Abstract
Cure rates for children and adolescents with acute lymphoblastic leukemia (ALL) have improved dramatically over the last few decades. With this success has come increasing recognition of the adverse late effects of treatment. The significant long-term sequelae in the earliest cohort of long-term survivors treated in the 1970s and 1980s are well documented. To reduce the incidence of these late effects, the majority of pediatric patients treated on more contemporary regimens receive less intensive treatment than did those treated 30-40 years ago. However, current therapies are not risk free; children treated with contemporary regimens remain at risk for developing long-term toxicities, including cardiac dysfunction, osteonecrosis, neurocognitive impairment, and second malignant neoplasms. One of the great challenges facing clinical investigators today is to identify interventions that will reduce the frequency and severity of long-term toxicities without adversely affecting cure rates. The use of dexrazoxane as a cardioprotectant (to prevent anthracycline-associated cardiotoxicity) and alternate-week dosing of dexamethasone (to reduce the risk of osteonecrosis) are examples of 2 such successful strategies. This article provides an overview of the long-term toxicities associated with current therapies and reviews results of clinical trials designed to minimize the burden of cure in long-term survivors.
Learning Objectives
To gain increased understanding of the late effects of treatment associated with current therapeutic approaches for childhood ALL
To identify factors predicting the development of late effects, including patient-related factors and specific agents/treatment modalities
To gain increased understanding of the results of clinical trials designed to reduce the frequency and severity of late effects in childhood ALL survivors
Introduction
The dramatic improvement in the prognosis of children with acute lymphoblastic leukemia (ALL) over the last 50 years is one of the great success stories of clinical oncology. Before the 1950s, the disease was incurable, with a median duration of survival from the time of diagnosis of only 2 months. With current regimens for the treatment of childhood ALL, >95% of patients achieve complete remission and ∼80%-85% are long-term event-free survivors1 (Figure 1). Overall survival, which includes patients who are salvaged after relapse, is now ∼90%.2 However, it is not all good news. Therapy for ALL involves 2-3 years of treatment with cytotoxic chemotherapy and is associated with numerous acute and long-term toxic effects. As more children with ALL become long-term survivors, there is an increasingly important need to understand the physical and emotional costs of cure and also to attempt to minimize the long-term impact of treatment.
EFS of 2999 Children Treated on DFCI ALL Consortium Trials from 1973–2010 by Decade.
EFS of 2999 Children Treated on DFCI ALL Consortium Trials from 1973–2010 by Decade.
Overview of late effects in ALL survivors
Most of the published studies of late effects in children with ALL focus on survivors who were treated in the 1970s and 1980s. These studies have documented a wide spectrum of morbidities, including second malignant neoplasms (SMNs), cardiac dysfunction, short stature, cataracts, neurocognitive impairment, and other neurologic toxicities. Adult survivors of childhood ALL treated during this era also appear to be at high risk of developing metabolic syndrome, a constellation of cardiovascular risk factors (including abdominal obesity, high triglyceride levels, reduced levels of high-density lipoprotein cholesterol, hypertension, and insulin resistance), which predisposes them to coronary artery disease and stroke.3 In a 25-year follow-up report from the Childhood Cancer Survivor Study (CCSS) of patients initially diagnosed between 1970 and 1986, childhood ALL survivors reported higher rates of chronic medical conditions, mental health problems, functional impairment, and activity limitations compared with siblings.4 In addition, the CCSS cohort of ALL survivors was also at increased risk of early mortality; they were 15 times more likely to die of a subsequent cancer, 7 times more likely to die from cardiac-related events, and 2.6 times more likely to die from other medical causes.5
The frequency and severity of late effects observed in survivors treated in the 1970s and 1980s led to changes in upfront therapy in the 1990s and 2000s. The majority of newly diagnosed pediatric patients are currently treated without many of the more morbid components of earlier therapy, including cranial radiation, high cumulative dosages of anthracyclines and alkylating agents, and epipodophyllotoxins. However, high-risk patients—including adolescents; those presenting with high leukocyte counts, adverse cytogenetic abnormalities, and T-cell phenotype; and those with high levels of minimal residual disease early in treatment—continue to receive intensified regimens that can lead to clinically significant late effects (Tables 1, 2). Even lower-risk patients remain at risk for long-term sequelae from the “nonintensive” components of treatment, including intrathecal chemotherapy, other CNS-directed therapies, and corticosteroids, among others (Tables 1, 2). The long-term risks associated with contemporary therapies are summarized in Table 1. Therefore, despite improvements that have been made over the last decades in ameliorating late effects of therapy, addressing long-term toxicities in the current cohort of ALL survivors is still essential.
