Abstract
Double-hit lymphomas (DHLs) are a heterogeneous group of mature B-cell lymphomas that harbor concurrent rearrangements of MYC and BCL2 or, occasionally, BCL6. Several studies have now shown that they are associated with a very aggressive clinical course and poor outcome after standard R-CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisone) therapy, with few patients surviving beyond 2 years. Due to their rarity, there is a paucity of data evaluating patient outcomes with alternative strategies to R-CHOP and no consensus on how they should be optimally managed. Recent studies have demonstrated that a significant proportion of diffuse large B-cell lymphoma (DLBCL) cases have high protein expression of MYC and BCL2 as detected by IHC. These so-called “double-expressor” DLBCLs are also associated with a poor outcome after R-CHOP, even when MYC and BCL2 rearrangements are absent. There is much interest in developing new strategies for DHL and better characterizing the underlying biology that drives their poor prognosis. Alternative chemotherapy platforms to R-CHOP, such as DA-EPOCH-R (dose-adjusted etoposide, prednisone, vincristine, cyclophosphamide, doxorubicin and rituximab), are under investigation for MYC-rearranged DLBCL, including DHL, and several novel small-molecule inhibitors of MYC and BCL2 are in development.
Learning Objectives
To understand the outcome with current standard treatment paradigms for “double-hit” and “double-expressor” lymphomas
To learn about novel treatment paradigms and promising targeted agents for these disease entities
Introduction
Diffuse large B-cell lymphoma (DLBCL) is now recognized as both a clinically and molecularly heterogeneous disease with disparate outcomes after standard therapy. Over the past 2 decades, significant improvements in overall survival (OS) have resulted from the addition of rituximab (R) to cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) chemotherapy.1 Despite this advance, standard therapy remains ineffective for a significant proportion of patients presenting with a new diagnosis of DLBCL. Although it has been established for some time that clinical characteristics such as those in the International Prognostic Index (IPI) score predict outcome, the impact of tumor characteristics on prognosis has been less well defined.2 Recently, novel insights in DLBCL have demonstrated that tumor biologic factors, independent of the IPI score, can identify subgroups of patients at higher risk of treatment failure. Gene expression profiling, for example, has demonstrated that DLBCL is not just one disease molecularly and most cases can be divided into a germinal-center B-cell (GCB) or activated B-cell (ABC) subtype.3 Although this is intriguing from a molecular standpoint, it is also a clinically relevant distinction. Patients with the ABC subtype, who have tumors with constitutive activation of the NF-κB pathway, are at higher risk of treatment failure after standard therapy.4 This recognition that DLBCL is not just one disease has led to the development of novel agents with selective biologic activity within specific subtypes.5 Based on this refined understanding of DLBCL, a randomized study testing one of these novel agents in combination with immunochemotherapy is under way in patients with newly diagnosed ABC-DLBCL.6
Recently, a group of diseases called “double-hit” lymphomas (DHLs) have received attention in the literature. They are associated with a poor prognosis and it is unclear how they should be approached therapeutically.7,8 “Double-hit,” strictly speaking, refers to cases that harbor a chromosomal breakpoint affecting the MYC/8q24 locus in combination with another recurrent breakpoint, which is typically BCL2 (t(14;18)(q32;q21)). However, BCL6+/MYC+ double-hit or BCL2+/BCL6+/MYC+ “triple-hit” lymphomas may also rarely occur.9 The majority of DHL cases fall into the histological categories of DLBCL or B-cell lymphoma, unclassifiable, with features intermediate between DLBCL and Burkitt lymphoma. Over the past few years, several groups have identified via immunohistochemistry (IHC) that a significant proportion of DLBCL cases have high protein expression of MYC and BCL2, but frequently without translocations.10-13 These “double-expressor” cases also have an inferior outcome (compared with cases not expressing high levels of MYC and BCL2) after standard R-CHOP therapy. Given the prognosis of these diseases, an important clinical question that now arises is how should DHLs be approached and should both cytogenetically and immunohistochemically defined double-expressor cases be treated with standard R-CHOP? This chapter reviews these categories of diseases with respect to current treatment paradigms that are being used and novel approaches that are being investigated in the clinical setting.
