Abstract
Cancer-associated thrombosis accounts for almost one-fifth of all cases of venous thromboembolism (VTE) and is a leading cause of death, morbidity, delays in care, and increased costs. Our understanding of risk factors for cancer-associated thrombosis has expanded in recent years, and investigators have begun to use biomarkers and clinical prediction models to identify those cancer patients at greatest risk for VTE. The Khorana Risk Model, which is based on easily obtained biomarkers and clinical factors, has now been validated in several studies. Recent clinical trials of prophylaxis and treatment of VTE in cancer patients are reviewed here. In addition, consensus guidelines and expert opinion regarding management of VTE in specific challenging situations are presented.
Epidemiology and impact of cancer-associated thrombosis
Approximately 20% of all cases of venous thromboembolism (VTE) occur in the setting of cancer,1 and cancer patients are 4- to 7-fold more likely to develop venous thrombotic events compared with patients without cancer.2 In a contemporary retrospective analysis, VTE occurred in 12.6% (2170/17 284) of ambulatory cancer patients receiving chemotherapy compared with only 1.4% (237/17 284) of matched controls without cancer.3 Cancer-associated thrombosis also results in increased morbidity, mortality, and cost of care. Thrombosis is a leading direct cause of death in cancer patients with fatal pulmonary embolism, being 3 times more common in cancer patients than in noncancer patients.4
VTE recurrence and bleeding complications during treatment are also major concerns in cancer patients. A prospective analysis of more than 800 patients with VTE revealed that the 12-month cumulative incidence of recurrent VTE was significantly higher in cancer patients compared with patients without cancer (20.7% vs 6.8%, hazard ratio [HR] = 3.2; 95% confidence interval [CI] = 1.9-5.4).5 Anticoagulant management of cancer-associated thrombosis is especially challenging because bleeding rates are also higher in cancer patients.6 A recent cost-analysis study based on medical claims data showed that cancer patients with VTE used significantly more health care resources compared with cancer patients without VTE.7 This included 3 times as many hospitalizations, increased inpatient and outpatient medical and prescription claims, and increased total health care costs per patient ($74 959 vs $41 691; P < .0001). That study estimated the total VTE-related health care costs to be $9247 per patient with VTE.
Risk factors for cancer-associated thrombosis
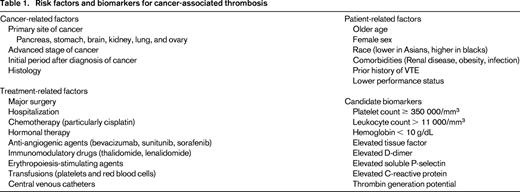
Many risk factors for cancer-associated thrombosis have been identified, including patient-related, cancer-related and treatment-related factors (Table 1). The evidence to support patient-related risk factors, including age, sex, and race, has been reviewed extensively.8 Cancer patients with increased medical comorbidities as measured by the Charlson Comorbidity Index3 and those with specific issues such as infection (odds ratio [OR] = 1.28), pulmonary disease (OR = 1.57), renal disease (OR = 1.41), and obesity (OR = 1.52) have higher rates of VTE.9 Cancer patients with a prior history of VTE have a 6- to 7-fold increased risk of developing VTE compared with patients with no history of VTE.10
Several cancer-specific factors, such as site, stage, and histologic subtype, are also risk factors for thrombosis. In pooled analyses of patients with many types of cancer, those with tumors originating in the pancreas, stomach, brain, kidney, uterus, lung, and ovary had the highest incidence of VTE.3,9,11 Advanced cancer stage is also a risk factor, and one population-based study showed significantly higher rates of VTE in patients with distant metastases (OR = 19.8; 95% CI = 2.6-149).12 Histological subtype also predicts for increased risk of cancer-associated VTE in some types of malignancy. In patients with non-small-cell lung cancer, 9.9% of patients with adenocarcinoma developed VTE in the first 6 months after diagnosis compared with 7.7% of patients with squamous cell carcinoma (HR = 1.9; 95% CI = 1.7-2.1).13 Several studies have demonstrated that the risk of VTE is highest in the initial time period after diagnosis of cancer.
