WP, a variable with a weak predictive power; SE, strong evidence; WE, weak evidence; CI, comorbidity index; . Gray shading indicates that results are not available.
Age
In the setting of high-dose conditioning before allogeneic HCT for young patients, age >40 years was shown to be associated with higher risks for NRM, as well as overall mortality, with 4-year OS rates of 45% versus 53% for younger patients.48 This finding was recently confirmed in a multicenter retrospective study that included all types of conditioning regimens.29 However, that same study suggested a lack of a prognostic impact of age beyond 40 years on HCT outcomes once other patient variables were taken into account.29 Several studies have indicated that RIC regimens minimized the role of age in outcomes. The European Group for Blood and Marrow Transplantation (EBMT) found similar 4-year rates of NRM (36% vs 39%, P = .23) and OS (34% vs 27%, P = .23) in patients age 50-60 and >60 years, respectively.49 The CIBMTR reported that 2-year rates of OS were 44%, 50%, 34%, and 36% in patients 40-54, 55-59, 60-64, or ≥65 years, respectively.50 A CIBMTR update using data from 3870 older AML patients receiving transplantations between 2008 and 2013 noted 2-year OS rates of 46%, 39%, 36%, and 37% in patients 60-64 (n = 1958), 65-69 (n = 1420), 70-75 (n = 457), and >75 (n = 35) years, respectively (Marcelo Pasquini, Center for International Blood and Marrow Transplant Research, personal communication). A recent study presented data from 372 patients prospectively enrolled into 21 different multicenter clinical trials using 2 Gy total body irradiation alone or with fludarabine 90 mg/m2.51 At 5 years, overall probabilities of relapse and NRM were 41% and 27%, respectively, whereas rates of DFS and OS were 32% and 35%, respectively. There were no statistically significant differences between age groups (60-64 vs 65-69 vs 70-83) in NRM and OS rates; 67% of living patients at 5 years have stopped all immunosuppressive medications.
It cannot be overemphasized that multivariate analyses have indicated that the importance of age is essentially due to its association with other covariates and that, after accounting for these, age has minimal/no effect on outcome of RIC/nonmyeloablative HCT. Rather, the important prognostic covariates have been advanced diseases at HCT and poor-risk cytogenetics for relapse and OS,49-51 comorbidities for NRM,51 and comorbidities and PS for OS.51,52 These results suggest that age per se should not be used to decide on suitability of allogeneic HCT in older adults. Conclusions must be tempered because the number of patients >70 years of age in the various datasets is limited. The referral rate of older patients to allogeneic HCT is also limited,52 raising questions about the applicability of reported results to all older AML patients. Even though the proportion of older patients receiving allogeneic HCT has doubled between the second (17%) versus the first half (8%) of the last decade,53 these proportions remain disproportionately low relative to the frequency of AML in older patients. This suggests a currently unmet need for tools and information to guide community physicians in identifying older patients suitable for allogeneic HCT. This need is being addressed in a large multicenter prospective observational study (www.ClinicalTrials.gov identifier #NCT01929408).
Comorbidities
Organ dysfunctions or medical problems other than primary cancer were long found to impact morbidity and mortality of cancer therapy. The HCT-Comorbidity Index (HCT-CI) was developed to capture the magnitude of comorbidity burden specifically before allogeneic HCT.54 The index comprises a mixture of clinical- and laboratory-based definitions for 17 different types and/or grades of comorbidities. The HCT-CI scores were found in several studies to stratify patients into multiple risk groups for prediction of NRM and OS.51,55,56 The discriminative capacity of the HCT-CI was rated by c-statistic estimates at 0.692 and 0.661 for the prediction of 2-year NRM and OS, respectively.54 The index has passed through the necessary steps to be applied widely. It has been validated in 2 large prospective studies in Italy and the United States.57,58 It was also recently complemented with standardized guidelines for its use so as to facilitate agreement on assignment of comorbidity scores by evaluators at different institutions.59 Indeed, investigators at various centers have confirmed the discriminative capacity of the index for mortality.55,60 Furthermore, the HCT-CI was recently shown to stratify patients into 3 risk groups for grades III-IV acute GVHD and to accurately predict post-GVHD mortality rates.61 Given its discriminatory ability, ease of use, and the increasing comorbidity burden associated with aging,62 the HCT-CI is recommended for evaluation of all older patients before allogeneic HCT. As a corollary, use of age alone to decide whether an older patient should receive HCT should be discouraged.
