Abstract
Two lingering problems regarding transplantation in older adults have been how to select patients appropriately and whether to use older sibling donors. Allogeneic hematopoietic cell transplantation (HCT) of older patients may result in long-term survival due to GVL, but the data remain observational and mostly restricted to those 50 to 69 years of age. Patients with excellent performance status and low comorbidity have the best long-term survival after HCT. Novel measures of health status such as self-report or performance-based functional measures allow “staging the age” and may inform candidacy for less robust patients. Older matched sibling donors should be preferred over matched unrelated donors (MUDs) because outcomes are equivalent to superior for matched sibling donors compared with MUD. However, MUDs also achieve acceptable outcomes and long-term disease control. An alternative donor can be considered based on institutional protocols and expertise. Very limited information is available in patients or related donors 70 years of age and older. Future efforts to more completely characterize patient health status before transplantation will allow better application of HCT in older adults.
Introduction
Older adults suffer a disproportionate burden of cases and mortality from hematologic malignancies. Surveillance Epidemiology and End Results (SEER) data from the United States reveal that the median age for the common transplantation indications of acute myeloid leukemia (AML), myelodysplastic syndrome (MDS), chronic lymphocytic leukemia, and non-Hodgkin lymphoma ranges from 64 to 72 years of age (http://seer.cancer.gov/). However, due to underreporting, the actual median age of diagnosis may be in the mid-70s.1 ALL is the exception, having a younger median age but a second incidence peak in older age. Adverse disease biology, reduced treatment tolerance, and shorter nonmalignancy survival interfere with long-term success. Optimal chemotherapy and supportive care in the cooperative group setting does not negate the adverse effects of older age.2
Older patients: choosing wisely
Definition of “older”
Over the past 15 years, the landscape of hematopoietic allografting changed from being rarely performed in patients 50 years or older to accounting for a little less than half of the transplantations reported to the Center for International Blood and Marrow Transplantation Registry (CIBMTR)3 (Figure 1). Nevertheless, very few allografts have been performed in those > 75 years of age. Therefore, in the context of hematopoietic cell transplantation (HCT), older will be defined here as 50 to 75 years of age.
Trends in autologous and allogeneic HCT from 1990 to 2010 showing a marked rise in transplantation for older patients.
Trends in autologous and allogeneic HCT from 1990 to 2010 showing a marked rise in transplantation for older patients.
Trends of transplantation in older patients
The reason for the marked rise in HCT among older adults varies and ranges from the introduction of lower toxicity conditioning regimens to the marked increase in the number of older adults4 (Table 15 ). Solid organ transplantation has witnessed a similarly increased utilization among older adults.6 In the past decade, HCT for patients 60 years and older has risen 4-fold (Table 2). AML and MDS have been the main transplantation indications in older adults.3 Nevertheless, only a small fraction of older adults with AML undergo HCT and even fewer for other hematologic diseases.7,8
Trends promoting HCT for older adults
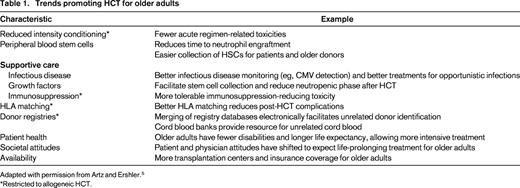
Adapted with permission from Artz and Ershler.5
*Restricted to allogeneic HCT.
Number of allogeneic HCT recipients 50 years of age and older registered with CIBMTR from 2000 through 2011* by year of transplantation and age group
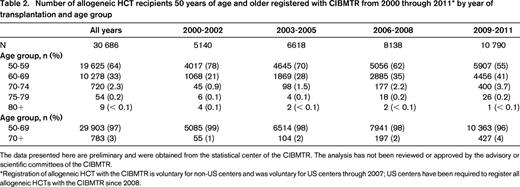
The data presented here are preliminary and were obtained from the statistical center of the CIBMTR. The analysis has not been reviewed or approved by the advisory or scientific committees of the CIBMTR.
Registration of allogeneic HCT with the CIBMTR is voluntary for non-US centers and was voluntary for US centers through 2007; US centers have been required to register all allogeneic HCTs with the CIBMTR since 2008.