Various treatments also increase the risk of VTE. Surgery is a major provoking factor for VTE in patients without cancer, and underlying cancer increases the risk of surgery related VTE by 2-fold.14 After adjusting for factors such as cancer type and stage, radiation therapy also increased the risk of VTE in a prospective observational study (HR = 2.3; 95% CI = 1.2-4.4; P = .012).15 Many systemic cancer treatments also increase VTE risk, including chemotherapy, which is associated with a 2- to 6-fold increased risk of VTE.16 Cisplatin, a DNA cross-linking agent, is associated with particularly high rates of VTE, with 18% of cisplatin-treated patients experiencing VTE in one study.17 Immunomodulatory drugs such as thalidomide and lenalidomide are also associated with increased risk of thrombosis. VTE occurs in 8% to 27% of myeloma patients treated with thalidomide and lenalidomide in combination with steroids or chemotherapeutic agents.18 Antiangiogenic agents have been noted to have particularly high risk of cancer-associated thrombosis. Several large clinical trials and meta-analyses have demonstrated that bevacizumab is associated with an ∼ 2-fold increased risk of arterial thromboembolic events19 and this agent may also increase VTE risk (although this is controversial).3,20,21 Oral tyrosine kinase inhibitors of VEGF, such as sunitinib and sorafenib, have been shown to increase the risk of arterial thrombotic complications (relative risk [RR] = 3.0; 95% CI = 1.25-7.37; P = .015), but not VTE.22
Supportive therapies used in cancer treatment may also increase VTE. A systematic review encompassing 37 trials and 6743 patients showed that erythropoietin-stimulating agents significantly increased the risk of thrombotic complications in cancer patients (RR = 1.7; 95% CI = 1.4-2.1).23 A meta-analysis of studies in which cancer patients were treated with hematopoietic growth factors suggested there is also an increased risk of thrombosis with the use of GM-CSF.24 Both arterial and venous thrombosis risk was related to red cell (OR = 1.60 for venous and OR = 1.53 for arterial) and platelet transfusion (OR = 1.20 for venous and OR = 1.55 for arterial) in a retrospective discharge database study.25 Indwelling central venous catheters were found to be associated with high incidence of symptomatic (0.3%-28%) and asymptomatic venography-detected catheter-related thrombosis (27%-66%) in a review of prospective clinical trials.26
Risk models and biomarkers
Efforts to identify cancer patients at greatest risk of VTE have focused on clinical factors, biomarkers, and risk prediction tools. A more refined approach to VTE risk stratification will allow for more targeted prophylaxis, which is particularly important in cancer patients, who have a heightened risk of bleeding complications.
Several biomarkers have been identified as potentially predictive of VTE in multivariate analyses (Table 1). Prechemotherapy elevation in leukocyte and platelet count and low hemoglobin levels are all predictive for chemotherapy-associated VTE.27 D-dimer was also predictive of cancer-associated VTE in colorectal cancer patients. Those with elevated D-dimer (defined as > 0.3 mg/L) had a 20% (95% CI = 12%-31%) 1-year incidence of deep vein thrombosis versus 5% (95% CI = 2%-12%) for other patients (adjusted HR = 6.53; 95% CI = 1.58-27.0).28 Elevated D-dimer was also associated with increased risk of VTE by multivariate analysis (HR = 1.8; 95% CI 1.0-3.2; P = .048) in the Vienna CATS registry.15 Studies on the predictive value of tissue factor in cancer-associated thrombosis have been conflicting, but tissue factor is particularly associated with increased VTE risk in pancreatic cancer patients.29 Other biomarkers, including soluble P-selectin, prothrombin fragment 1.2, factor VIII, and thrombin generation potential, have been studied and the results are summarized elsewhere.30
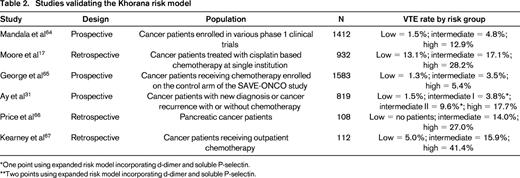
A recently developed risk score sometimes referred to as the Khorana Risk Score can accurately stratify cancer patients into low, intermediate, and high risk for developing VTE (Figure 1).27 This risk score was originally derived from a development cohort of 2701 patients and was then validated in an independent cohort of 1365 patients from a prospective registry. Observed rates of VTE in the development and validation cohorts were 0.8% and 0.3% in the low-risk category, 1.8% and 2% in the intermediate-risk category, and 7.1% and 6.7% in the high-risk category, respectively. This model has now been validated in several other studies (Table 2), including the prospective Vienna CATS study, which expanded the study and improved prediction by including biomarkers (D-dimer and soluble P-selectin).31
The Khorana Risk Score model. The Khorana Risk Score was originally derived from a development cohort of 2701 ambulatory cancer patients initiating chemotherapy and was then validated in an independent cohort of 1365 patients from a prospective registry. Rates of VTE in the derivation and validation cohorts are shown at the top.27