Late effects associated with contemporary treatment regimens for newly diagnosed childhood ALL

Other rare complications include: infertility (with testicular radiation), intracranial vascular malformations and stroke (cranial radiation), and cirrhosis/portal hypertension (6-thioguanine).
IT, Intrathecal.
Therapies associated with potential late effects on recently conducted clinical trials in children and adolescents with ALL
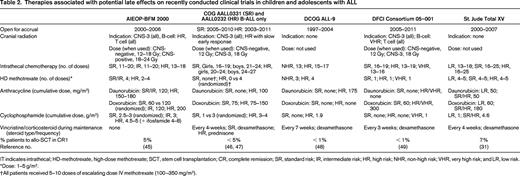
IT indicates intrathecal; HD-methotrexate, high-dose methotrexate; SCT, stem cell transplantation; CR, complete remission; SR, standard risk; IR, intermediate risk; HR, high risk; NHR, non-high risk; VHR, very high risk; and LR, low risk.
*Dose: 1–5 g/m2.
†All patients received 5–10 doses of escalating dose IV methotrexate (100–350 mg/m2).
Anthracycline-associated cardiac late effects
Anthracyclines, primarily doxorubicin and daunorubicin, have been key components of multiagent therapy for childhood ALL for several decades. However, these agents have been associated with cardiotoxicity in long-term survivors, including left ventricular wall thinning and depressed function.6 The severity of cardiac dysfunction is correlated with higher cumulative doses of anthracycline and higher dose rates. Patients treated at a young age, females, and those with Down syndrome appear to be more vulnerable to developing anthracycline-associated cardiac toxicity.7,8
Over the last several decades, therapeutic regimens have been modified so that patients receive lower cumulative dosages of anthracyclines (Table 2), resulting in a decreased frequency of symptomatic congestive heart failure.9 However, it is not clear whether there is a “safe” dose of anthracycline in pediatric patients. Even with lower cumulative doses, a large proportion of patients develop asymptomatic echocardiographic changes that may progress over time and ultimately lead to symptomatic ventricular dysfunction.10 Therefore, despite the use of lower doses of anthracycline and a marked reduction in the risk of acute congestive heart failure with current treatment regimens, there remains a need to identify cardioprotective strategies in pediatric ALL patients. Because congestive heart failure may be a very late manifestation of the cardiac injury that occurs during therapy, echocardiographic measures indicative of cardiotoxicity are typically used as end points for these investigations.
Several potentially cardioprotective strategies have been investigated in pediatric ALL patients, including continuous anthracycline infusion (rather than bolus administration) and the concomitant use of dexrazoxane, an iron-chelating agent. In a randomized study of high-risk ALL patients (all of whom received a 360 mg/m2 cumulative dose of doxorubicin) conducted by the Dana-Farber Cancer Institute (DFCI) ALL Consortium between 1991 and 1995, 48-hour continuous infusion was compared with bolus dosing; there was no difference in event-free survival (EFS) and both groups demonstrated cardiac abnormalities on posttreatment echocardiograms without any advantage noted in patients who received continuous infusion.11 Similarly, in a report by the UK-ALL group, survivors who had received daunorubicin by 6-hour continuous infusion demonstrated subclinical echocardiographic abnormalities (increased left ventricular end-systolic stress, impaired left ventricular function) that were not significantly different from those observed in survivors who had received the same cumulative dose of daunorubicin (180 mg/m2) by bolus.12 Therefore, although continuous infusion dosing appears to reduce the frequency of acute cardiotoxicity in adult cancer patients, there is no evidence that it prevents late cardiotoxicity in pediatric ALL patients.
