Abstract
The medical effects of transfusing stored RBCs is an area of significant concern that has received substantial attention in recent years. Retrospective trials show all possible outcomes, including sequelae from transfusing older RBCs, no difference between older and fresher RBCs, and a benefit to older RBCs. Several prospective clinical trials are under way to further investigate potential untoward effects of stored RBCs. Thus far, the issue of potential sequelae from transfusing stored RBCs remains a highly controversial issue. However, what is not controversial is that RBC storage is an unnatural state during which a series of substantial changes take place to the stored RBCs. These changes result in the formation of cellular and chemical entities known to have biological activities in other settings, giving rise to several distinct hypotheses by which stored RBCs may alter recipient biology. Herein, the clinical background and basic science of RBC storage are reviewed, with a particular focus on factors that may complicate hypothesis testing and obfuscate underlying biologies. The complexity of the RBC storage lesion, donor-to-donor variation, and the diversity of recipient pathophysiologies remain a challenge to prospective trials assessing the safety of stored RBCs.
Introduction
In recent years, there has been an evolving and escalating debate regarding a fundamental issue in transfusion medicine: what is the effect of storing blood products on outcomes in transfusion recipients? Blood storage is currently a logistical necessity to maintain an adequate blood supply. RBCs are collected in an acidic solution and then stored in a plastic bag filled with sugar at 4°C. During storage (up to 42 days), RBCs are metabolically active in the absence of any waste disposal system (ie, no kidneys or liver), so the stored cells are essentially marinated in a bath of ever-increasing waste products (eg, lactate). What seems clear is that storage is a distinctly unnatural state to which we expose blood cells for various periods of time. The overall issue breaks down to 2 major questions: (1) to what extent do stored blood cells maintain their desired function and therefore constitute an efficacious product?, and (2) are there untoward effects of storage based changes that result in medical sequelae due to toxic substances that accumulate in the blood?
Although the above questions are conceptually simple and straightforward, generating meaningful answers is a considerable challenge with which the field of transfusion medicine has been wrestling. These difficulties stem from several sources, including the current lack of optimal assays and thus the inability to observe certain biologies, substantial donor-to-donor differences in blood storage, and the wide variety of disorders for which transfusion is given. This landscape renders the question “is stored blood efficacious?” unclear and the more generalized concern, “is stored blood bad?” a grossly oversimplified question for which a meaningful answer may not easily be generated. However, breaking down the question into more focused inquiries regarding subsets of patients and categories of blood aging may allow a more accurate analysis.
Is stored blood efficacious?
The Food and Drug Administration criteria by which storage solutions are approved, which are the same criteria that are generally used in clinical research, do not measure the function of the blood products being transfused. RBC storage criteria include hemolysis of less than 1% and average 24 hour posttransfusion recoveries of 75% or greater, which remain the only useful metrics that are accurately assessed.1 These metrics are by no means illogical; indeed, an RBC that does not survive storage or cannot circulate upon transfusion will de facto not be able to deliver oxygen, collect carbon dioxide, or participate in other RBC functions. Attempts are certainly made to measure parameters that may correlate to function. For example, RBC storage studies routinely measure rheological properties of RBCs, some RBC surface molecules, and RBC metabolism (including 2,3-DPG levels that regulate oxygen affinity curves). Furthermore, additional molecular changes have been described that may diminish an RBC's ability to deliver oxygen to tissues, not only through oxygen dissociation, but also through additional factors such as regulating vasodilatation through the oxygen-sensing and nitric oxide (NO)–modulating properties of hemoglobin.
There is a strong argument to be made that stored RBCs have decreased function after transfusion.2-4 Some have even expressed the view that RBCs have no function immediately after transfusion and do not improve oxygenation.2-4 This extreme view appears to be contradicted by the observation that patients who are severely anemic and symptomatically hypoxic improve clinically after transfusion. Similarly, some patients undergo massive transfusion protocols in which essentially every circulating RBC was recently transfused. The observation that such patients live indicates that stored RBCs do have at least some function posttransfusion. Finally, chronically anemic patients and those requiring hematological support during reconstitution after BM transplantation do not survive in the absence of transfusion. However, such observations do not indicate that stored blood cells function optimally (or even well), especially in the early times after transfusion. It is also worth noting that even if the transfused blood cells were functioning optimally, this does not necessitate that transfusions would have a positive effect upon medical outcome in a variety of settings in which the indication for transfusion itself is questioned.5
In aggregate, it seems that current storage technologies allow the transfusion of blood cells that retain some of their native function. That having been noted, it is likewise true that the current metrics by which we measure RBC storage quality do not include the ability to deliver oxygen or regulate vascular tone. Rather, circulation (without any measure of function) is the current in vivo criterion that is used. Therefore, as ongoing efforts are made to characterize and improve blood storage conditions, the field must remain mindful of the disconnect between what is measured as metrics of quality and the in vivo function that we are seeking to introduce with transfusion.