PS
PS scales have the advantages of simplicity and ease of use. They further refine the decision making process.63-65 However, PS scales do not differentiate between functional impairment due to AML, which is potentially responsive to AML therapy, and that due to other health impairments that are possibly contradictive to intensive therapies. Further, their use as an exclusion criterion (cut-point varied between 50%–70%) for many RIC allogeneic HCT protocols has limited their capacity to predict outcome (Table 2). The greatest benefit of PS scales appears to result when they are combined with a comorbidity index.64,65 This was shown in a study of 339 recipients of nonmyeloablative conditioning and allogeneic HCT at 5 institutions.65 Spearman rank correlation was weak between HCT-CI and Karnofsky performance status (KPS), suggesting that each tool captured a different type of health impairment. However, in multivariate regression models, the HCT-CI was stronger in predicting NRM (P = .0002) and OS (P = .0002) than was PS (P = .13 and P = .05, respectively). A combined model was designed that further stratified patients with HCT-CI scores of 0-2 or ≥3 based on KPS percentages of >80% vs ≤80% into 4 risk groups for NRM and OS (Table 3). It is advisable to use a PS scale alongside the HCT-CI during pre-HCT assessment for older patients.
Outcomes of patients of 60 y or older after allogeneic HCT as stratified by composite risk models
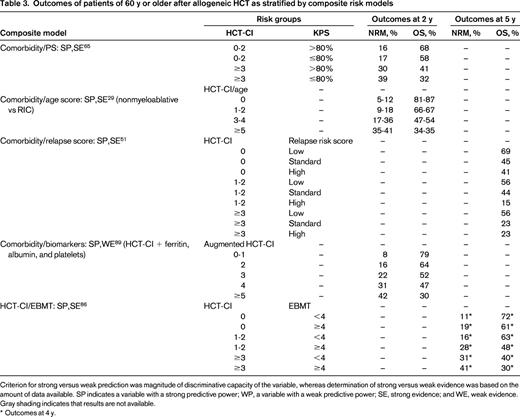
Criterion for strong versus weak prediction was magnitude of discriminative capacity of the variable, whereas determination of strong versus weak evidence was based on the amount of data available. SP indicates a variable with a strong predictive power; WP, a variable with a weak predictive power; SE, strong evidence; and WE, weak evidence. Gray shading indicates that results are not available.
* Outcomes at 4 y.
Geriatric assessment and frailty measures
The health care of an older patient with cancer should include domains beyond the traditional management of cancer. Although evaluation of comorbidities and PS are critical components, they can be complemented by other domains of geriatric assessment (GA) evaluating physical, cognitive, affective, social, financial, and other factors that could affect the health of an older adult. The National Comprehensive Cancer Network, together with the International Society for Geriatric Oncology, have recommended the routine use of a GA for patients ≥65 years of age who are diagnosed with cancer.66 Benefits ranging from prediction of toxicity and OS to assistance in treatment decisions and improving overall well-being of older cancer patients have been proven in nontransplantation settings.67-69 Whereas comorbidities provide data on organ-specific physiologic changes, frailty indices assess the often declining physiologic reserve of multiple systems that is as a feature of aging. Frailty can be manifested by weight loss, weakness, relative immobility, exhaustion, and decrease in physical activity. It can be captured by different tools; one of the most commonly used and proven sensitive for prediction of OS of cancer patients is the Frailty Index.70
Functional status is a component of GA. It is traditionally assessed by PS scales in transplantation settings. However, results in geriatric oncology have shown PS scales to underestimate the magnitude of functional impairment in older patients compared with the more comprehensive assessments provided by use of basic activities of daily living and instrumental activities of daily living.71,72 The latter, in particular, was shown to predict OS of older patients with newly diagnosed AML independent of age, PS, or cytogenetics.71,72 Measurement of gait speed is a simple and objective tool that proves to be very useful for predicting functional decline, life expectancy, and mortality after therapy.73 It was also shown to be as informative as a physical performance battery.74 Likewise, assessments of cognition, nutrition, psychosocial status, and social support have all been shown to predict treatment tolerance, OS, and ability to comply with treatment instructions.