Dexrazoxane is thought to mitigate anthracycline-induced cardiac damage by preventing free radical formation. It has been shown to prevent acute congestive heart failure in adult cancer patients who have already received a cumulative doxorubicin dose of 300 mg/m2 or higher. However, this dosing strategy (ie, waiting until patients have already received a relatively high total dose of dexrazoxane before initiating dexrazoxane) may not be optimal in pediatric patients, in whom subclinical cardiomyocyte damage can occur with even the first dose. In another randomized study of children with high-risk ALL conducted by the DFCI ALL consortium (1996-2000), doxorubicin was administered to a total cumulative dose of 300 mg/m2 by bolus with or without the use of dexrazoxane before each dose, starting with the first dose of doxorubicin. Dexrazoxane reduced the incidence of doxorubicin-associated myocardial injury as measured by cardiac troponin-T during treatment.13 Five years after completion of doxorubicin, echocardiograms revealed fewer and less severe cardiac abnormalities in dexrazoxane-treated patients compared with those who received doxorubicin alone14 (Table 3). Importantly, the cardioprotection associated with dexrazoxane did not come with any increase in relapse risk: with a median follow-up of 8.7 years, there was no difference in EFS between those treated with and without dexrazoxane14 (Figure 2).
Mean echocardiographic left ventricular Z-score measurements 5 years after completing treatment in HR ALL patients randomized to receive doxorubicin alone or with dexrazoxane (DFCI Consortium Protocol 95–001)

Z-score represents the number of SDs above or below predicted values in healthy children. A Z-score of zero indicates that the mean of the observed values is at the expected mean for a normal population. Adapted with permission from Lipshultz et al, 2010.14
EFS of 205 high-risk patients treated on DFCI ALL Consortium Protocol 95-01 (1996-2000) by randomized treatment group (doxorubicin given alone or with dexrazoxane). At a median follow-up of 8.7 years, EFS was 77% (95% confidence interval, 67-84) in the doxorubicin group, and 76% (95% confidence interval, 67-84) in the doxorubicin plus dexrazoxane group (P = .99). Adapted with permission from Lipshultz et al, 2010.14
EFS of 205 high-risk patients treated on DFCI ALL Consortium Protocol 95-01 (1996-2000) by randomized treatment group (doxorubicin given alone or with dexrazoxane). At a median follow-up of 8.7 years, EFS was 77% (95% confidence interval, 67-84) in the doxorubicin group, and 76% (95% confidence interval, 67-84) in the doxorubicin plus dexrazoxane group (P = .99). Adapted with permission from Lipshultz et al, 2010.14
Results of a single trial in pediatric Hodgkin patients raised concern that dexrazoxane might be associated with an increased risk of secondary acute myelogenous leukemia (AML)/myelodysplastic syndrome.15 However, the DFCI Consortium observed only one case of secondary AML (5-year cumulative incidence of 0.24%) in 553 high-risk ALL survivors who were treated with dexrazoxane between 1996 and 2010.16
Dexrazoxane appears to provide significant cardioprotection in high-risk ALL patients without compromising antileukemic efficacy or increasing the risk of secondary leukemia. However, it may not be sufficient to fully eliminate cardiac late effects. Additional or alternative therapeutic interventions need to be identified. Liposomal anthracyclines appear to be less cardiotoxic in adults than standard formulations of doxorubicin and daunorubicin, but the long-term cardiac consequences of these formulations, and whether they are as effective in treating leukemia as standard formulations, remains to be tested in the pediatric population.17
Osteonecrosis
Osteonecrosis is a disabling bony toxicity that frequently involves multiple joints. Symptomatic osteonecrosis has been observed in 2%–9% of children treated for ALL and can lead to significant pain and loss of function.18,19 In a study conducted by the Children's Cancer Group (CCG), 62 of 143 (43%) high-risk ALL patients with osteonecrosis underwent surgical procedures, including total joint replacements (33% of procedures) and core decompression with or without bone grafting (31% of procedures).20 Osteonecrosis typically presents during treatment, but symptoms may persist years after the completion of therapy. In a series from the Dutch Childhood Oncology Group (DCOG), 35 ALL patients were followed for a median of 4.9 years after the diagnosis of osteonecrosis: 60% had persistent symptoms, with 20% of patients reporting interference with activities of daily living due to pain and dysfunction.18
Several risk factors have been identified for the development of osteonecrosis, including higher total doses of glucocorticoids and age at diagnosis. Rates of osteonecrosis are significantly higher in adolescents than in younger children.18-20 Interestingly, adults treated for ALL do not seem to have as high an incidence of symptomatic osteonecrosis as teenagers, suggesting that the hormonal and physiologic changes of puberty may render adolescents more susceptible to this complication.21 In addition to age, other reported risk factors for the development of osteonecrosis include female sex and high body mass index.18,20 In a study from the St. Jude Children's Research Hospital (SJCRH), lower albumin levels, elevated cholesterol, and poor dexamethasone clearance were all linked to osteonecrosis, as were polymorphisms of the ACP1 gene, which regulates lipid levels and osteoblast differentiation.22 Therefore, several host-related features, including age, sex, body habitus, and genetic polymorphisms, may help to identify patients at high risk for this complication for whom preventative strategies may be targeted.