Is stored blood bad?
Several highly provocative retrospective studies have reported that transfusing older RBC units results in significantly worse medical outcomes compared with transfusing fresher RBC units. However, careful analysis of the literature has also revealed a substantial number of retrospective studies indicating no difference between fresh and old blood, and even some indicating that older blood is safer than younger blood.6-8 It has been argued that the data are sufficiently conflicting and the independent studies so variant in approach so as to preclude formal meta-analysis.9 Given this ambiguity and the intrinsic problems of retrospective analyses, several prospective randomized trials have been launched in the hope of clarifying this issue (Table 1)10-12 ; however, at the current time, this issue remains a matter of dispute and debate.
Ongoing randomized controlled trials testing effects of RBCs stored for longer versus shorter periods of time
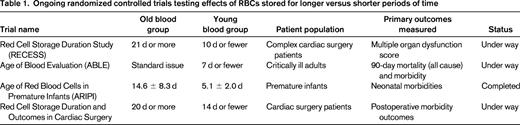
It has been noted that, due to the substantial number of transfusions annually (1 of every 70 Americans), even small effects on medical outcome will have a substantial impact. In this context, it has been argued that the statistical power of the ongoing prospective trials, while sufficient to test larger effects, may miss smaller differences that would be medically meaningful.13 Nevertheless, prospective randomized clinical trials are the best approach with which to resolve the fundamental question: does the transfusion of stored RBCs result in increased medical sequelae? The field is eagerly awaiting the outcome of the ongoing trials.
Recent reporting of the outcome of the Age of Red Blood Cells in Premature Infants (ARIPI) trial showed no difference between “fresh” and “standard” RBCs.14 This was a double-blind, randomized control trial. However, due to the standard practice of giving fresher blood to neonates, the mean age of RBCs in the “fresh” and “standard” groups was 5.1 and 14.6 days, respectively. Therefore, it is unclear whether ARIPI had a group with old enough RBCs to test the hypothesis in a broader context. However, what is clear is that in the standard of practice for transfusing premature infants, no effect of RBC storage age was detected.
Biological changes during RBC storage
Although the potential toxicities and sequelae of transfused RBCs remain controversial, it has been unequivocally demonstrated that a distinct series of biochemical changes occur during storage that gives rise to a litany of chemical and cellular entities known to have biological activities in other contexts. It is thus reasonable to predict that such entities will have effects upon transfusion recipients. Of course, reason does not always predict what is empirically observed, but the extensive experimental evidence on these changes is one driving force behind the strength of opinion that stored RBCs are problematic. These substances can be divided into several different categories.
Effectors of vascular tone
Free hemoglobin (from in-bag hemolysis) can scavenge NO, which may increase vascular tone by depriving blood vessels of the NO required to relax appropriately.15 Frank hemolysis is not required for this because hemoglobin in microparticles can scavenge NO as efficiently as free hemoglobin.15 Finally, it has been reported that asymmetric dimethylarginine can originate from animal and human RBCs16,17 and is known to inhibit NO synthesis. In addition to NO pathways, it has been documented that stored blood products accumulate leukotrienes, which can likewise affect vascular tone in complicated ways.18 It has been reported recently in both reductionist aortic ring studies and hospitalized patients using the “flow-mediated dilation assay” that stored (but not fresh) RBCs produce a decrease in vasodilatory response by NO-mediated pathways.19,20
Induction of cytokine storm
It has been reported in both mice and dogs that transfusion of stored (but not fresh) RBCs results in recipients having systemic inflammatory cytokine release.21,22 Moreover, the induction of baseline inflammation in recipient mice with lipopolysaccharide before transfusion induced a synergistic effect with greatly enhanced cytokine storm after transfusion of stored but not fresh RBCs.22 A controlled trial in healthy volunteers showed no induction of cytokines after transfusion of autologous units that had been stored for 42 days.23 However, careful analysis of the animal data demonstrates that a single unit of RBCs only gives a slight induction of cytokines and multiple units are required for a large effect.22 Moreover, all of the human volunteers were healthy.23 The above data raise the possibility that human biology simply differs from murine and canine biology in this regard. However, equally consistent with the data are the hypotheses that multiple units must be given and that the effect would be augmented in sick patients with baseline innate immune activation. Recent studies lend some support to this latter notion in patients experiencing trauma.24 It has also been reported recently that multiple serum cytokines increased after transfusion in neonates, although fresh RBCs were not compared with older RBCs in that study.25 In addition, it has been reported recently that IL-6 was induced after transfusion of RBCs into a pediatric population, with less induction from washed units.26
In addition to inducing cytokines in mice and dogs, transfusion of older units of RBCs resulted in plasma factors that support the growth of ferrophilic bacteria in either mice or human specimens.21,22,27,28 Contributing significantly to this line of investigation, Solomon et al recently reported a study on dogs receiving a massive exchange transfusion after a pulmonary inoculation with Staphylococcus aureus.29 Strikingly, dogs receiving 42-day-old RBCs had increased mortality and more severe pathology surrounding the lung infection, with increased tissue necrosis. These findings coincided with findings indicating hemolysis in vivo, such as increased NO consumption and decreased haptoglobin. The above findings and reports are generating a body of evidence implicating older RBCs in systemic inflammation and the potential promotion of bacterial infection in nonhuman animal models.
