Abstract
Heparin-induced thrombocytopenia (HIT) is a prothrombotic disorder caused by antibodies that recognize complexes of platelet factor 4 (PF4) and heparin. HIT is frequently considered in the differential diagnosis of thrombocytopenia occurring in patients on heparin therapy. HIT is a challenging diagnosis because of routine heparin use in hospitalized patients, the common occurrence of thrombocytopenia, and high rates of anti-PF4/heparin seroconversions in patients treated with heparin. Our diagnostic approach to HIT is presented here, underscoring critical elements of clinical and laboratory evaluation.
Introduction
Heparin-induced thrombocytopenia (HIT) is a potentially life-threatening immune complication that occurs after exposure to unfractionated heparin (UFH) or, less commonly, to low-molecular-weight heparins (LMWHs).1 It is characterized by declining platelet counts beginning 5 to 14 days after heparin exposure and occurs in isolation (isolated HIT) or concurrently with new arterial and venous thrombotic complications.2 HIT is caused by antibodies directed against complexes formed by a platelet protein, platelet factor 4 (PF4), and heparin (H). Circulating immune complexes containing IgG and PF4/H complexes bind to platelet and monocyte Fc receptors and promote cellular activation, leading to procoagulant microparticle release and thrombin generation.3,4
Historically, the challenge associated with HIT was a lack of awareness of the syndrome and its pursuant complications; the challenge now is in the overdiagnosis and treatment of HIT. With the widespread availability of screening immunoassays and the desire of clinicians to avoid the thrombotic consequences associated with true disease, many patients without HIT now suffer needless morbidity due to bleeding complications from the use of alternative anticoagulants. To avoid a reflexive diagnosis of HIT in the heparinized thrombocytopenic patient, clinicians must have a sound understanding of the clinical and laboratory diagnostic elements essential for a diagnosis of HIT. Here, we review our diagnostic and management strategy in evaluating the common presentation of thrombocytopenia in a heparinized patient.
Diagnosing HIT: the clinical challenge
HIT is a challenging clinical diagnosis. The increasing use of UFH/LMWH for thromboprophylaxis in hospitalized patients5 coupled with the frequency of thrombocytopenia, particularly among critically ill patients,6 results in a significant overlap of patients suspected of HIT. In a recent registry of ∼1000 patients treated with thrombo-prophylactically dosed heparin, 19% (n = 190) met thrombocytopenia criteria compatible with a diagnosis of HIT (as defined by a platelet count < 150 × 109/L or a > 50% decrease in platelet counts), but only 5% of patients were diagnosed with HIT.6 This study and clinical experience suggest that other causes of thrombocytopenia, such as infection, medications, and/or intravascular devices, are more likely to be etiologic for thrombocytopenia than HIT.
Prospective and retrospective studies indicate that HIT occurs in < 0.1% to 5% of exposed patients and disease incidence varies by drug- and/or host-related risk factors. The incidence of HIT is 10-fold higher with UFH compared with LMWH, whereas fondaparinux is rarely associated with HIT.7 To a lesser extent, other drug-associated risk factors include: duration of therapy (≥ 6 days) and source of heparin (bovine > porcine).2 Among host variables, surgical patients (trauma and orthopedic patients) appear to be at greater risk of HIT (2%-5%) compared with patients exposed to heparin for medical indications (0.8%-3%).8 HIT is exceedingly rare in pediatric patients, obstetric patients, and patients on chronic hemodialysis.1
The reasons for developing the PF4/H immune response and subsequent HIT are poorly understood. Recent studies indicate that PF4/H antibody sensitization may be associated with prior bacterial infections,9 whereas murine studies suggest that the biophysical features of circulating PF4/H complexes also contribute to immunogenicity.10
Our approach to the heparinized patient with thrombocytopenia
The evaluation of thrombocytopenia in a heparinized patient is a common consult request in both the academic and community hospital. Because > 94% of patients develop absolute or relative thrombocytopenia in the context of HIT,11 the first diagnostic element to establish is the presence of thrombocytopenia and/or new thrombosis in the setting of heparin therapy. This diagnostic criteria is fulfilled with a ≥ 30% decrease from baseline platelet count resulting in an absolute thrombocytopenia (≤ 150 × 109/L) or even a normal platelet count.12 When absolute thrombocytopenia occurs in HIT, the counts are moderately decreased (50-70 × 109/L). Severe thrombocytopenia (< 20 × 109/L) is infrequent in HIT and is often associated with disseminated intravascular coagulation or severe thrombotic HIT.11
Patients who have recently undergone surgery may experience a rebound thrombocytosis and, in this situation, the postoperative rebound count should be considered baseline. In exceptional circumstances, thrombosis may occur in the absence of thrombocytopenia, as in patients with heparin-induced skin necrosis.13 Patients are considered to have isolated HIT when thrombocytopenia is the only manifestation of HIT. Because thromboembolic complications occur in ∼ 17% to 53% of patients who present with isolated HIT,11,14 these patients should be evaluated for new or occult thrombosis.