The prognostic significance of GA has not been adequately tested in the transplantation setting. One preliminary small study of 166 patients has shown that GA could uncover additional impairments in health beyond those captured by PS.75 However, the study collected data on patients ≥50 years of age, and only 4 of these were ≥70 years of age. Identifying those components of GA that might add prognostic information to current pre-HCT assessment tools while not adding a substantial burden on patients or transplantation teams requires an adequately sized national study. Such a study has been recently proposed to the Bone Marrow Transplant Clinical Trial network.76
Other important variables
Older patient preferences regarding the possibility of prolonged survival versus a possibly diminished quality of life attendant to use of curative therapy almost certainly vary and should be assessed systematically to aid the decision making process. In a small study in AML patients, patient and physician estimates of cure differed significantly, and patients reported not being offered alternative treatment options.77 Effective communication between patients and physicians during the critical time of decision making is of a prime importance for success of therapy.
Socioeconomic status of cancer patients can affect choice of treatment, access to specialized care, and/or OS. African Americans were shown to be less likely to be offered allogeneic HCT than Caucasians.78 They also have higher risks for mortality that could be due to limited access to care79 or unidentified genetic polymorphisms.80 In a study from CIBMTR, lower income levels were associated with increased mortality regardless of race.81 Although race and socioeconomic status could affect outcomes of allogeneic HCT, their role independent from comorbidities and other health impairments has not yet been well established. Other factors that might influence risks for NRM and overall mortality and should be taken into account include recipient CMV serology status and number of prior chemotherapy regimens.47 Several single nucleotide polymorphisms (SNPs) were found to be associated with critical post-HCT morbidities and thus mortality.82,83 The use of SNPs in pre-HCT risk assessment will require further validation, but can potentially improve our methods to select appropriate candidates for allogeneic HCT.76 The majority of these studies included both younger and older patients. Confirmation of the impacts of these variables on HCT outcomes specifically in older patients is warranted.
Composite models for risk assessment (Table 3)
The previously described risk factors could be used individually or in aggregate to build composite models that would improve our ability to assign patients to the most appropriate treatment. The Pretransplantation Assessment of Mortality (PAM) score incorporates 8 different patient and transplantation risk variables to estimate probabilities of OS.84 Validation cohorts from the same sample used to design the model were disease specific. Only 4% of patients were ≥60 years of age, which together with lack of independent cross-validation studies limits PAM as a risk assessment tool in older patients. The EBMT risk score, which was originally developed in patients with chronic myeloid leukemia, accounts for age, disease stage, donor type, time from diagnosis to HCT, and donor/recipient sex combinations. The risk score was also validated in AML, but largely in young recipients of high-dose conditioning.85 Recently, the EBMT risk score was combined with the HCT-CI in a group of 1616 patients, among whom 42% were recipients of RIC/nonmyeloablative regimens. The HCT-CI/EBMT model further refined risk stratification with an improved c-statistic estimate of 0.630 for OS compared with either model alone (0.613 for EBMT score and 0.558 for HCT-CI, P<0.0001 for each).86
The strong influence of pre-HCT comorbidities on NRM and OS prompted their combination with other risk variables. Age was added to the HCT-CI as an additional variable, acquiring a weight of 1 for patients ≥40 years of age. Results were cross-validated in data from 5 collaborating sites.29 Although age per se was shown to be a poor prognostic factor, its incorporation into the HCT-CI gives a better understanding of the (often small) effect of age in a given patient, an important consideration given that pre-HCT assessment has been greatly biased against older patients.87 The integration of the KPS with the HCT-CI allowed better outcome stratification in recipients of nonmyeloablative HCT.65 A relapse risk score that was specifically developed for recipients of nonmyeloablative HCT88 was integrated with the HCT-CI to enhance prediction of OS among patients ≥60 years. The combined comorbidity/relapse score stratified older patients into 9 risk groups, with 5-year OS rates ranging from 69% in those with an HCT-CI score of 0 and AML in CR1 to 23% in those with HCT-CI scores of ≥3 and advanced or secondary AML.51 Biomarkers could improve decision making by increasing the objectivity and precision of current health measures. The HCT-CI has been augmented by the addition of serum albumin as a measure of nutritional impairment, serum ferritin as a measure of inflammation and/or iron overload, and platelet count as a measure of BM recovery.89 Validation of this augmented comorbidity model in other cohorts, as well as testing the inclusion of other biomarkers could greatly improve risk stratification before HCT.