Procoagulant effect
It has been reported that stored RBCs acquire procoagulant activities.30-33 These activities include changes in Russell viper venom time,30 clotting activity,32 increased thrombin generation,31 and prothrombinase and tenase activities.33 Although the mechanisms of these activities are not entirely elucidated, there are data to implicate microparticles, the exposure of phospholipids, and a potential role for tissue factor. Although provocative, the results of these in vitro studies have yet to be transitioned into in vivo observations.
Advanced glycation end products
One of the effects upon exposure of proteins to glucose is a reaction between the aldehyde group of glucose with free amino groups, leading to a Schiff base that rearranges into a series of advanced glycation end (AGE) products, including carboxy-methyl-lysine. Additional chemistries, including cross-linking of glycans, proceed from this point. Overall, AGEs constitute a complex class of molecular glycation with diverse structures. There are several receptors that have been described with the capacity to recognize AGEs of different composition. Most notably is the receptor for advanced glycation end products (RAGE). RAGE plays an active role in inflammation and innate immune activation. Because RBCs are stored in supraphysiological levels of glucose, it has been hypothesized that AGEs would be increased as a result of storage. Indeed, it has been reported that stored RBCs have increased AGEs (ie, carboxy-methyl-lysine) and that they are capable of ligating RAGE, leading to alterations of cultured endothelial cells.34 These findings have led to the hypothesis that AGEs on stored RBCs may play a role in inflammatory pathologies posttransfusion, such as transfusion-related acute lung injury.
Testing the central hypothesis
It seems clear, or at least very likely, that retrospective approaches have been used to their fullest benefit. The result of combined analyses of retrospective findings leads to a murky view with an equivocal outcome. Accordingly, ongoing analysis must involve prospective trials. However, for such a prospective trial to be meaningful, it is necessary to ask the correct questions and collect the appropriate data. Above all else, it is essential to frame the hypotheses in a fashion that is amenable to being tested and susceptible to rejection.
Of course, the more generalizable knowledge is, the more useful it is. Therefore, the very general hypothesis that “older blood results in worse medical outcomes” covers the essence of what one would want to know about potential sequelae of transfusing stored blood products. However, the more generalized a hypothesis, the more one risks oversimplifying complex landscapes. The result can lead to questions that are essentially meaningless in substance and impossible to answer. Although it is not clear that this is the case with testing the hypothesis that “older blood results in worse medical outcomes,” it is necessary to give careful attention to this issue and full consideration to its implications.
One source of generalization in the question of whether “older blood results in worse medical outcomes” is the lumping together of disparate recipient groups. Blood transfusions are given for a wide number of different indications. The physiological effects of exposure to the biochemical changes that occur in a stored blood product may be vastly different depending upon the pathophysiology of the transfusion recipient. Some retrospective trials have looked at all hospitalized patients who were transfused within a defined period of time and juxtaposed generalized markers of medical outcome between groups of patients receiving RBCs of different storage times. However, other retrospective trials are certainly more focused and prospective trials are inevitably so due to the need to limit sample size as a practical matter. Nevertheless, even in the narrower context, the problem persists.
Consider a trial that is focused on patients in a particular category of disease (eg, trauma patients arriving at the emergency department, patients with sickle cell disease, patients admitted to the intensive care unit, patients undergoing cardiac surgery, etc). Even within these definitions, which are clearly narrower than generalized populations, there is a distinct heterogeneity of recipient pathology that may alter the effects of stored blood transfusion. For example, consider patients admitted to the intensive care unit. For the sake of example, let us also assume that free hemoglobin in stored RBCs is of a sufficient dose that it scavenges enough NO from the recipient such that vascular relaxation is impaired. One subset of intensive care patients may be suffering from insufficient blood flow to a vital organ (eg, thrombotic disease, atherosclerotic stenosis, etc). In such a patient, it is reasonable to predict that impairing vascular relaxation would exacerbate their condition and may lead to a worse outcome. However, another subset of intensive care patients may have a pathophysiology in which insufficient vessel tone is playing a role (eg, some forms of shock, sepsis, disease with an anaphylactic component, etc). In this case, it is possible that the scavenging of NO may do no harm and may even have a therapeutic effect, essentially acting as a vasopressor, the latter being a standard pharmacological approach to treating shock. Similarly, procoagulant activity in stored RBCs may be harmful to a patient with atherosclerotic or thrombotic complications, but therapeutic in a patient who is hemorrhaging. Indeed, biologies of this nature could go a long way toward explaining why, in some settings, fresher blood seems to be worse than older blood.