In some studies, dexamethasone (when used instead of prednisone) has been associated with a higher risk of osteonecrosis, particularly in adolescents.19 However, several clinical trials have demonstrated that dexamethasone is associated with lower relapse risk and superior EFS.19,23,24 On most contemporary regimens, dexamethasone is the principal corticosteroid that is used, especially during postinduction treatment phases (Table 3). The challenge for clinical investigators is to find a way to preserve the beneficial aspects of dexamethasone treatment while preventing its bone complications.
Results from the CCG Protocol 1961 (1996-2002) for pediatric patients with high-risk ALL suggest that altering the dosing schedule of dexamethasone may reduce the risk of osteonecrosis without adversely affecting antileukemic outcome. In that study, high-risk patients were randomized to receive either 1 or 2 delayed intensification (DI) phases. Patients who received only 1 DI phase received dexamethasone continuously for 21 days (days 0-20) during that phase; patients receiving 2 DI phases received dexamethasone discontinuously during each of the phases (days 0-7 and then again days 14-20). For patients aged 10-21 years, alternate-week dosing of dexamethasone (ie, 2 DI phases) was associated with a significantly lower cumulative incidence of osteonecrosis compared with continuous dosing/1 DI phase (8.7% vs 17.0%, P = .0005), even though patients receiving 2 DI phases received a higher total cumulative dose of dexamethasone.20 There was no significant difference in EFS in patients randomized to 1 versus 2 DI phases,25 indicating that the reduced frequency of osteonecrosis associated with alternate-week dosing of dexamethasone did not come at the cost of reduced efficacy.
Neurocognitive sequelae
The neurocognitive impact of ALL treatment has been a major focus of late effects research. Low and low average IQs were frequent findings in survivors of ALL treated in the 1970s, most of whom received cranial radiation at higher doses (24-28 Gy) than are typically administered with current regimens. With 5-10 years of follow-up, survivors from this era were found to have a high frequency of learning disabilities related to a slow speed of processing information, distractibility, and difficulty in dealing with complex or conceptually demanding material.26,27 With longer follow-up, cognitive issues continue to be identified. Investigators from SJCRH performed cognitive testing on long-term survivors (median age of 41 years) and found that those who had received 24 Gy cranial radiation exhibited impairments in both immediate and delayed memory, suggestive of early onset of cognitive aging.28
Radiation-associated neurocognitive impairment appears to be dose related. Long-term survivors treated with 18 Gy radiation (especially those who were 3 years of age or older at diagnosis) appear to have less severe neurocognitive sequelae than those who received higher doses of radiation. In some studies, the overall cognitive functioning (including global IQ scores) in survivors who received 18 Gy cranial radiation does not differ from age-expected norms, although subtle effects are observed with detailed neuropsychological testing.29,30 In the SJCRH study of memory in long-term survivors, those who had received 24 Gy radiation had twice the rate of memory impairment compared with those who had received 18 Gy radiation.28 It is possible that, with longer follow-up, those treated with lower-dose radiation may also develop these problems, but at a median of 25.6 years after having received cranial radiation, survivors who received 18 Gy radiation did not have any statistically significant impairment in immediate or delayed memory. The long-term neurocognitive impact of 12 Gy radiation, the dose most frequently used when radiation is administered in contemporary protocols (Table 3), has not yet been determined, but, given the dose-related findings observed thus far in irradiated survivors, it would not be expected to be worse than that associated with 18 Gy radiation and may even be less severe.