Transplantation outcomes in older adults
The results for HCT among older adults can be derived from observational and retrospective comparisons; no studies have prospectively compared transplantation to no transplantation in a rigorous fashion (eg, donor versus no donor analysis) for patients > 55 years of age. Moreover, only scant data are available for diseases other than AML and MDS. McClune et al for the CIBMTR described reduced intensity conditioning transplantations for AML in remission and for MDS in patients 40 and older after reduced intensity conditioning and found similar to slightly worse outcomes with advancing age.9 Specifically, AML patients 60 to 64 years of age and those 65 years and older had overall survival (OS) of 34% (95% confidence interval [CI]: 25%-43%) and 36% (95% CI: 24%-49%), respectively. For MDS patients 60 to 64 years of age and those 65 years and older, the 2-year OS was 45% (95% CI: 36%-54%) and 38% (95% CI: 25%-51%), respectively. Devine et al demonstrated a similar 2-year OS of 46% and a 2-year disease-free survival of 39% among 123 AML patients 60 years and older undergoing related or unrelated donor transplantation in the cooperative group setting.10 Other series have reported equivalent results in older patients and lower relapse rates compared with chemotherapy only in case-control studies.11-14
Morbidity and long-term outcomes
Sorror et al reported on morbidity and long-term outcomes among 372 patients 60 years and older who underwent nonmyeloablative transplantation for hematologic neoplasms.12 Of these, 33 (8.9%) were 70 to 74 years of age. Only 6% to 16% suffered grade IV nonhematologic toxicity in the first 100 days, likely reflecting the excellent tolerance to this nonablative strategy. GVHD appeared to be a significant morbidity because 39% of survivors remained on immunosuppression. Nevertheless, the median Karnofsky performance status (KPS) at last contact was 90% (range 60%-100%). The 5-year OS was 35% (95% CI: 30%-40%).
Patient-specific factors
Uncertainty about tolerance and nonmalignancy life expectancy in older adults hinders more widespread use of HCT.
Stage the age
A frequently recited mantra has been that “age is not a barrier” to HCT and clinicians should consider “physiologic aging.” That said, although use is rising, HCT is still rarely performed in those 70 years and older. How to appropriately evaluate the health of older patients to establish whether they will have acceptable transplantation-related morbidity and mortality remains unclear. A comprehensive evaluation of health conditions through the Geriatric Assessment (GA) can address the heterogeneity of health. GA is a set of instruments designed to measure limitations or vulnerabilities, essentially “staging the age.” The building blocks of GA derive from limitations that influence morbidity or long-term survival in older adults.14,15
Comorbidity
Sorror et al established the importance of comorbidity by the Hematopoietic Cell Transplantation-Comorbidity Index (HCT-CI) on transplantation-related morbidity and mortality.17 This was an early example of “staging the age” by modifying a tool developed to prognostic long-term mortality in nontransplantation patients, the Charlson Comorbidity Index. Subsequent studies have validated the HCT-CI in general18 and, more recently, in transplantation recipients 60 years and older.12 The HCT-CI alone is not sufficient because patients 60 years and older with high HCT-CI and standard relapse risk, which includes AML in first remission, achieved 5-year OS of 38% (95% CI: 29%-47%). Importantly, patients with high comorbidity also have worse nontransplantation outcomes.19
Functional assessment
Physician-rated performance status, often by KPS, has been the primary measure of functional status. Lower KPS prognosticates for adverse outcome, but the majority of older adults undergoing HCT have a preserved KPS of 80% to 100%.12,20,21 A recent study showed inferior survival in AML patients 50 years and older having a KPS of 80% or less.22
Novel factors to stage the age
Older adults entering transplantation with a KPS of 80% or an HCT-CI of 3 or more can be acceptable transplantation candidates; conversely, candidates who score well by KPS and HCT-CI may be poor candidates. Performance status may be quite difficult to accurately and reproducibly assign. GA may provide additional objective data and further prognostic discrimination. Domains from a GA include functional status, comorbidity, cognition, nutritional state, social functioning, emotional state, polypharmacy, and others.23,24
GA and transplantation
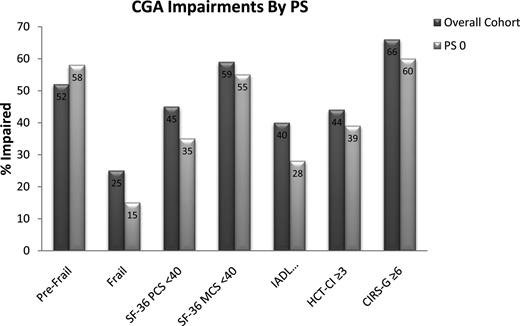
We recently reported 166 HCT recipients 50 to 73 years of age prospectively evaluated by GA before conditioning.25 Almost all patients had a Karnofsky Performance Status (KPS) of 0 to 1 (n = 164 of 166). Similar to the Sorror study of adults 60 years and over, 44% demonstrated HCT-CI scores of 3 or more. GA identified a high frequency of limitations even in the presence of HCT-CI scores of 0 to 2 or preserved performance status (Zubrod PS of 0 or KPS equivalent of 90%-100%; Figure 2). The Cumulative Illness Rating Scale-Geriatrics, a more sensitive comorbidity index, documented more comorbidity than HCT-CI, with a mean of 7.3 per patient. Applying the Fried Frailty Index, a widely accepted tool to phenotype frailty, 25% met the formal definition of frail and another 51% were pre-frail.16 Therefore, formally staging the age paints a picture of heterogeneous health before HCT for older adults, similar to the disease variability now appreciated for normal karyotype AML.