In the above scenario, it is easy to see that even very real effects on outcome of disease treatment will be difficult to observe due to the combination of disparate groups with alternate biological factors into a single test population. To make matters worse, this same concern can be applied to each of the above known cellular and chemical changes that occur during RBC storage. Therefore, the simultaneous effect of multiple changes in the blood unit and the differential effect that each one may have on alternative pathophysiologies may lead to waters too muddy to see through.
The problems of oversimplification through the combining of disparate groups into a single category is not limited to patient populations. There is an additional and very serious concern regarding the definition of the age of the stored unit. On the surface, this seems like a very straightforward notion indeed; one counts the time (in this case days) from when the unit was drawn and an unequivocal age is determined as a function of time. However, it has been well described that there is substantial donor-to-donor variation in how RBCs store. Donor variation is perhaps best described with regard to posttransfusion recovery and survival of 51Cr-labeled RBCs. However, donor variation has also been described with regard to in vitro “in the bag” hemolysis before transfusion, microparticle accumulation, and accumulation of leukotrienes. The basis of this variation appears to have a genetic component and shows patterns of heritability in humans. This same pattern of donor variability is observed in animal models of RBC storage between genetically distinct strains of inbred mice.35 Recently, even the sex of the donor has been shown to play a substantial role in this process, both in humans and in mice.36,37 This donor variation in RBC storage underscores the fundamental problem in testing the medical effect of stored RBCs based upon chronological age. One donor's blood may be pristine after 42 days of storage, whereas another donor's blood may have much free hemoglobin, leukotriene synthesis, microparticles, AGEs, and induce a cytokine storm after only 7 days of storage. The only way to remedy this problem is to measure quantitatively the characterized biology of stored RBC units before transfusion and posttransfusion survivals/recoveries, which may not be logistically feasible in human prospective trials and is clearly impossible in retrospective studies.
An additional theoretical problem has been generated by studies in animal models of stored RBCs. It has been reported in murine systems that storing RBCs both increases recipient cytokine storm upon transfusion and also increases the immunogenicity of at least one antigen on the stored RBCs.38 Somewhat surprisingly, when the same recipient is transfused with fresh RBCs and then old RBCs (through different tail veins), the presence of the fresh RBCs mitigates both the increased cytokines and increased immunogenicity of the stored RBCs.39 The explanation for this phenomenon is unclear and it is also unclear whether a similar biology occurs in humans. However, should such biology exist in human transfusion, it further complicates trials in which groups receive an age range of RBCs. For ethical concerns, groups are often established that receive “fresh blood” versus “standard issue,” because it is unclear whether purposefully giving a group uniform older blood is ethically appropriate.
Summary
The answer to the question of whether there is a difference between fresh and old blood seems to be an unequivocal “yes.” The storage lesion is a clear process by which RBCs degrade over time. On the contrary, the question of “does it matter” remains unclear and is likely to remain so for some time. Retrospective studies give a variety of answers. Ongoing prospective trials have the potential to shed great light on this issue and represent an excellent step forward in these studies. However, many confounding problems are unavoidable in these first prospective trials (as detailed above) such that a negative finding will not resolve the issue. Due to the sheer magnitude of transfusions given each year (1/70 Americans), even small effects on outcome may have widespread medical significance both in patient outcome and cost of care. Blood transfusion is clearly a lifesaving maneuver in many cases. Nevertheless, the accumulating data showing that restrictive transfusion practices often result in better medical outcomes provide a rational basis for the understanding that RBC transfusions may do more harm than good in certain settings.40 The extent to which the age of the stored RBCs plays a role in this process is an essential question because the answer will guide both medical practice and basic research into storage technologies that improve outcomes focusing on the health of the transfusion recipient.
Disclosures
Conflict-of-interest disclosure: The author has received research funding from Terumo and Immucor, has consulted for Haemonetics, and has received honoraria from Cerus. Off-label drug use: None disclosed.
Correspondence
James C. Zimring, MD, PhD, Puget Sound Blood Center Research Institute, 1551 Eastlake Ave E, Seattle, WA 98102; Phone: 206-568-2200; Fax: 206-587-6056; e-mail: jzimring@psbc.org.