One of the major goals of omitting cranial radiation in childhood ALL treatment is to avoid neurocognitive late effects. However, the other CNS-directed therapies used to substitute for cranial radiation (such as extra doses of intrathecal chemotherapy and high-dose IV methotrexate) also may lead to neurocognitive impairments. In the SJCRH Total XV trial (2000-2007), all patients were treated without cranial radiation; those considered at higher risk of relapse received more doses of intrathecal chemotherapy and more intensive systemic chemotherapy than lower-risk patients.31 The overall EFS of that trial was relatively favorable (5-year EFS of 86%), although some patients did significantly worse, including those with CNS-3 disease at diagnosis. Comprehensive neurocognitive testing of patients treated on that protocol performed 2 years after the completion of consolidation therapy revealed that most survivors performed well on global measures of cognitive ability; however, 40% of the tested cohort performed below average on a measure of sustained attention regardless of age, sex, or treatment intensity.32 Higher treatment intensity was associated with worse performance on measures of process speed and academic abilities, as well as greater parent report of learning problems, outcomes that are not dissimilar to those reported for survivors who had received 18 Gy cranial radiation in other clinical trials.
Results of trials that randomized patients to receive cranial radiation or not during upfront therapy indicate that neurocognitive functioning appears similar in irradiated and nonirradiated survivors treated on contemporary regimens. In a DFCI ALL Consortium trial (1996-2000), standard-risk patients were randomized to receive 18 Gy cranial radiation or more frequent dosing of intrathecal chemotherapy without radiation. EFS was not significantly different between the arms.33 Neuropsychological testing (completed a median of 6 years from diagnosis) revealed that cognitive function was solidly in the average range, with no differences between the groups in cognitive skills or in the frequency of children receiving special education.30 In general, both irradiated and nonirradiated survivors exhibited similar patterns of strengths and weaknesses on the battery of tests, although children who had received radiation exhibited less adaptability in their daily activities and slower information processing. Similar results were reported from the UK ALL clinical trials group, which conducted a randomized trial comparing high-dose methotrexate and 24 Gy cranial radiation in high-risk patients (1990-1997); at 3 and 5 years after treatment, there were no significant differences in IQ scores in irradiated and nonirradiated high-risk patients, nor was there any difference in the proportion of patients with IQ scores <80.34
Many studies of neuropsychological functioning in ALL survivors are limited by small patient numbers and relatively short follow-up. Because it appears that neurocognitive function may decline over time in ALL survivors,35 longer follow-up is necessary before making definitive conclusions. In one study of 567 survivors who were 10 more years from initial diagnosis, neurocognitive impairment rates (measured by testing and self-report) increased as a function of cranial radiation dose, with the lowest rates observed in nonirradiated patients.36 However, the most profoundly affected patients in this series had received 24 Gy radiation. The nonirradiated and 18 Gy-treated survivors both demonstrated high rates of impairment in all neurocognitive domains, but there were few statistically significant differences noted between the 2 groups.36 Therefore, the omission of cranial radiation alone does not appear sufficient to eliminate neuropsychologic sequelae in long-term survivors and investigation of interventions that may prevent late neurocognitive sequelae in children with ALL (including those treated without cranial radiation) should remain a priority.