MYC- and BCL2-rearranged DLBCL: outcome after R-CHOP
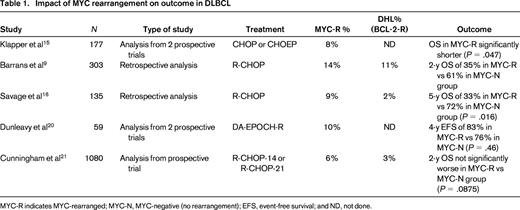
Although BCL2 or BCL6 are each rearranged in ∼1/3 of DLBCL cases, a MYC rearrangement is detected by FISH in 5%–14% of cases.9,14 Despite some conflicting reports, a negative prognostic impact of a BCL2 or BCL6 rearrangement alone has not been well established. However, several reports from both the pre- and post-rituximab eras have demonstrated that a MYC rearrangement confers a worse prognosis after treatment with CHOP or R-CHOP.9,15,16 In contrast to Burkitt lymphoma, in which an “MYC-simple” karyotype is typically present and associated with mutations of the TCF3/ID3 pathway, DLBCL cases harboring a MYC rearrangement often have a complex karyotype with many additional molecular alterations.17-19 It is likely that these additional hits and the added genetic complexity of the tumor contribute to its poor prognosis. Many groups have now assessed the clinical features of DHL and the median age at diagnosis in most series is in the 7th decade of life.7,9 This is important because therapies more intensive than R-CHOP may be poorly tolerated in this population. In addition, most groups have identified advanced-stage disease, elevated lactate dehydrogenase levels, and extranodal sites of disease in a high proportion of patients. Prior indolent lymphoma has only been documented in a minority of cases.
Several studies have looked at the prognostic effect of a MYC and other rearrangements (Table 1).9,15,16,20 One early study by Barrans et al aevaluated 303 untreated DLBCL patients who received R-CHOP in the majority of cases.9 They detected MYC rearrangements in 14% using FISH and, interestingly, 83% of the cases also harbored either BCL2 (74%) or BCL6 rearrangements; therefore, 83% were double-hit or triple-hit. Cases with a MYC rearrangement were more likely to be associated with a high IPI score and a tumor of GCB origin compared with cases without a rearrangement. The 2-year OS for MYC-rearranged patients was significantly inferior, with 35% alive compared with 61% in the FISH-negative group. In another study, the British Columbia Cancer Agency (BCCA) reported 135 cases of DLBCL treated with R-CHOP and identified 9% of cases with a MYC rearrangement; 25% (3/12) had a concurrent BCL2 translocation.16 The 5-year OS for MYC-rearranged cases was just 33% compared with 72% for those without a rearrangement. MYC rearrangement was associated with higher tumor proliferation and a higher likelihood of central nervous system relapse. The BCCA recently reported on 167 R-CHOP treated DLBCL patients and identified concurrent MYC and BCL2 translocations in 5% of cases. This was associated with a poor OS of just 27% at 5 years and most patients had tumors of GCB origin.10 In contrast to these reports, a study in the United Kingdom comparing 14- versus 21-day cycles of R-CHOP in patients >60 years of age found that a MYC rearrangement was not negatively prognostic when adjusted for other clinical factors.21 Interestingly, in that study, the presence of an additional BCL2 translocation did not portend a worse OS at 2 years (double-hit 63% vs no double-hit 85%; P = .06). A non IG-MYC translocation partner is found in a high proportion of cases: one recent study detected it in 41% of MYC-translocation-positive lymphomas other than molecular Burkitt lymphoma.22 The prognostic impact of the MYC translocation partner has not been well studied and is a subject of current investigation.23
Concurrent protein expression of MYC and BCL2 by IHC