In the majority of patients with HIT, however, thrombosis occurs concomitantly with thrombocytopenia.11 Venous thrombotic events occur more often than arterial (frequency of 2.4-4:1),11 with lower limb deep venous thrombosis and pulmonary embolism as the predominant complications.11 Rare and unusual sites for thrombosis, such as adrenal vein thrombosis, cerebral vein thrombosis, skin necrosis, and venous limb gangrene, should prompt clinical consideration for HIT.13 Because thrombophilias (associated with deficiencies in protein C, protein S, antithrombin III, or mutations leading to factor V Leiden and prothrombin gene mutations) are not correlated with thrombosis in HIT,15 we see no reason to order tests of hypercoagulability in patients suspected of HIT.
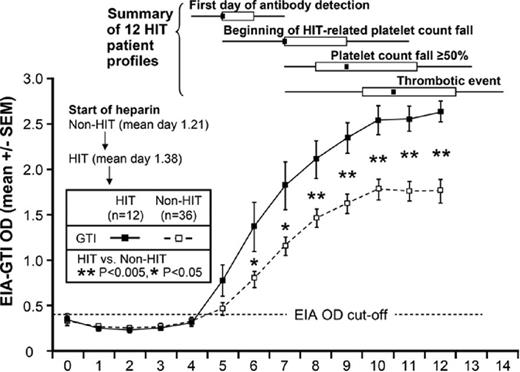
The second major diagnostic element to document in the evaluation of the heparinized, thrombocytopenic patient is the timing of thrombocytopenia and thrombosis in relation to heparin therapy. A detailed evaluation of recent and remote heparin exposure should be obtained and correlated in a flow sheet with the onset of thrombocytopenia. In heparin-naive patients, thrombocytopenia and/or thrombosis occurs 5 to 14 days after the initiation of heparin therapy in the wake of newly formed PF4/H antibodies.14 For patients who have received heparin in the last 100 days, thrombocytopenia can develop rapidly (median = 10.5 hours) due to circulating PF4/H antibodies.16 In a temporal variant termed “delayed-onset HIT,” thrombocytopenia and/or thrombosis can present days to weeks after heparin discontinuation.17 Because thrombocytopenia and thrombosis occur as a consequence of circulating PF4/H antibodies, PF4/H seroconversion should be present at the time of thrombocytopenia and/or thrombosis. The development of thrombocytopenia and/or thrombosis before PF4/H seroconversion is unlikely to be HIT, because PF4/H antibody seroconversions usually predate thrombocytopenia and/or thrombosis by 2 to 4 days (Figure 1).14
Evolution of the HIT immune response in relation to clinical manifestations. Twelve patients with HIT (■) and 36 control patients who were PF4/H seropositive but did not have HIT (□) were monitored after orthopedic surgery for PF4/H antibodies, thrombocytopenia, and thrombosis. The time course of PF4/H seroconversions are shown on the x-axis and OD levels are shown on the y-axis. The difference in OD between the HIT patients and the seropositive non-HIT controls was statistically significant (P < .05 by unpaired t test). Four key events for the 12 patients with HIT are shown: first day PF4/H antibody was detectable, onset of HIT-related decrease in platelet count, decrease in platelet count ≥ 50%, and thrombotic event. These events are shown as medians (small black squares within rectangles), interquartile ranges (length of open rectangles), and ranges (ends of thin black lines). Adapted with permission from Warkentin et al.14
Evolution of the HIT immune response in relation to clinical manifestations. Twelve patients with HIT (■) and 36 control patients who were PF4/H seropositive but did not have HIT (□) were monitored after orthopedic surgery for PF4/H antibodies, thrombocytopenia, and thrombosis. The time course of PF4/H seroconversions are shown on the x-axis and OD levels are shown on the y-axis. The difference in OD between the HIT patients and the seropositive non-HIT controls was statistically significant (P < .05 by unpaired t test). Four key events for the 12 patients with HIT are shown: first day PF4/H antibody was detectable, onset of HIT-related decrease in platelet count, decrease in platelet count ≥ 50%, and thrombotic event. These events are shown as medians (small black squares within rectangles), interquartile ranges (length of open rectangles), and ranges (ends of thin black lines). Adapted with permission from Warkentin et al.14