Decision making and prognostic risk schema (Table 4)
Risk-adapted approach integrating knowledge about patient-related, AML-related, and HCT-related risk factors comparing outcomes after chemotherapy/autologous HCT versus allogeneic HCT in older patients with AML
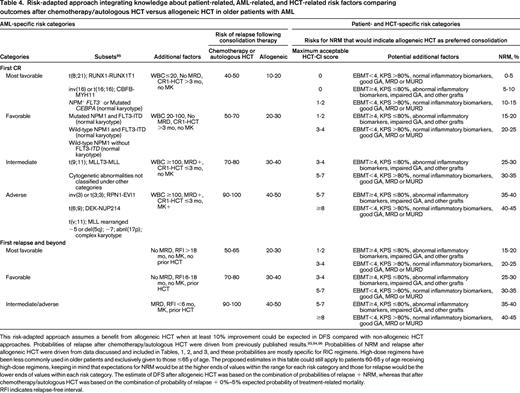
This risk-adapted approach assumes a benefit from allogeneic HCT when at least 10% improvement could be expected in DFS compared with non-allogeneic HCT approaches. Probabilities of relapse after chemotherapy/autologous HCT were driven from previously published results.93,94,96 Probabilities of NRM and relapse after allogeneic HCT were driven from data discussed and included in Tables, 1, 2, and 3, and these probabilities are mostly specific for RIC regimens. High-dose regimens have been less commonly used in older patients and exclusively given to those ≤65 y of age. The proposed estimates in this table could still apply to patients 60-65 y of age receiving high-dose regimens, keeping in mind that expectations for NRM would be at the higher ends of values within the range for each risk category and those for relapse would be the lower ends of values within each risk category. The estimate of DFS after allogeneic HCT was based on the combination of probabilities of relapse + NRM, whereas that after chemotherapy/autologous HCT was based on the combination of probability of relapse + 0%–5% expected probability of treatment-related mortality.
RFI indicates relapse-free interval.
“Genetic randomization” based on the availability of an HLA-identical sibling donor has frequently been used to test the value of allogeneic HCT in younger patients with AML in CR1. A recent meta-analysis of 6000 patients <60 years of age who received high-dose HCT in CR1 in studies comparing allogeneic HCT with autologous HCT or conventional chemotherapy used such donor/no donor analysis and found an OS benefit for allogeneic HCT compared with autologous HCT or conventional chemotherapy in patients with either unfavorable [HR (HR) = 0.69] or intermediate cytogenetics (HR = 0.76), but not favorable cytogenetics (HR = 1.06).90
Similar studies are lacking in older patients. Such patients are more likely to receive HCT from unrelated donors than are younger patients. The relative complexity of the unrelated donor search process makes donor/no donor analyses more difficult to do with unrelated donors and requires use of Mantel Byar statistical methodology.91 Therefore, we are left to rely on retrospective studies to assess the role of allogeneic HCT among older patients; few of these address the value of allogeneic HCT versus chemotherapy among patients in CR1. One study compared outcomes in patients 50-70 years of age according to whether they received allogeneic HCT (n = 152) or chemotherapy only (n = 884).92 Landmark analyses excluded 46 patients from the chemotherapy group because they relapsed or died within 60 days of achieving CR1. Allogeneic HCT was associated with a significantly lower 3-year rate of relapse (22% vs 62%, P < .001), which was not offset by the significantly higher probabilities of NRM (21% vs 3%, P < .001), resulting in better rates of OS (62% vs 51%, P = .012) and DFS (56% vs 29%, P < .001), respectively. The OS benefit primarily reflected results in patients with intermediate-risk cytogenetics (67% vs 54%, P = .024), per SWOG classification,18 rather than unfavorable risk (47% vs 35%, P = .2), in whom the post-HCT relapse rate was 41%. In a CIBMTR study, recipients of RIC allogeneic HCT who were 60-70 years of age had better OS rates than recipients of conventional chemotherapy.93 OS benefit was found in all cytogenetic risk groups, as classified by a consensus panel on behalf of the European LeukemiaNet.94 In patients in first relapse, 4 variables (length of relapse-free interval after CR1, cytogenetic risk, age, and prior high-dose HCT) were combined to stratify patients into favorable, intermediate, and unfavorable risk groups, with 5-year OS rates of 46%, 18%, and 4%, respectively.95 Allogeneic HCT seemed to convey superior OS at 5 years in each of the 3 groups.
The feasibility of allogeneic HCT was addressed in a prospective study of patients ≥50 years of age. Ninety-nine (38%) of a total of 259 enrolled patients achieved remission, of whom 26 had suitable HLA-matched donors and only 14 (5%) actually underwent HCT.52 One or more chemotherapy pair-mates were found for each patient who underwent transplantation based on cytogenetic risks, age, and lead time bias. There was a >99% probability that the outcome after RIC was superior to that seen in patients not receiving HCT. Clearly, this study suffered from small sample size of the patients, lacked assessment of other donor types, and did not examine the role of comorbidities and other health status measures in the decision not to perform HCT.