Although earlier studies mainly focused on MYC and BCL2 rearrangements, it is recognized that MYC and BCL2 can be activated through other mechanisms, leading to high expression of the protein products. FISH is not always readily available and is costly and time consuming, so recent studies have looked at the prognostic impact of high MYC and BCL2 protein expression detected by IHC: the double-expressor cases10-13,24,25 (Table 2). One Danish study evaluated 193 patients with DLBCL who were uniformly treated with R-CHOP and performed both FISH for MYC and BCL2 rearrangements and IHC for protein overexpression.11 Although, expectedly, a poor outcome was observed in the 6% of patients who were DHL by FISH, it was interesting that in the double-hit score (DHS) 2 group, those with overexpression of MYC and BCL2 by IHC and comprising 29% of patients, there was a similarly significantly inferior OS. In addition, when the investigators compared patients who were DHL by FISH with FISH-negative patients in the DHS 2 group, outcomes were similarly poor. What was interesting in this analysis was that, whereas almost all patients with DHL by FISH (91%) had tumors of GCB origin, the opposite was true in the FISH-negative DHS 2 group: the majority (73%) of these cases had non-GCB tumors. The DHS maintained its prognostic significance in both the GCB and non-GCB group. The BCCA did a similar analysis in a group of homogeneously treated (R-CHOP) patients with de novo DLBCL and identified 21% with concurrent high expression of MYC and BCL2 by IHC.10 Again, these double-expressor cases had a significantly inferior OS compared with cases not doubly overexpressing the proteins, even after adjusting for the presence of high-risk clinical factors and concurrent MYC/BCL2 translocations. Interestingly, as with the Danish study, whereas most cytogenetically defined DHL cases were of GCB origin, most cytogenetically negative double-expressor cases fell into the ABC group (by gene expression profiling). Others have demonstrated a similar poor prognostic impact of high MYC and BCL2 expression after R-CHOP.12,13 One study from the International DLBCL Rituximab-CHOP Consortium Program analyzed 466 patients treated with R-CHOP. High MYC/BCL2 coexpression was associated with an aggressive clinical course and inferior outcome and was more common in the ABC subtype.13
Impact of double expression of MYC and BCL2 on outcome in DLBCL

PFS indicates progression-free survival; and HR, hazard ratio.
*Results from the training cohort remained significant in the validation cohort.
So should double-expressor cases be approached differently and strategies other than R-CHOP used? In considering that question, it is important to cautiously interpret the aforementioned various studies that have looked at IHC concurrent protein overexpression. Although the detection of a MYC or BCL2 translocation by FISH is robust and highly reproducible, IHC techniques and scoring have been reported to be quite variable and optimal cutoff points have sometimes not been well established. At the same time, several studies performed by many different groups demonstrate a significant and independent negative impact of the immunohistochemical score on prognosis and it is critical to study this prospectively. There appear to be distinct mechanisms of MYC and BCL2 activation within subtypes of DLBCL and these should be elucidated further to guide the development of effective novel agents and strategies.
Management of DHLs: beyond R-CHOP
Although several studies demonstrate poor efficacy of R-CHOP in DHLs, with short OS, other strategies have not been well studied and there are no prospective data due to the rarity of these tumors. Considering that these lymphomas harbor a MYC rearrangement, testing approaches that are effective in Burkitt lymphoma make sense. However, one big challenge in that regard is the median age of this group of patients. Most are at least in the 7th decade of life and approaches that are commonly used for the treatment of Burkitt lymphoma are poorly tolerated and not typically feasible in this age group.26 Although there are several small reports using alternative strategies to R-CHOP, bigger series are rare (Table 3).
Treatment and outcome of double-hit lymphoma
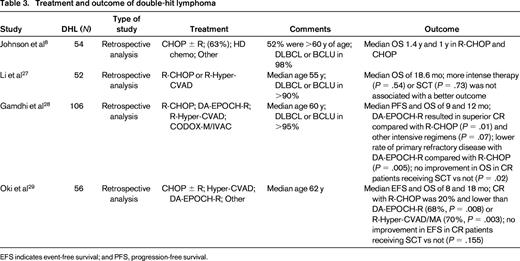
EFS indicates event-free survival; and PFS, progression-free survival.