The Khorana Risk Score model. The Khorana Risk Score was originally derived from a development cohort of 2701 ambulatory cancer patients initiating chemotherapy and was then validated in an independent cohort of 1365 patients from a prospective registry. Rates of VTE in the derivation and validation cohorts are shown at the top.27
Prophylaxis for cancer-associated thrombosis
Postoperative setting
Prevention of cancer-associated thrombosis must be addressed separately in 3 distinct populations: postoperative patients, hospitalized nonsurgical patients, and ambulatory patients receiving chemotherapy. Several consensus guidelines have outlined specific recommendations for prophylaxis in these situations.32-35 Many older studies comparing placebo with heparin-based prophylaxis in surgical patients have been summarized in meta-analyses.36 A review of prophylaxis in patients undergoing gynecologic cancer surgery showed that heparin prophylaxis significantly reduced VTE compared with placebo (RR = 0.58; 95% CI = 0.30-0.95) without increasing bleeding complications.36 A meta-analysis of prophylaxis in patients undergoing major abdominal surgery also showed a significant reduction in VTE in patients receiving low-molecular-weight heparin (LMWH) compared with placebo (RR = 0.29; 95% CI = 0.14-0.51; P = .009), and this reduction was greatest in the subgroup of patients undergoing cancer surgery.37 These meta-analyses36,37 suggest that there is no significant difference in efficacy between unfractionated heparin and LMWH prophylaxis, but there is a nonsignificant trend toward decreased bleeding with LMWH. The ENOXACAN38 study compared standard duration to extended 28-day prophylaxis in cancer patients undergoing major abdominal surgery and demonstrated a significant reduction in venous detected VTE (12.0% vs 4.8%; P = .02) without increased bleeding. A systematic review of extended prophylaxis in cancer patients also supports this approach in high-risk patients.39
Hospitalized nonsurgical patients
Unfortunately, there are no cancer-specific prophylaxis trials for the prevention of VTE in nonsurgical hospitalized cancer patients. Three large randomized, double-blinded, placebo-controlled trials in acutely ill medical patients demonstrated reduced rates of VTE with the use of prophylactic LMWH or fondaparinux, with an absolute risk reduction of 2% to 10% in the incidence of VTE over a 3-month follow-up period and no significant increases in bleeding complications.40-42 The percentage of patients enrolled in these trials with cancer was 5% to 15% and subgroup analysis of the MEDENOX study demonstrated a RR of 0.5 in cancer patients with prophylaxis.43 This reduction was not statistically significant due to inadequate power in this subgroup, but the results suggest that prophylaxis offers clinical benefit in hospitalized medically ill cancer patients. Two major prospective randomized studies have examined extended prophylaxis in medically ill patients. The Extended Clinical Prophylaxis in Acutely Ill Medical Patients (EXCLAIM) study44 showed that extended enoxaparin prophylaxis decreased VTE events including asymptomatic deep vein thrombosis (2.5% vs 4%), but it also resulted in increased major bleeding complications (0.8% vs 0.3%). A specific subgroup analysis of the 15% of patients with active or prior cancer was not done, but the reduced VTE rates were most significant in patients > 75 years old and those with level 1 immobility, both of which are common in cancer patients. The recently published MAGELLAN trial,45 which included 7% cancer patients, demonstrated significant reduction in VTE with extended oral rivaroxaban prophylaxis compared with standard duration enoxaparin (4.4% vs 5.7%; P = .02) but, again, the extended prevention arm also experienced more major bleeding events (0.6% vs 0.3%; P = .03). A formal subgroup analysis of cancer patients from the MAGELLAN trial has not been reported.
Ambulatory patients receiving chemotherapy
Most cancer treatment occurs in the outpatient setting, and several recent trials have focused on thromboprophylaxis for solid tumor patients receiving systemic outpatient therapy (Table 3). SAVE-ONCO, the largest and most recent outpatient prophylaxis trial, compared once daily semuloparin with placebo in ∼ 3000 patients with solid tumors and showed a significant reduction in VTE (1.2% vs 3.4%; HR = 0.36; P < .001).46 Similarly, the Prophylaxis of Thromboembolism during Chemotherapy Trial (PROTECHT) study showed that daily nadroparin reduced VTE (2.0% vs 3.9%; P = .02) in patients with “high-risk” sites of cancer, including those with locally advanced or metastatic lung, gastrointestinal, pancreatic, breast, ovarian, and head/neck cancers receiving chemotherapy.47 The low event rate and trend toward increased bleeding events has tempered general application of these findings. A smaller but more recent study examined apixaban, a novel oral anticoagulant, as a prophylactic agent in a mixed cohort of cancer patients and suggested efficacy with a reduced VTE rate (0 vs 10.3%) without increased bleeding.48 Other more focused prophylaxis studies in patients with lung,49 breast,49 and pancreatic cancers50,51 suggest that prophylaxis may benefit high-risk patients such as those with pancreatic cancer and stage IV lung cancer.