Second malignancies
Long-term survivors of childhood ALL are at risk for developing SMNs, including brain tumors, AML, non-Hodgkin's lymphomas, and carcinomas of the parotid and thyroid glands.37-39 The overall cumulative incidence of SMNs reported in the literature ranges from 1% to 6%, depending on the treatment regimen and length of follow-up.37,38 In a retrospective study of 2169 patients treated at SJCRH between 1962 and 1998 (median follow-up, 18.7 years), the overall cumulative incidence of SMN was ∼4% at 15 years and 11% at 30 years.38 Many of the late-developing SMNs were “benign” neoplasms (basal cell carcinoma and meningioma); when these diagnoses were excluded, the cumulative incidence of SMNs at 30 years was ∼6%.
Cranial and craniospinal radiation markedly increase the risk of developing secondary solid tumors, including malignant gliomas and meningiomas.37,38 The cumulative incidence of malignant gliomas appears to plateau ∼15-20 years after diagnosis; conversely, even with 30 years of follow-up, a plateau in the incidence of meningiomas was not observed in the SJCRH study.38 Lower-dose radiation may not be associated with as high a risk of SMNs; in one study, patients who had received 18 Gy cranial radiation appeared to be at lower risk than those who had received 24 Gy radiation.37 The risk of secondary brain tumors with 12 Gy cranial radiation, the dose most commonly used in current protocols in those patients still receiving radiation (Table 3), has not yet been elucidated.
Cranial radiation has also been associated with the development of vascular malformations, which can lead to neurological symptoms and intracranial hemorrhage.40 Therefore, the omission of cranial radiation from upfront regimens is likely to substantially reduce the risk of CNS SMNs and secondary vasculopathies. To that end, on most current regimens, 90% or more of newly diagnosed patients are treated without radiation (Table 3). For the remaining high-risk patients, including those with CNS-3 status at diagnosis and some T-ALL patients, determining whether the benefit of omitting cranial radiation is outweighed (or not) by a significant increase in relapse risk and/or decrease in overall survival rates remains the focus of clinical investigation.
Secondary AML can also develop in long-term survivors, with significantly higher rates observed in those who were treated with higher (and/or more frequent) doses of epipodophyllotoxins and alkylating agents.41-43 Therefore, current regimens include relatively low total doses of cyclophosphamide and other alkylators (Table 3); epipodophyllotoxins are typically administered in the upfront setting only to patients considered to be at very high risk of relapse and cumulative doses are kept relatively low.
Some studies have suggested that secondary leukemia risk is increased in patients who receive higher starting doses of mercaptopurine during the maintenance phase (75 mg/m2/d instead of 50 mg/m2/d).39 Whether patients with homozygous or heterozygous deficiencies in thiopurine methyltransferase (an enzyme involved in the metabolism of mercaptopurine) are more at risk for SMNs remains controversial, but may depend in part on mercaptopurine dose intensity and/or duration.44
Summary and future directions
With contemporary therapy, cure rates for childhood ALL are quite favorable, but late effects of therapy remain a major concern. Changes in therapy have reduced the frequency and severity of many of the late effects that have been documented in survivors who were treated in the 1970s and 1980s; however, currently treated patients remain at risk for developing neurocognitive impairment, subclinical but potentially progressive cardiotoxicity, and long-term pain and joint dysfunction from osteonecrosis. It is likely that the rate of SMNs in long-term ALL survivors will decrease as an increasing proportion of patients are treated without radiation; however, confirmation awaits longer follow-up. The use of dexrazoxane to prevent cardiotoxicty and alternate-week dosing of dexamethasone are examples of therapeutic interventions that reduced the risk of late effects without compromising efficacy. Testing strategies to further decrease late effects remains a priority, but should be done in the context of clinical trials that carefully measure the impact of the intervention both on the toxicities of interest and on relapse incidence and overall survival rates. In addition, an increased understanding of host factors that predispose to toxicities (such as pharmacogenomics) may help to target these interventions to those patients at highest risk for developing late effects.
Disclosures
Conflict-of-interest disclosure: The author declares no competing financial interests. Off-label drug use: None disclosed.
Correspondence
Lewis B. Silverman, Department of Pediatric Oncology, Dana-Farber Cancer Institute, 450 Brookline Avenue, Boston, MA 02215; Phone: (617)632-6191; Fax: (617)632-5710; e-mail: lewis_silverman@dfci.harvard.edu.