Li et al evaluated 52 patients with MYC/BCL2 DHL and the majority received high-dose therapy, primarily R-Hyper-CVAD (rituximab, hyperfractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone). However, the median OS was just 18.6 months and treatment intensity or stem cell transplantation did not affect outcome.27 One recently reported large, multicenter, retrospective analysis across 15 centers looked at the impact of induction regimen and consolidative stem cell transplantation in patients with DHL.28 The definition of DHL was not confined histologically to DLBCL and a high proportion of cases had B-cell unclassifiable with features intermediate between DLBCL and Burkitt lymphoma and a few cases were reported as Burkitt-like. In an early report of the analysis, 106 patients were included and the majority of these (89%) had MYC and BCL2 rearrangements. Primary refractory disease was the primary predictor of OS. Alternatives to R-CHOP, including R-Hyper-CVAD, R-CODOX-M/IVAC (rituximab, cyclophosphamide, vincristine, doxorubicin, methotrexate, ifosfamide, etoposide, cytarabine), and DA-EPOCH-R (dose-adjusted etoposide, prednisone, vincristine, cyclophosphamide, doxorubicin and rituximab) were used. DA-EPOCH-R resulted in a higher rate of complete responses compared with R-CHOP (P = .01) or other intensive regimens (P = .07). In addition, primary refractory disease occurred less frequently in patients treated with DA-EPOCH-R compared with R-CHOP (P = .05) or other regimens (P = .03). Overall, patients achieving a complete response did not appear to benefit from consolidative stem cell transplantation, but the numbers were low. The M.D. Anderson group also recently presented their experience with DHL over 15 years.29 They identified 56 cases, most of whom harbored a MYC and BCL2 rearrangement. They did not identify an optimal regimen retrospectively and the survival of patients undergoing frontline stem cell transplantation was not significantly different compared with patients who did not. In both of these series, there were a significant number of patients with a prior history of an indolent lymphoma, which is different from earlier series.
Although these studies highlight the poor prognosis of DHL histology, they do not provide any definitive guidance about how to manage these patients. However, it is interesting that higher complete response rates and lower rates of primary refractory disease were associated with more intensive regimens than R-CHOP. Given that MYC rearrangements are associated with high tumor proliferation, strategies that can address this and overcome it may be more effective in MYC-driven DLBCL. CHOP-like therapy has low efficacy in DLBCL tumors with high proliferation and in Burkitt lymphoma.30,31 Because DA-EPOCH-R is very effective in Burkitt lymphoma and in GCB-DLBCL cases with high tumor proliferation, the prognostic role of a MYC rearrangement was retrospectively assessed in a National Cancer Institute (NCI) and Cancer and Leukemia Group B (CALGB) study.20 Of 59 DLBCL cases treated with the regimen, MYC rearrangements were detected in 10% and clinical and IPI characteristics were similar in both the MYC-rearranged and MYC-negative groups. At a median follow-up of 4 years, event-free survivals were similar in both groups (83% in MYC-rearranged and 76% in MYC-negative: P = .46). This analysis is currently being expanded to include more cases and the contributing role of BCL2. The finding that an MYC rearrangement did not portend a poor prognosis is the basis for including a MYC-rearranged DLBCL arm in a national NCI-Intergroup study that is currently assessing the regimen in Burkitt lymphoma in a multicenter setting. The study's eligibility also includes the category called “B-cell lymphoma with features intermediate between Burkitt lymphoma and diffuse large B-cell lymphoma,” where many DHLs lie. Unlike “standard” Burkitt lymphoma regimens, DA-EPOCH-R is well tolerated and feasible in elderly patients.31,32 This study will provide a larger and multicenter prospective assessment of the regimen in these diseases and evaluate the contribution of a BCL2 rearrangement and protein overexpression in MYC-rearranged DLBCL.
Approach to IHC-defined overexpressing MYC and BCL2 cases
As with DHL cases, outcomes after standard approaches for double-expressor cases are poor and optimal management approaches remain to be defined. Although a small proportion of these cases represent cytogenetically defined DHL histology and are associated with the GCB subtype, the majority do not have a MYC or BCL2 rearrangement and appear to be of non-GCB derivation according to the aforementioned studies. It is important to recognize this in terms of considering distinct treatment approaches for these IHC-defined cases. High protein expression of MYC and BCL2 within different subtypes is likely to be driven by different tumor biology and therefore cell of origin needs to be carefully considered. However, given the potential pitfalls of IHC, its prognostic role regarding MYC and BCL2 should be validated prospectively. Albeit a small series, concurrent expression of MYC and BCL2 by IHC was assessed in DLBCL cases treated with DA-EPOCH-R and did not correspond with a statistically inferior outcome.25 However, non-GCB cell of origin was predictive of an inferior outcome and MYC+/BCL2+ cases segregated with the non-GCB subtype as in other series.