VTE prophylaxis studies in cancer patients

CRNM indicates clinically relevant nonmajor bleeding.
*Thirty-two patients to 5 mg, 30 patients to 10 mg, 33 patients to 20 mg.
†In stage IV lung cancer patients, VTE was significantly reduced (3.5% vs 10.2%; odds ratio, 0.32; 95% CI 0.09-0.98; P = .032).
Multiple myeloma and myeloproliferative neoplasms
Multiple myeloma poses a unique challenge in thrombosis prevention. Rates of both venous (3.6%) and arterial (7.9%) thrombotic events in the 5-year period after diagnosis of myeloma are significantly higher than rates in matched controls without myeloma (HR = 4.6 and 1.5, respectively).52 Patients treated with thalidomide and lenalidomide in combination with steroids or other chemotherapeutic agents experience very high rates of VTE. There are no placebo-controlled trials of prophylaxis in multiple myeloma patients, but a recent prospective study compared the efficacy of LMWH, low-dose aspirin (acetylsalicylic acid [ASA]) or low-fixed-dose warfarin in 667 newly diagnosed myeloma patients.53 The incidence of VTE was 5% in the LMWH group, 6.4% in the ASA group, and 8.2% in the warfarin group (P = NS). The investigators concluded that LMWH, warfarin, and ASA are likely to be similarly effective prophylactic regimens except in elderly patients, in whom warfarin showed less efficacy than LMWH. Current consensus guidelines do not recommend routine outpatient VTE prophylaxis, but many do suggest prophylaxis in myeloma patients receiving high-risk regimens (Table 4).
Guidelines for cancer-associated thrombosis
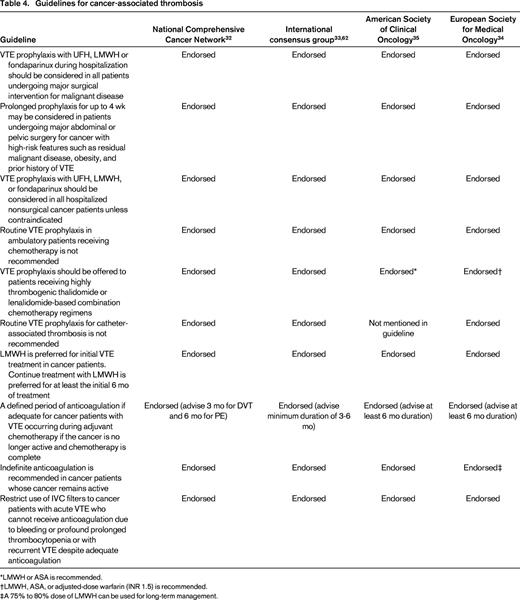
*LMWH or ASA is recommended.
†LMWH, ASA, or adjusted-dose warfarin (INR 1.5) is recommended.
‡A 75% to 80% dose of LMWH can be used for long-term management.