The final and most difficult diagnostic element in the evaluation of the heparinized, thrombocytopenic patient is excluding other causes of thrombocytopenia. Depending on the duration of the hospitalization and comorbidities, this last criterion is often the most likely to stump even the HIT-expert hematologist. Causes of thrombocytopenia such as nonheparin medications (IIb/IIIa inhibitors, antibacterial and antiviral agents, chemotherapeutic agents), infection (bacterial, viral, or fungal), mechanical devices (intraaortic balloon pump, left-ventricular assist device, extracorporeal membrane oxygenation), and consumption can occur concurrently. In these cases, additional testing, such as a blood film review for the presence of Döhle bodies in white blood cells (indicating infection) or schistocytes (indicating mechanical fragmentation) or positive microbial cultures may provide support for other causes of thrombocytopenia. It is also in this setting, in which there are one or more causes of thrombocytopenia and a persistent clinical suspicion for HIT, that laboratory evaluation for HIT antibodies becomes essential.
We advocate using clinical scoring systems for the systematic application of the diagnostic criteria reviewed above. The “4Ts” scoring system developed by Warkentin et al is the easiest to use and has been validated in numerous studies for excluding HIT (Table 1). The 4Ts assesses the presence of 4 typical features of HIT: (1) magnitude of Thrombocytopenia, (2) Timing of the decrease in platelet count or complication in relation to heparin exposure, (3) presence of Thrombosis or other HIT-associated complication, and (4) no oTher explanation for thrombocytopenia present. Each of these 4 features is assigned a score of 0, 1, or 2, for a total score ranging from 0 to 8. Scores of 0 to 3 are consistent with a low pretest probability for HIT, scores of 4 to 5 with intermediate probability, and scores of 6 to 8 with high probability.18 In a recent meta-analysis of studies using the 4Ts scoring system, a low-probability 4Ts score (0-3) was associated with a high negative predictive value (0.998; 95% confidence interval, 0.970-1.000) and reliably excluded patients without serologic evidence of HIT regardless of the study population, prevalence of HIT, or the expertise of the scoring party.18 In this study, the positive predictive value of an intermediate score (4-5) was 0.14 (0.09-0.22) and a high score (> 6) was 0.64 (0.40-0.82). The positive predictive value of the 4Ts score was reduced in settings in which the prevalence of HIT was low (≤ 0.10)18 and was dependent on the expertise of the scoring party.19 Therefore, the strength of the 4Ts scoring system is in excluding HIT when a low-probability score is obtained, allowing the clinician to continue heparin therapy and to forego further unnecessary testing or treatment.18
4Ts scoring system

Total score of ≥ 6 points, high probability of HIT; 4-5 points, intermediate probability of HIT; ≤ 3 points, low probability of HIT.
To improve on the diagnostic utility of the 4Ts clinical scoring system, a more detailed scoring system, the HIT expert probability score (HEP) was developed recently (Table 2). This system was developed using broad expert opinion to assign 8 clinical features of HIT each a point ranging from −3 to +3 based on diagnostic relevance. The HEP scoring system provides more detailed diagnostic criteria, particularly with regard to “other causes of thrombocytopenia” and may be particularly useful for general providers who lack familiarity with HIT. In the original study describing the HEP score, the scoring system yielded 100% sensitivity and 60% specificity when applied to a validation cohort of patients with and without HIT. Compared with the 4Ts score, the HEP score demonstrated improved correlation with serologic HIT testing and improved interobserver agreement.20 Prospective validation of the HEP score is currently under way.