Double-expressor cases represent a treatment dilemma and it is not clear whether they should be approached differently from those without double overexpression. Because several studies demonstrate that a high proportion of these are of non-GCB or ABC origin, clinical trials that incorporate novel agents directed against NF-κB and other ABC targets should be considered for non-GCB/ABC cases. Moving forward, it is important that prospective studies incorporate strict and reproducible pathological inclusion criteria for double-expressor cases and attempt to better elucidate the underlying biology of these diseases using robust assays/predictors of cell of origin.33
Novel agents in DHL
Targeted agents that may be promising in this group of patients include specific inhibitors of MYC and BCL2. With regard to MYC, recent work demonstrates that there is a loss of expression of the tumor suppressor phosphatase and tensin homolog (PTEN), leading to MYC up-regulation by constitutive activation of the PI3K/AKT pathway in a high proportion of GCB-DLBCL cases.34 PI3K inhibition was selectively toxic to PTEN-deficient GCB-DLBCL models, suggesting that PI3K inhibitors could have activity in these diseases. Several PI3K inhibitors directed against different isoforms of PI3K are in development and have shown promising activity in other lymphoid diseases.35 Small-molecule inhibitors of the bromodomain and extraterminal (BET) domain proteins, such as JQ1 and I-BET 151, are also interesting with respect to MYC. Although they have demonstrated impressive preclinical activity in cancers with chromosomal translocations of MYC, they also appear to have activity in nontranslocated cancers in which MYC is deregulated at the posttranscriptional level.36 Recent studies found that JQ1 treatment can suppress the expression of MYC in DLBCL cell lines and, interestingly, that BET and histone deacetylase inhibitors can synergize to kill in MYC-induced murine lymphoma.37,38 This suggests that combinations of these agents may be promising in these diseases. Aurora kinase inhibitors are also interesting agents because aurora kinase function has been shown to be required for the maintenance of MYC-driven lymphoma. Inhibitors of aurora kinase are in development and one of these, alisertib, a selective aurora kinase A inhibitor, recently demonstrated a modest response rate in relapsed and refractory aggressive B- and T-cell lymphoma. One patient with Burkitt lymphoma and one with a transformed lymphoma with high expression of MYC and BCL2 by IHC were among the responders.39 Other potential therapeutic targets that may be important in MYC-overexpressing cancers include human mitochondrial peptide deformylase and the mitochondrial sirtuin SIRT 4.40,41
BCL-2 is a druggable target and several inhibitors of the BCL-2 family are in development. Navitoclax is being investigated in several phase 2 studies and, recently, ABT-199, a platelet-sparing BCL-2 inhibitor, has also been tested.42,43 In a recent early report of a phase 1 study of ABT-199, some responses were observed in DLBCL at higher doses.44 Small-molecule inhibitors of BCL-6 are also in development.45 One such compound binds to the corepressor-binding groove of the BCL6 BTB domain and is effective in killing BCL6-positive DLBCL cell lines. It is likely that rational combinations of these agents with more effective chemotherapy platforms than CHOP will offer the highest chance of altering the therapeutic outcome of DHLs.
Conclusions
DHLs represent a huge therapeutic challenge. They are clinically aggressive diseases with complex karyotypes and have unacceptably poor cure rates with standard approaches. Although the definition of DHL has usually been restricted to cases harboring MYC and BCL2 rearrangements, there is recent recognition that cases overexpressing MYC and BCL2 by IHC but without rearrangements are also associated with a poor outcome. These double-expressor cases appear to cluster with the non-GCB subtype of DLBCL. At this point, we have not yet defined what the optimal therapeutic management of these diseases should be. New approaches under investigation include alternative immunochemotherapy platforms and novel small-molecule inhibitors, including those targeting MYC, BCL2, and BCL6. Given the rarity of DHL, it is critical to study these diseases in multicenter prospective studies that incorporate pertinent tumor biology analyses.
Acknowledgment
This work was supported by the intramural program of the National Cancer Institute.
Disclosures
Conflict-of-interest disclosures: The author declares no competing financial interests. Off-label drug use: None disclosed.
Correspondence
Kieron Dunleavy, MD, Lymphoid Malignancies Branch, National Cancer Institute, 9000 Rockville Pike, Building 10, Room 4N-115, Bethesda, MD 20892; Phone: (301)435-1007; Fax: (301)480-1105; e-mail: dunleavk@mail.nih.gov.