While awaiting results from ongoing observational (www.ClinicalTrials.gov identifier #NCT01929408) and randomized (www.ClinicalTrials.gov identifier #NCT00766779) studies between transplantation and chemotherapy, we are left to rely on information from previous retrospective studies for decision making. The European LeukemiaNet AML Working Party recently suggested an integrated dynamic risk-adapted approach to decide between HCT and chemotherapy in younger patients with AML. Allogeneic HCT was favored whenever an improvement in DFS of at least 10% could be suggested based on the risk-adapted approach.27 A similar approach was recently recommended for older patients.96 Table 4 expands on these recommendations and bases the allogeneic HCT versus chemotherapy/autologous decision on the comparative probabilities of relapse after these modalities and the risk of NRM after allogeneic HCT, noting that death in AML with or without HCT is generally accompanied by persistent AML. In general, the greater the risk of relapse after chemotherapy/autologous HCT and the greater the decrease in this risk after allogeneic HCT, the higher the maximum HCT-CI score and, accordingly, the higher the risk of NRM that still lead to a decision to perform allogeneic HCT based on a DFS benefit of at least ∼10%. Based on this risk schema, the only group of older patients to be potentially denied allogeneic HCT because of lack of DFS benefit is the group with HCT-CI scores of ≥8 plus impairment in one or more of the additional patient-specific risk factors. In this context, it must be stressed that the same factors associated with a low risk of relapse (leading to a decision not to offer HCT) in younger patients [inv(16), t (8;21), NPM-1+/FLT3-ITD−] are associated with considerably higher risks of relapse in patients ≥60-65 years of age. Of course, it is important to be aware that our ability to predict relapse risk even after accounting for the cytogenetic and molecular covariates listed in Table 4 is quite imperfect and likely to be aided by an emphasis on post- rather than pretreatment variables. Because of limited data, the table regards various factors as having equivalent risks when this is probably an oversimplification. It also must be acknowledged that there is very little information about HCT in patients >70 years of age. The possibility of selection biases influencing the data we do have must be borne in mind. Nonetheless, the favorable benefit-risk ratio plausibly associated with HCT compared with no HCT in these patients at the least seems to warrant further, more systematic study of HCT in older patients; only in this way will we get a more accurate appraisal of the value of HCT in this population. In our practice, we emphasize the importance of RIC-HCT, particularly in patients with unfavorable cytogenetics, particularly complex cyogenetics, or a monosomal karyotype and in patients with MRD as determined by multiparameter flow cytometry, particularly after completion of a reasonable course of postremission chemotherapy.
Summary and future directions (Table 5)
Summary of risk factors for outcomes following allogeneic HCT for AML in older adults
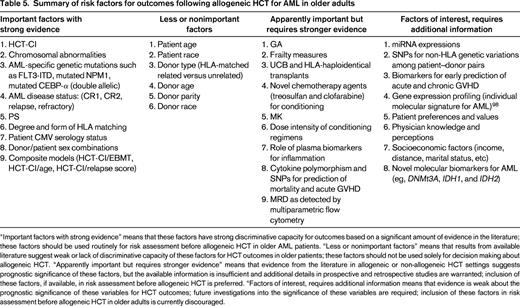
“Important factors with strong evidence” means that these factors have strong discriminative capacity for outcomes based on a significant amount of evidence in the literature; these factors should be used routinely for risk assessment before allogeneic HCT in older AML patients. “Less or nonimportant factors” means that results from available literature suggest weak or lack of discriminative capacity of these factors for HCT outcomes in older patients; these factors should not be used solely for decision making about allogeneic HCT. “Apparently important but requires stronger evidence” means that evidence from the literature in allogeneic or non-allogeneic HCT settings suggests prognostic significance of these factors, but the available information is insufficient and additional details in prospective and retrospective studies are warranted; inclusion of these factors, if available, in risk assessment before allogeneic HCT is preferred. “Factors of interest, requires additional information means that evidence is weak about the prognostic significance of these variables for HCT outcomes; future investigations into the significance of these variables are required; inclusion of these factors in risk assessment before allogeneic HCT in older adults is currently discouraged.