HEP score
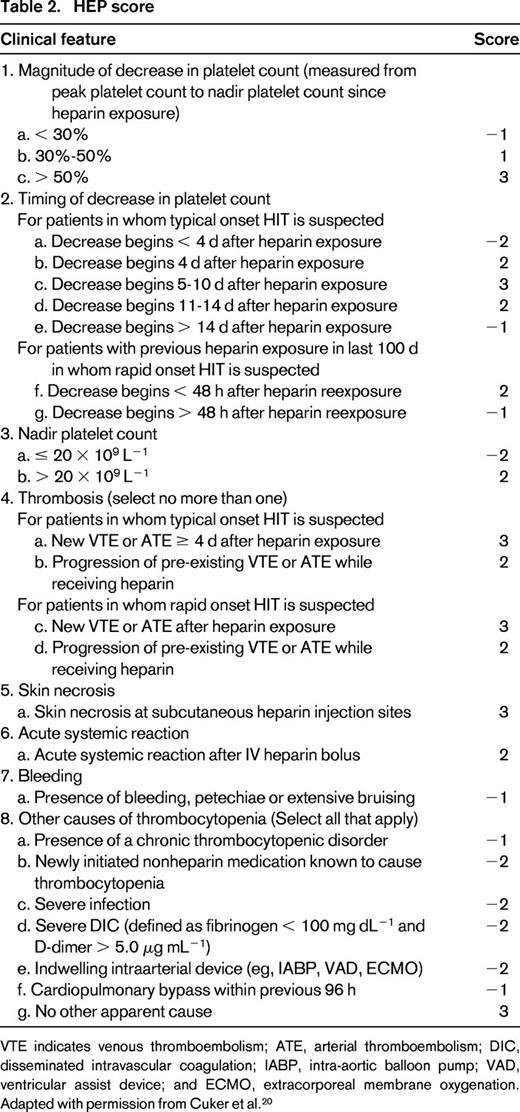
VTE indicates venous thromboembolism; ATE, arterial thromboembolism; DIC, disseminated intravascular coagulation; IABP, intra-aortic balloon pump; VAD, ventricular assist device; and ECMO, extracorporeal membrane oxygenation. Adapted with permission from Cuker et al.20
Diagnosing HIT: the laboratory challenge
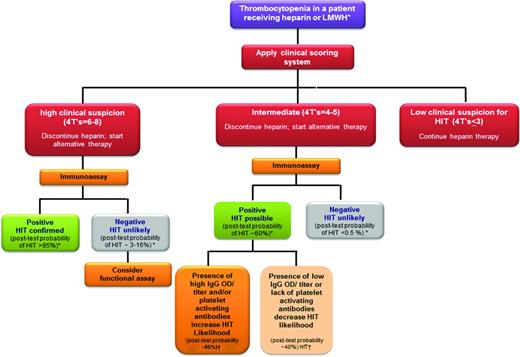
The diagnosis of HIT requires both clinical and laboratory evaluation (Figure 2). As stated above, in the meta-analysis of studies applying the 4Ts for the evaluation of HIT, the positive predictive value of a high score was only 64%. Even in cases of diagnostic certainty based on clinical evaluation, we strongly recommend laboratory testing to confirm the presence of PF4/H antibodies, not only for documentation in the medical record, but also for future indications when antibody levels may need to be followed (such as for planning of cardiac surgery or other interventions).
Diagnostic algorithm. Shown is our diagnostic approach for HIT using clinical and laboratory evaluation. *Based on Pouplard et al.44 †Based on Nellen et al.35
Our institution, like most institutions around the country, offers only an immunoassay onsite for HIT testing. Immunoassays use immobilized PF4/H complexes on microtiter plates or gel particles to identify antigen-specific antibodies in plasma or serum. These immunoassays are widely available, have a rapid turnaround time, and are highly sensitive (> 99%).2,21 However, they are notorious for poor specificity (30%-70%) due to their detection of nonpathogenic antibodies caused by exposure to UFH, LMWH, or fondaparinux.2
To overcome their poor specificity, immunoassays have been “tweaked” to be more clinically useful through: (1) detection of IgG antibodies, (2) quantification of the antibody level or optical density (OD), and (3) utilization of high heparin concentrations to dissociate or compete with binding of antibodies to PF4/H complexes.