Myeloproliferative neoplasms such as essential thrombocythemia, polycythemia vera, and primary myelofibrosis are associated with increased risk of arterial and venous thrombotic events. In these patients, age > 60 years, history of prior thrombosis, cardiovascular risk factors (hypertension, diabetes), leukocytosis, and Jak2 mutation are associated with higher risk of thrombotic complications.54 The European collaboration on Low-dose Aspirin in Polycythemia Vera (ECLAP) trial demonstrated that ASA significantly reduces thrombotic outcomes in patients with polycythemia vera (RR = 0.40; 95% CI = 0.18-0.91; P = .03).55 Cytoreduction is also important to reduce thrombotic risk in high-risk patients (those > 60 years of age or with a prior history of thrombosis), and hydroxyurea has been shown to be superior to anagrelide for thrombosis risk reduction.56 A recent randomized trial showed that an aggressive cytoreduction strategy with phlebotomy and hydroxyurea for a goal HCT < 45% significantly reduced thrombotic outcomes compared with a less stringent phlebotomy threshold.57
Treatment of cancer-associated thrombosis
Subgroup analysis of studies comparing unfractionated heparin with LMWH for the initial treatment of VTE reported no difference in recurrent VTE, suggesting that either can be used safely and effectively to treat cancer patients with acute VTE.58 However, after the initial 3- to 6-month course of anticoagulation, more extended treatment is needed to prevent recurrence. The Comparison of LMWH versus Oral anticoagulant Therapy for prevention of recurrent venous thromboembolism in patients with cancer (CLOT) study compared dalteparin with oral vitamin K antagonist therapy.59 In this trial, patients in the dalteparin arm received 200 units/kg for the first month, followed by dose reduction to approximately 150 units/kg. Over the 6-month study period, 27 of 336 (8.0%) patients in the dalteparin group compared with 53 of 336 (15.7%) patients in the oral anticoagulant group experienced recurrent VTE (HR = 0.48; 95% CI = 0.30-0.77; P = .0017) with a nonsignificant trend toward increased major (3.6% and 5.6%; P = .27) and reduced total bleeding events (18.5% vs 13.6%; P = .09) in dalteparin-treated patients (P = .09). Based largely on the results of the CLOT trial and other smaller trials summarized in a meta-analysis,60 long-term therapy with LMWH should be viewed as the standard of care in the management of patients with cancer-associated thrombosis. No clinical trials have yet prospectively examined the appropriate length of anticoagulant therapy in patients with cancer and VTE, but in patients with active cancer and minimal bleeding risk, it is reasonable to continue anticoagulation beyond 6 months. In most patients who are in cancer remission, anticoagulation can be discontinued after 6 months, but there may be some cases where continued anticoagulation is needed due to high estimated risk of recurrence.
VTE recurrence during anticoagulant therapy is 3-fold more common in cancer patients than in patients without cancer,5 and recent consensus guidelines33 have addressed how to manage recurrent VTE. These guidelines advocate a 20%-25% dose escalation in patients already receiving full-dose LMWH, return to standard treatment dose of LMWH in patients who had been dose reduced in the maintenance phase of treatment, and conversion to full treatment dose LMWH in patients receiving warfarin. This recommendation is at least partly based on one retrospective study suggesting this approach results in acceptable VTE control.61 In this study, 70 patients with recurrent VTE (67% of patients were receiving LMWH and 33% were receiving warfarin) were managed with this approach and, at 3 months of follow-up, 8.6% had experienced a second VTE recurrence and 4.3% had a bleeding complication. Many guidelines recommend considering inferior vena cava (IVC) filter placement for recurrent VTE despite adequate anticoagulation, but evidence to support benefit from an IVC filter in this setting is lacking and the risk of filter-associated thrombosis in prothrombotic conditions such as cancer may be higher (Table 4). Although several novel oral-targeted anticoagulants have been shown recently to be effective for the treatment and prevention of VTE, there are insufficient data to support the use of these agents in cancer patients at this time. Bleeding and thrombocytopenia are common complications in cancer patients, which make VTE management problematic. These situations must be considered on an individual basis with accurate and ongoing assessment of the risk of bleeding and recurrent or progressive thrombosis. Guidelines support the consideration of an IVC filter when bleeding precludes the use of anticoagulation, but when bleeding risk has resolved, filter removal and/or initiation of anticoagulation should be promptly arranged. There are insufficient data to mandate a precise platelet threshold at which to stop anticoagulation, but many consider full-dose anticoagulation unsafe when the platelet count is consistently below 50 000/μL. Intermediate or prophylactic doses of anticoagulants can sometimes be used when the platelet count is in the 20 000-50 000/μL range.
Guidelines regarding the prevention and management of cancer-associated thrombosis
Many guidelines have been developed and published over the past 5 years addressing important issues in the prevention and management of cancer-associated thrombosis.32,33,62,63 The major recommendations from these guidelines are summarized in Table 4. On most major points pertaining to prevention and management of cancer-associated thrombosis, the major guidelines are in agreement.
Conclusions
Cancer-associated thrombosis is a common problem with various risk factors including patient-, cancer-, and treatment-related issues. Biomarkers and risk models such as the Khorana model will be important in identifying those patients who will benefit most from prophylaxis strategies, and clinical trials are currently under way using such strategies. There are also many challenges encountered in managing cancer patients with VTE, most notably the balance between adequate anticoagulation and bleeding risk.
Disclosures
Conflict-of-interest disclosure: C.W.F. has received research funding, has consulted for, and has received honoraria from Eisai. G.C.C. declares no competing financial interests. Off-label drug use: None disclosed.
Correspondence
Charles W. Francis, MD, 601 Elmwood Avenue, Box 704, Rochester, NY 14642; Phone: 585-273-3258; Fax: 585-273-1042; e-mail: charles_francis@urmc.rochester.edu.