Proportion with a limitation by select tools from a comprehensive geriatric assessment (CGA) before allogeneic HCT for all patients and the subset with fully preserved PS (Zubrod PS of 0).25
Proportion with a limitation by select tools from a comprehensive geriatric assessment (CGA) before allogeneic HCT for all patients and the subset with fully preserved PS (Zubrod PS of 0).25
We also found that many of these limitations by GA adversely influenced outcomes among 203 patients 50 years and older undergoing HCT for any hematologic condition. (Muffly et al, unpublished data, 2013). After adjusting for disease status, age, and regimen intensity, limitations in instrumental activities of daily living (hazard ratio [HR] = 2.38; P < .001), slow walk speed (HR = 1.80; P = .01), high HCT-CI (HR = 1.59; P = .02), low self-report mental health (HR = 1.67; P = .01), and elevated serum C-reactive protein above 10 mg/L (HR = 2.59; P < .001) were associated with inferior OS. Likewise, others have shown that limitations in instrumental activity of daily living predict for worse survival in older AML patients after accounting for performance score and older age.26
Clinically available biomarkers such as serum ferritin and C-reactive protein have emerged as important prognostic markers before transplantation and are amenable to testing interventions to mitigate adverse consequences.27,28 To the extent that such markers are later validated and independent of GA, they also will likely supplement GA in promoting creation of a much needed risk score for transplantation-related morbidity and mortality for older adults.
Summary of selecting older patients for transplantation
Transplantation should be considered early in the disease course for older patients with preserved performance status because disease-free survivals are frequently short. Fully evaluating and optimizing patient health if not donor selection (see next section) may require more time than with younger patients. For nonmalignant diseases, a similar approach of balancing disease risks against transplantation tolerance can be used. Staging the age more appropriately through GA or similar tools should promote more accurate estimates of outcome and even identify limitations that may be amenable to therapeutic strategies (eg, physical therapy for slow walk speed, minimizing certain medications for patients with mild cognitive impairment).
Choosing wisely: older donors
Older sibling donors
Older patients will on average have older siblings being considered as donors. Harvesting most often involves G-CSF mobilization of peripheral blood stem cells (PBSCs) and collection by leukapheresis. Older age strongly correlates with mobilizing fewer CD34+ cells in the peripheral blood and therefore lower CD34+ cell yields (Table 3).29,30 The reason for this age effect has not been elucidated clearly. A reduction in HSCs is not likely implicated because preclinical models show that older mice not only have more HSCs but also mobilize HSCs better.31 However, with normal human aging, mutations that might interfere with HSC mobilization accumulate.32 The potential clinical consequences of donor leukemia or graft failure have not been identified as a problem from older sibling donor grafts.
Influence of clinical factors on G-CSF progenitor cell mobilization29
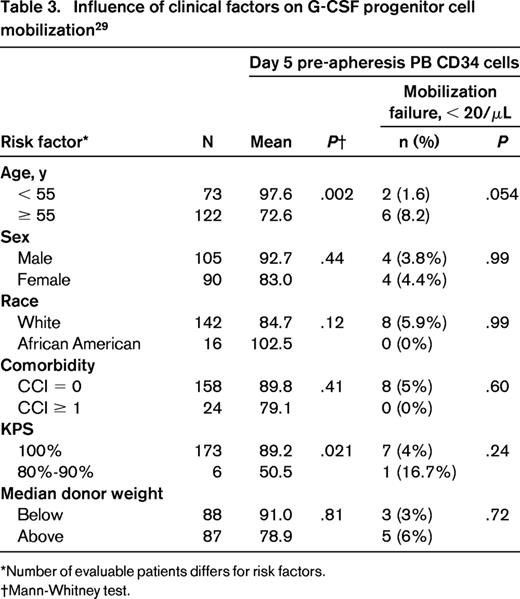
*Number of evaluable patients differs for risk factors.
†Mann-Whitney test.
Comorbid conditions do not reduce the capacity to mobilize CD34+ cells, whereas worse donor performance score may.30 Medically cleared older sibling donors 50 to 70 years of age will generally have adequate PBSC CD34+ cells for transplantation.29,30,33,34 The influence of severe comorbid conditions, impaired functional status, or age > 70 years for donors remains unknown.