The detection of IgG antibodies improves the specificity of immunoassays by identifying antibodies that elicit platelet activation. In a pooled analysis of tests performed on 3366 patients, IgG-specific ELISAs were associated with greater specificity compared with polyspecific ELISAs (93.5% vs 89.4%, respectively). However, the improved specificity occurred at the expense of slightly lower sensitivity (95.8% vs 98.1%, respectively).21 By relying on IgG-specific assays, there is the possibility that some patients with true HIT will have a negative IgG assay. In these exceptional cases in which clinical suspicion remains high in spite of a negative IgG assay, the availability of a polyspecific assay may provide reassurance. If the polyspecific assay is positive, this may reveal the presence of HIT IgA or IgM antibodies, which in some reports have been associated with HIT.22,23 The magnitude of the OD is increasingly being used as a surrogate marker for identifying antibodies with pathogenic potential, and we routinely rely on this serologic feature in our diagnostic assessment. In several studies evaluating the diagnostic performance of a variety of immunoassays, there appears to be a linear correlation between the OD value and the presence of platelet-activating antibodies, the likelihood of HIT, and the risk for subsequent thromboembolism.24,25 However, simply increasing the cutoff of the OD value to improve diagnostic specificity invariably compromises the sensitivity and leads to unacceptable numbers of false negatives.26,27
The use of a high heparin “confirmatory” step is yet another serologic parameter that has been investigated for improving assay specificity. Because HIT antibodies show heparin-dependent binding, the addition of excess heparin (2-100 U/mL) competes and/or disrupts binding of HIT antibodies to preformed PF4/H complexes. A positive result (consistent with the presence of heparin-dependent antibodies) is present when a ≥ 50% reduction in OD is seen in the presence of high-dose heparin (100 units). Application of the high-heparin step improves the specificity of immunoassays,28 with the exception of HIT antibodies with strong ELISA reactivity, which may not be inhibited by high doses of heparin.27 Our institution provides OD values for the polyspecific and IgG-specific immunoassays, as well as results of the heparin confirmatory step, to guide the laboratory evaluation of HIT. For the majority of patients with suspected HIT, the clinical evaluation combined with the serologic results from these assays provides sufficient information to forgo testing by functional assays (see “Combining the clinical and laboratory evaluation” below).
In cases in which there is intermediate clinical suspicion and immunoassays are not particularly helpful because of mixed serologic features (high polyclonal, but negative IgG or high IgG OD and negative excess heparin step), we send samples out for functional assay testing. Historically, before the availability of PF4/H immunoassays, functional assays were used routinely to establish the presence of heparin-dependent antibodies. In functional assays, platelets from normal donors are incubated with patient plasma in the presence of buffer or various doses of heparin (low- or high-dose heparin). In the presence of HIT antibodies, low-dose heparin, but not buffer or high-dose heparin, activates platelets. The end point used for detecting platelet activation can vary. Functional end points of light transmission (platelet aggregation or visualization of magnetic beads), release of radioactivity (platelets labeled with radioactive serotonin), or membrane activation markers (flow cytometry) are described in the literature.
The functional assays, in particular the serotonin release assay, have high specificity (> 95%)29 and a high positive predictive values (89%-100%).8 However, the sensitivity of these assays is largely dependent on the type of functional end point used (serotonin release assay > flow cytometry > light transmission; 62%-100%)30,31 and is heavily influenced by technical variables, including baseline platelet reactivity and complexity of assay. For these reasons, functional assays are limited to major commercial laboratories or referral laboratories at academic medical centers.
The “ideal” assay for detecting HIT antibodies would combine the sensitivity and technical feasibility of the immunoassays with the specificity of functional assays. Such hybrid assays are now in development. One recently described assay uses the expression of exogenous FcγRIIA receptors on lymphocytes32 and another measures proteolysis of the FcγRIIA receptor33 as a surrogate marker for platelet activation. Both of these assays hold promise for improving the specificity of and simplifying functional assays.