In light of the infrequency with which RIC-HCT is performed in older patients (particularly those >70 years of age), it is important to stress the potential benefits of the procedure compared with non-HCT treatments. First, the latter is associated with high relapse rates, even in patients who would be viewed as “favorable” if younger. Second, NRM rates after HCT have been declining in all age groups, and indeed age itself appears to have no effect on NRM, at least up to age 75. Therefore, it is plausible that RIC-HCT will improve survival in most, if not all, cytogenetic and molecular groups. Perhaps the most important future direction would be examination of whether this hypothesis will prove to be correct in larger numbers of older patients, especially those >70 years of age as best as possible in the absence of selection bias.
Crucial to more appropriate allocation of patients to HCT is discovery and use of new molecular markers to aid in assessing the parallel risks of relapse and NRM, the quantification of which underlies the decision to recommend HCT. Whole-genome sequencing, gene expression profiling,97 and expression of miRNAs98 are likely to improve prediction of relapse in patients with intermediate cytogenetics. Incorporation of information gained only after induction and postremission chemotherapy, for example, MRD as assessed by flow cytometry or PCR, will likely also be important. To date, factors such as a monosomal karyotype or FLT3-ITD that predict relapse after chemotherapy also predict relapse after HCT, although, on average, the risk of relapse is considerably lower after the latter. It will be important to determine whether there are disease features in which the relative risks of relapse after HCT versus chemotherapy are much lower (or higher) than this average. Molecular data may also be useful in assessing risks of NRM. Such data include SNPs,82 non-HLA genetic variants,99 and biomarkers predictive of development of acute GVHD,100,101 which could further reduce risks for NRM, particularly among patients who are older or medically infirm.61
Results suggest that HCT from HLA-matched identical siblings and unrelated donors are associated with relatively similar outcomes. However, evidence is still lacking on the relative merits of HLA-mismatched unrelated, UCB, and haploidentical grafts in older patients compared with HLA-matched grafts, despite the assumption from available studies that the former is relatively less well tolerated in older patients. Comorbidities, as captured by the HCT-CI, provide the best prediction of NRM after allogeneic HCT; therefore, their evaluation is crucial before HCT and could be further stratified by PS or EBMT risk score. Future directions include evaluation/confirmation of the possibilities that information about biomarkers for inflammation, GA, and frailty measures and SNPs will supplement information provided by comorbidity indices. Most importantly, these “estimates for biological age” should replace sole reliance on chronological age when a decision is made about HCT. Current evidence suggests that, per se, age between 60 and 75 years lacks prognostic significance for HCT outcomes. An important future direction will be to test that hypothesis by performing more HCT in fit patients >75-80 years of age, particularly if they are at high risk of relapse without HCT, as noted in Table 4. Testing the hypothesis is particularly important given the increasing number of older individuals and therefore the expected increase in the number of cases of AML.
It should also be noted that HCT is not static. New preparative regimens such as those using radiolabeled monoclonal antibodies may decrease the risk of relapse while patients with persistent MRD after HCT could be enrolled in trials investigating tyrosine kinase inhibitors of FLT3, vaccination against leukemia-specific antigens,102 or graft engineering.103 Of course, chemotherapy is not static either, and it will be crucial to reevaluate relative risks of relapse and NRM after chemotherapy and HCT as new modalities are investigated. Although it has been difficult to conduct randomized trials in HCT or for the purpose of comparing HCT and chemotherapy, such trials might be beneficial.
Current data from the CIBMTR show that 30% of AML patients >75 years of age survive for 3 years after allogeneic HCT. This reflects both advances in risk assessment before HCT and a decline in NRM after HCT due to better management of HCT-related complications. Current efforts are focused on the effects of allogeneic HCT, not only on OS, but also on quality of life (www.ClinicalTrials.gov identifier #NCT01929408). These efforts are also emphasizing prospective evaluation of the reasons that older patients, with their physicians, decide to undergo or not undergo HCT.
Acknowledgments
This work was supported by the National Institutes of Health–National Heart, Lung and Blood Institute (Grant HL088021), Research Scholar Grant RSG-13-084-01-CPHPS from the American Cancer Society, and Contract CE-1304-7451 from the Patient-Centered Outcome Research Institute. The authors thank Dr. Frederic Appelbaum for his insightful contribution to this work.
Disclosures
Conflict-of-interest disclosures: The authors declare no competing financial interests. Off-label drug use: None disclosed.
Correspondence
Mohamed L. Sorror, MD, Clinical Research Division (D1-100), Fred Hutchinson Cancer Research Center, 1100 Fairview Ave. N, Seattle, WA 98109-1024; Phone: (206)667-2765; Fax: (206)667-6124; e-mail: msorror@fhcrc.org.