Early data showing that unrelated donor age < 30 years for BM grafts achieved optimal outcomes have led to the notion that older donor age for unrelated if not related donors is an adverse factor. Interestingly, a larger registry study of > 900 unrelated donor PBSC grafts revealed that unrelated donor age did not enter the model (ie, it had no effect on recipient outcome).35 However, very few MUDs are > 50 years of age. We explored the influence of donor age among sibling donor transplantations (98% matched siblings) at our institution.36 Older related donor age did not influence engraftment, cytopenias for the first year after transplantation, or the risks of acute GVHD, nonrelapse survival, progression-free survival, or OS. After adjusting for recipient age and other prognostic factors, nonrelapse mortality was lower for older related donor age compared with younger related donor age (HR = 0.64 per decade; P = .013) and survival (HR = 0.76 per decade; P = .045). Although clearly limited by the strong correlation of donor and recipient age, which precludes adequate adjustment, the study supports older sibling donors providing an adequate allograft. A CIBMTR analysis by Alousi et al (discussed in the next section) suggested a threshold whereby matched sibling donors 67 years or older translated into worse survival compared with matched sibling donors 50 to 66 years of age, although excluding an effect or interaction of recipient age is difficult.22
Older matched sibling donor versus MUD
The more germane question has been the optimal choice between a young MUD or an older matched sibling, should they both be available.
A CIBMTR analysis by Alousi et al analyzed transplant outcomes of 2172 recipients 50 years and older with leukemia or lymphoma from sibling donors at least 50 years of age against younger MUDs (mostly 10/10 HLA match) in the largest report to date.22 Matched sibling donors provided statistically improved OS and reduced acute GVHD relative to MUDs. Most other series show at least similar outcomes after matched sibling donors compared with MUD among older patients although sample sizes have been smaller.12 One group found a possible benefit of young MUD in MDS patients, but the above CIBMTR analysis and another study suggested the same to better outcomes using matched siblings.37,38
The above studies have important drawbacks: MUD transplantations were from earlier time periods, outcomes after unrelated donor allografts continue to improve, and data for related donors > 70 years of age remains sparse. In the Alousi study, only 69 of the sibling donors were 70 years or older and 33 patients were at least 70 years of age. Reduced toxicity regimens using alternative donor sources of haploidentical or cord blood transplantation are becoming more frequent and readily applied to older patients.39,40 Series restricted to older adults remain limited.41
Evaluation of older sibling donors: a clinical approach
I advocate performing HLA typing on all siblings without a clear contraindication to donation. Most patients have few siblings and serial HLA typing of the healthiest or youngest siblings first delays the process of clearing older donors who may be the only HLA match and may delay an unrelated donor search. PBSCs from siblings is usually the preferred source, especially in donors 60 years or older, because PBSC yields are more predictable relative to BM.
Older siblings should be evaluated thoroughly for health conditions by a physician knowledgeable in the risk of stem cell harvest but independent of the recipient's need. Special attention should be paid to hematologic abnormalities such as macrocytic anemia or lymphocytosis, which could represent an incipient precursor donor hematologic malignancy. An upper age limit cannot be defined, although donor age > 70 years warrants scrutiny. When medically clearing donors, one should consider the toxicity profile for harvest. Hypertension may be exacerbated by G-CSF–induced bone pain, electrolyte disturbances from diuretics may be worsened from leukapheresis, and antiplatelet agents and anticoagulation may present challenges should a central line be required for harvest. Further discussion with the donor's regular physician or an independent physician at the transplantation center is invaluable in weighing the risks of collection in light of donor health conditions.
Matched sibling donors are preferred over MUDs based on ready availability, lower cost, and possibly improved outcomes. That said, a MUD source still achieves acceptable and nearly equivalent results.
Conclusions
Allogeneic HCT can achieve long-term disease-free control in older adults with high-risk hematologic malignancies. Older recipient or donor age does not obviate potent GVL effects. Patients 50 to 70 years of age with high-risk hematologic malignances, good performance status, and no severe comorbidities may be considered for transplantation early in the disease course. In addition to comorbidity and performance status, “staging the age” through a GA may uncover occult vulnerabilities, allow better risk stratification, and permit enhanced generalization of results. Similar to younger adults, matched sibling donors when medically cleared should be the first donor option. Alternative donor transplantations are also increasingly being studied in older adults. Ongoing and planned prospective studies will explore the risks and benefits of allogeneic HCT in older adults with MDS and AML relative to nontransplant approaches.
Disclosures
Conflict-of-interest disclosure: The author declares no competing financial interests. Off-label drug use: G-CSF to mobilize stem cell donors (most transplantation drugs are not approved to use in transplantation).
Correspondence
Andrew S. Artz, MD, Division of Hematology-Oncology, The University of Chicago Medicine, 5841 S Maryland Ave MC 2115, Chicago, IL 60637; Phone: 773-702-4400; Fax: 773-702-3163; e-mail: aartz@medicine.bsd.uchicago.edu.