Combining the clinical and laboratory evaluation
Our approach to evaluating the thrombocytopenic patient for HIT combines clinical assessment (using the 4Ts or the HEP score) with laboratory evaluation (Figure 2 shows the diagnostic algorithm). For patients with a low clinical suspicion of HIT, we do not recommend testing due to the high negative predictive value (NPV) of scoring systems (NPV = 0.998).18 For patients with a high clinical suspicion (eg, 4Ts ≥ 6), we consider a positive immunoassay as confirmation of HIT. Studies have shown that patients with a high 4Ts score and a positive immunoassay have a posttest probability of HIT approaching 100% (Table 1).34,35 Although rare, in our clinical experience, some patients can present with a high clinical suspicion but have negative immunoassay results. Although the NPV of immunoassays is high (false negative rate of < 0.5%, NPV = 99%),22,34 in patients with high clinical suspicion, there remains a quantifiable likelihood of HIT. In one study of 213 patients evaluated by the polyvinyl sulfate ELISA and the particle gel-immunoassay, patients with a high suspicion of HIT and a negative immunoassay had a posttest probability of HIT of < 3% with the polyvinyl sulfate ELISA and 16% with the negative particle gel immunoassay.34 Because patients with high clinical suspicion and negative immunoassay results may have heparin-dependent antibodies to other related antigens, such as IL-8 or neutrophil activating protein-2 (NAP-2), a functional assay may be helpful in further evaluating these patients.
The majority of patients we evaluate fall into the “intermediate” category (4Ts = 4-5) of clinical suspicion for HIT. Serologic information (polyspecific vs IgG, OD, and a high heparin “confirmatory” step) and/or functional assay results are particularly helpful for the evaluation of this group of patients (Figure 2). A negative immunoassay in a patient with intermediate clinical suspicion virtually eliminates the possibility of HIT because of the high NPV of immunoassays. The posttest probability for this group of patients is estimated at ∼ 0.6% to 10%, depending on the type of immunoassay.34,35 Conversely, a positive immunoassay for patients with intermediate clinical suspicion increases the likelihood of HIT to 40% to 60%.34,35 Additional testing in this group of patients, either by functional assay and/or evaluation of serologic features (IgG, OD, or excess heparin) could further refine the risk estimate for HIT. For instance, in a recent study by Nellen et al, patients were risk stratified using the 4Ts and tested by both immunoassays and functional assays.35 Posttest probabilities were calculated using positivity in the functional assay and application of Bayes' theorem. Patients in the intermediate category with a high titer in the particle gel assay (titer = 8) had a posttest probability of 65%, compared with 41% for patients who only met the threshold for positivity in the assay (titer = 4). Additional investigations on the impact of combining clinical suspicion with the various serologic variables (IgG isotype, OD, and high heparin) are likely to further clarify the utility of immunoassays and may even one day obviate the need for functional assays.
Management of the heparinized patient with thrombocytopenia
The management of patients suspected of HIT begins at the time of consultation, often long before results of laboratory testing are available (Figure 2). For patients with a low clinical suspicion of HIT, we do not obtain testing and recommend continuation of heparin therapy. For patients with an intermediate or high clinical suspicion of HIT, we discontinue heparin and initiate an alternative anticoagulant. Argatroban is the only nonheparin anticoagulant currently approved by the Food and Drug Administration for the treatment of HIT, but other agents such as bivalirudin and fondaparinux are increasingly used based on successful clinical experience. Due to space limitations, we will not review the pharmacology, dosing, and clinical experience of the nonheparin anticoagulants in HIT. The reader is referred to recent excellent comprehensive reviews on these topics.1,36
The choice of alternative anticoagulant is primarily driven by comorbidities and half-life considerations. We prefer to use parenteral direct thrombin inhibitors (DTI) in the critically ill patient, often due to the need for procedural interventions and/or underlying bleeding risk in these patients, in whom a shorter half-life is desirable. We recommend judicious use of these alternative anticoagulants due to high hemorrhagic potential and lack of an antidote. If laboratory evaluation later reveals a low likelihood of HIT, we discontinue alternative anticoagulants and resume heparin therapy.
For patients with a laboratory-confirmed diagnosis of isolated HIT, we recommend ultrasound imaging of upper and lower extremities to rule out subclinical thrombosis, because findings of thromboembolic complications would alter the duration of anticoagulation therapy. Once the patient is anticoagulated on an alternative therapy and platelet counts have increased back to baseline, we initiate warfarin therapy at a low dose (5 mg). Current guidelines recommend up to 4 weeks of anticoagulation with warfarin for patients with isolated HIT and a minimum of 3 months for patients with HIT complicated by thrombosis.1 For patients with refractory or progressive thromboses on DTIs, we use plasmapheresis with fresh-frozen plasma replacement as salvage therapy to reduce antibody burden.37 It should be noted that the use of plasmapheresis in HIT is not a categorized indication by the American Society of Apheresis.37
In the remainder of the discussion, we highlight clinical questions that require further study. We bring attention to these questions not only to define best clinical practice, but also to help move the management of HIT from the realm of case series and expert opinion to evidence-based standards.
1. Can PF4/H sensitized patients be exposed or reexposed to heparin?
Whether sensitized patients with or without HIT can receive future heparin therapy is unresolved. Because data are lacking, current guidelines advise limiting heparin reexposure in patients with a history of HIT.1 The PF4/H immune response peaks between 5 and 14 days after heparin exposure and wanes over time (120-360 days).16,38 In one case series, HIT patients were safely reexposed to heparin months to years (10 months to 13 years) after their initial diagnosis, without disease recurrence.16 It is also recognized that 8% to 15% of patients treated with UFH and/or LMWH for medical indications and > 50% of patients undergoing cardiac surgery become sensitized to heparin without developing HIT.8 Because sensitized patients are at theoretical risk for rapid-onset HIT, it is not clear if heparin therapy can be safely continued in this patient population. Another unanswered question is whether these sensitized patients are at higher risk for HIT or PF4/H seroconversions on subsequent heparin exposure. These questions can be readily answered through well-designed prospective observational or randomized studies.
2. What is the optimal intensity and duration of anticoagulation in patients with isolated thrombocytopenia in HIT?
Patients with HIT and isolated thrombocytopenia are hypercoagulable and are at high risk for developing new thromboses (∼ 17%-53% over a 30-day period).39,40 Although there is consensus among experts that patients with isolated HIT should be treated in the short term with alternative anticoagulants, there is no firm consensus on the intensity or duration of anticoagulation for this patient population. Current American College of Chest Physicians guidelines, based on expert opinion, recommend 4 weeks of anticoagulation therapy.1 Prospective multicenter studies could readily establish the optimal intensity and duration of therapy in this patient group.
3. What is the optimal management strategy in cardiac surgery patients with HIT or who are suspected of HIT?
The management of patients with HIT who require cardiac surgery is not well defined. For patients requiring cardiac surgery with a remote history of HIT or a diagnosis of HIT > 3 months ago, we repeat PF4/H serologies. If seronegative, patients can be safely reexposed to heparin during cardiac surgery. If patients are still seropositive by immunoassay, additional testing by functional assays is recommended if time allows. Alternatively, for patients with acute HIT and/or emergent indications, cardiac surgery can be performed with the use of DTIs.41,42 The bleeding risk with DTIs during cardiac surgery, however, is not trivial. At our institution, some surgeons deem the bleeding risk unacceptable and will not perform surgery using DTIs. For these patients, management approaches extend from delaying surgery, if possible, to the use of plasmapheresis.43 Prospective studies could help to delineate optimal management strategies for this high-risk patient population.
Acknowledgments
This work was supported by the National Institutes of Health (grants HL110860, HL109825, and AI101992 to G.M.A. and grants 2T32HL007057-36 and 1F32-AI108118-01 to G.M.L.).
Disclosures
Conflict-of-interest disclosure: G.M.A. has received research funding from Teva Pharmaceuticals. G.M.L. declares no competing financial interests. Off-label drug use: None disclosed.
Correspondence
Gowthami M. Arepally, MD, Associate Professor, Medicine, Division of Hematology, Department of Medicine, Duke University Medical Center, DUMC Box 3486, Room 301 Sands Building, Durham, NC 27710; Phone: 919-668-1550; Fax: 919-684-2420; e-mail: arepa001@mc.duke.edu.